New Approach for the One-Pot Synthesis of 1,3,5-Triazine Derivatives: Application of Cu(I) Supported on a Weakly Acidic Cation-Exchanger Resin in a Comparative Study
Abstract
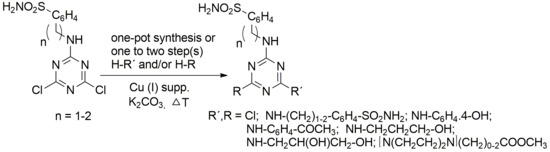
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Information
3.2. Catalyst Preparation and Characterization
3.3. General Synthetic Procedures
3.3.1. General Method for Synthesis of Disubstituted Derivatives of Cyanuric Chloride as a Step-by-Step Reaction
3.3.2. General Method for Synthesis of Trisubstituted Derivatives of Cyanuric Chloride as a Step-by-Step Reaction
3.3.3. General Method for Synthesis of Trisubstituted Derivatives of Cyanuric Chloride as a One-Pot Reaction
3.3.4. Characterization of New Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al-Turki, D.A.; Abou-Zeid, L.A.; Shehata, I.A.; Al-Omar, M.A. Therapeutic and toxic effects of new NSAIDs and related compounds: A review and prospective study. Int. J. Pharm. 2010, 6, 813–825. [Google Scholar] [CrossRef]
- Chen, X.; Zhan, P.; Liu, X.; Cheng, Z.; Meng, C.; Shao, S.; Pannecouque, C.; Clercq, E.D.; Liu, X. Design, synthesis, anti-HIV evaluation and molecular modeling of piperidine-linked amino-triazine derivatives as potent non-nucleoside reverse transcriptase inhibitors. Bioorg. Med. Chem. 2012, 20, 3856–3864. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.R.; Modh, R.P.; Chikhalia, K.H. Privileged s-triazines: Structure and pharmacological applications. Future Med. Chem. 2014, 6, 463–477. [Google Scholar] [CrossRef]
- Singla, P.; Luxami, V.; Paul, K. Triazine as a promising scaffold for its versatile biological behavior. Eur. J. Med. Chem. 2015, 102, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; Supuran, C.T. An overview of the selectivity and efficiency of the bacterial carbonic anhydrase inhibitors. Curr. Med. Chem. 2015, 22, 2130–2139. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; Supuran, C. Inhibition of bacterial carbonic anhydrases as a novel approach to escape drug resistance. Curr. Top. Med. Chem. 2017, 17, 1237–1248. [Google Scholar] [CrossRef]
- Supuran, C.T.; Alterio, V.; Di Fiore, A.; D’ Ambrosio, K.; Carta, F.; Monti, S.M.; De Simone, G. Inhibition of carbonic anhydrase IX targets primary tumors, metastases, and cancer stem cells: Three for the price of one. Med. Res. Rev. 2018, 38, 1799–1836. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin. Drug Discov. 2016, 12, 61–88. [Google Scholar] [CrossRef]
- Supuran, C. Carbonic Anhydrases and metabolism. Metabolites 2018, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Carta, F.; Garaj, V.; Maresca, A.; Wagner, J.; Avvaru, B.S.; Robbins, A.H.; Scozzafava, A.; McKenna, R.; Supuran, C.T. Sulfonamides incorporating 1,3,5-triazine moieties selectively and potently inhibit carbonic anhydrase transmembrane isoforms IX, XII and XIV over cytosolic isoforms I and II: Solution and X-ray crystallographic studies. Bioorg. Med. Chem. 2011, 19, 3105–3119. [Google Scholar] [CrossRef]
- Huthmacher, K.; Most, D. Cyanuric acid and cyanuric chloride. In Ullmann’s Encyclopedia of Industrial Chemistry; Elvers, B., Ed.; Wiley: Weinheim, Germany, 2000. [Google Scholar]
- Garaj, V.; Puccetti, L.; Fasolis, G.; Winum, J.-Y.; Montero, J.-L.; Scozzafava, A.; Vullo, D.; Innocenti, A.; Supuran, C.T. Carbonic anhydrase inhibitors: Synthesis and inhibition of cytosolic/tumor-associated carbonic anhydrase isozymes I, II, and IX with sulfonamides incorporating 1,2,4-triazine moieties. Bioorg. Med. Chem. Lett. 2004, 14, 5427–5433. [Google Scholar] [CrossRef] [PubMed]
- Garaj, V.; Puccetti, L.; Fasolis, G.; Winum, J.-Y.; Montero, J.-L.; Scozzafava, A.; Vullo, D.; Innocenti, A.; Supuran, C.T. Carbonic anhydrase inhibitors: Novel sulfonamides incorporating 1,3,5-triazine moieties as inhibitors of the cytosolic and tumour-associated carbonic anhydrase isozymes I, II and IX. Bioorg. Med. Cem. Lett. 2005, 15, 3102–3108. [Google Scholar] [CrossRef] [PubMed]
- Afonso, C.; Lourenco, N.; Rosatella, A. Synthesis of 2,4,6-Trisubstituted-1,3,5-Triazines. Molecules 2006, 11, 81–102. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Dao, P.; Laporte, A.; Garbay, C. High yielding microwave-assisted synthesis of 2-(arylmethyl)amino-4-arylamino-6-alkyl-1,3,5-triazines. Tetrahedron Lett. 2010, 51, 3174–3176. [Google Scholar] [CrossRef]
- Alkalay, D.; Volk, J.; Bartlett, M.F. Conversion of biguanides into substituted s-triazines assayable by GC or mass fragmentography. J. Pharm. Sci. 1976, 65, 525–529. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Wang, F.; Wu, C.; Shi, T.; Min, D.; Chen, H.; Zhang, W. Synthesis of 1,3,5-triazines via Cu(OAc)2-catalyzed aerobic oxidative coupling of alcohols and amidine hydrochlorides. Org. Biomol. Chem. 2015, 13, 6723–6727. [Google Scholar] [CrossRef]
- Zhang, C.; Ban, M.-T.; Zhu, K.; Zhang, L.-Y.; Luo, Z.-Y.; Guo, S.-N.; Cui, D.-M.; Zhang, Y. Copper-catalyzed synthesis of substituted 2,4-diamino-1,3,5-triazines from 1,1-dibromoalkenes and biguanides. Org. Lett. 2017, 19, 3947–3949. [Google Scholar] [CrossRef] [PubMed]
- Kore, N.; Pazdera, P. New stable Cu(I) catalyst supported on weakly acidic polyacrylate resin for green C-N coupling: Synthesis of N-(pyridin-4-yl)benzene amines and N,N-bis(pyridine-4-yl)benzene amines. Molecules 2017, 22, 2. [Google Scholar] [CrossRef]
- Kore, N.; Pazdera, P. New stable Cu(I) catalyst supported on weakly acidic polyacrylate resin for “click” chemistry: Synthesis of 1,2,3-triazole and novel synthesis of 1,2,3-triazol-5-amine. Curr. Org. Synth. 2018, 15, 552–565. [Google Scholar] [CrossRef]
- Reddy, K.R.; Kumar, N.S.; Sreedhar, B.; Kantam, M.L. N-Arylation of nitrogen heterocycles with aryl halides and arylboronic acids catalyzed by cellulose supported copper(0). J. Mol. Catal. A Chem. 2006, 252, 136–141. [Google Scholar] [CrossRef]
- Ouali, A.; Laurent, R.; Caminade, A.-M.; Majoral, J.-P.; Taillefer, M. Enhanced catalytic properties of copper in O- and N-arylation and vinylation reactions, using phosphorus dendrimers as ligands. J. Am. Chem. Soc. 2006, 128, 15990–15991. [Google Scholar] [CrossRef] [PubMed]
- Choudary, B.M.; Sridhar, C.; Kantam, M.L.; Venkanna, G.T.; Sreedhar, B. Design and evolution of copper apatite catalysts for N- Arylation of heterocycles with chloro- and fluoroarenes. J. Am. Chem. Soc. 2005, 127, 9948–9949. [Google Scholar] [CrossRef] [PubMed]
- Kantam, M.L.; Venkanna, G.T.; Sridhar, C.; Shiva Kumar, K.B. Copper fluorapatite catalyzed N-arylation of heterocycles with bromo and iodoarenes. Tetrahedron Lett. 2006, 47, 3897–3899. [Google Scholar] [CrossRef]
- Rout, L.; Jammi, S.; Punniyamurthy, T. Novel CuO nanoparticle catalyzed C−N cross coupling of amines with iodobenzene. Org. Lett. 2007, 9, 3397–3399. [Google Scholar] [CrossRef] [PubMed]
- Son, S.-U.; Park, I.-K.; Park, J.; Hyeon, T. Synthesis of Cu2O coated Cu nanoparticles and their successful applications to Ullmann-type amination coupling reactions of aryl chlorides. Chem. Commun. 2004, 7, 778–779. [Google Scholar] [CrossRef]
- Dutta, P.K.; Sen, S.; Saha, D.; Dhar, B. Solid supported nano structured Cu-catalyst for solvent/ligand free C 2 amination of azoles. Eur. J. Org. Chem. 2018, 2018, 657–665. [Google Scholar] [CrossRef]
- Islam, S.M.; Salam, N.; Mondal, P.; Roy, A.S.; Ghosh, K.; Tuhina, K. A highly active reusable polymer anchored copper catalyst for C–O, C–N and C–S cross coupling reactions. 2014, 387, 7–19. J. Mol. Catal. A Chem. 2014, 387, 7–19. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, K.; Li, T.; Tan, B.; Gu, Y. Functionalized hypercrosslinked polymers with knitted N-heterocyclic carbene–copper complexes as efficient and recyclable catalysts for organic transformations. Catal. Sci. Technol. 2016, 6, 4345–4355. [Google Scholar] [CrossRef]
- Benaskar, F.; Patil, N.G.; Rebrov, E.V.; Ben-Abdelmoumen, A.; Meuldijk, J.; Hulshof, L.A.; Hessel, V.; Schouten, J.C. Micro/Milliflow Processing with selective catalyst microwave heating in the Cu-catalyzed ullmann etherification reaction: A μ2-process. ChemSusChem 2013, 6, 353–366. [Google Scholar] [CrossRef]
- Likhar, P.R.; Roy, S.; Roy, M.; Kantam, M.L.; De, R.L. Silica immobilized copper complexes: Efficient and reusable catalysts for N-arylation of N(H)-heterocycles and benzyl amines with aryl halides and arylboronic acids. J. Mol. Catal. A Chem. 2007, 271, 57–62. [Google Scholar] [CrossRef]
- Choplin, A.; Quignard, F. From supported homogeneous catalysts to heterogeneous molecular catalysts. Coord. Chem. Rev. 1998, 178–180, 1679–1702. [Google Scholar] [CrossRef]
- Ahluwalia, V.K.; Aggarwal, R. Organic Synthesis: Special Techniques, 2nd ed.; Alpha Science International: Oxford, England, 2006. [Google Scholar]
- Pazdera, P. Chapter 1. Emerging ubiquity of green chemistry in engineering and technology. In Handbook on Applications of Ultrasound-Sonochemistry for Sustainability, 1st ed.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2011. [Google Scholar]
- Barbaro, P.; Liguori, F. Ion exchange resins: Catalyst recovery and recycle. Chem. Rev. 2009, 109, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Keiji, O.; Mutsumi, S. Method for producing hydroxylamine compound using platinum catalyst fixed on ion-exchange resin. U.S. 020060106254 Al, 18 May 2006. [Google Scholar]
- Herrmann, W.A.; Kratzer, R.M.; Bliimel, J.; Friedrich, H.B.; Fischer, R.W.; Apperley, D.C.; Mink, J.; Berkesi, O. Polymer supported catalyst for the effective autoxidation of cumene to cumene hydroperoxide. J. Mol. Catal. A Chem. 1997, 120, 109–116. [Google Scholar]
- Yu, L.; Chen, D.; Li, J.; Wang, P.G. Preparation, characterization, and synthetic uses of Lanthanide(III) catalysts supported on ion exchange resins. J. Org. Chem. 1997, 62, 3575–3581. [Google Scholar] [CrossRef]
- Havránková, E.; Csollei, J.; Vullo, D.; Garaj, V.; Pazdera, P.; Supuran, C.T. Novel sulfonamide incorporating piperazine, aminoalcohol and 1,3,5-triazine structural motifs with carbonic anhydrase I, II and IX inhibitory action. Bioorg. Chem. 2018, 77, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Sambiago, C.; Marsden, S.P.; Blacker, A.J.; McGowan, P.C. Copper catalyzed Ullmann type chemistry: From mechanistic aspects to modern development. Chem. Soc. Rev. 2014, 43, 3525–3550. [Google Scholar] [CrossRef]
- Chassaing, S.; Bénéteau, V.; Pale, P. When CuAAC “click chemistry” goes heterogeneous. Catal. Sci. Technol. 2016, 6, 923–957. [Google Scholar] [CrossRef]
Sample Availability: Samples of the catalyst are available from the author Pavel Pazdera (pazdera@chemi.muni.cz, Department of Chemistry, Faculty of Science, Centre for Syntheses at Sustainable Conditions and Their Management, Masaryk University). |


| Compound | n | R | R′ | Without Catalyst | Catalyst-Supported Cu(I) b | One-Pot Reaction; Catalyst-Supported Cu(I) b | |||
|---|---|---|---|---|---|---|---|---|---|
| t (h) | %yield | t (h) | %yield | t (h) | %yield | ||||
| 1 a | 1 | [N(CH2CH2)2N]COOCH3 | Cl | 168 | 63.4 | 10 | 67.2 | - | - |
| 2 a | 0 | [N(CH2CH2)2N]COOCH3 | Cl | 64 | 89.3 | 4 | 87.9 | - | - |
| 3 a | 1 | [N(CH2CH2)2N]CH2COOCH3 | Cl | 144 | 44.3 | 8 | 54.8 | - | - |
| 4 a | 0 | [N(CH2CH2)2N]CH2COOCH3 | Cl | 50 | 86.9 | 3 | 88.1 | - | - |
| 5 a | 1 | [N(CH2CH2)2N]CH2CH2COOCH3 | Cl | 72 | 29.8 | 2 | 59.9 | - | - |
| 6 | 1 | NH-CH2CH2CH2OH | Cl | 23 | 64.1 | 7 | 57.4 | - | - |
| 7 | 2 | NH-CH2CH2CH2OH | Cl | 27 | 97.9 | 3 | 95.2 | - | - |
| 8 | 2 | NH-CH2CH(OH)CH2OH | Cl | 13 | 87.6 | 5 | 77.2 | - | - |
| 9 | 2 | [N(CH2CH2)2N]CH2CH2COOCH3 | Cl | 33 | 77.8 | 5 | 78.1 | - | - |
| 10 a | 1 | NH-CH2CH2-C6H4(1,4)-4-SO2NH2 | NH-CH2CH2-C6H4(1,4)-4-SO2NH2 | 22 | 32.0 | 2 | 51.9 | 12 | 56.9 |
| 11 a | 0 | NH-CH2CH2-C6H4(1,4)-4-SO2NH2 | NH-CH2CH2-C6H4(1,4)-4-SO2NH2 | 24 | 68.3 | 2 | 71.2 | 8 | 73.2 |
| 12 a | 1 | NH-CH2-C6H4(1,4)-4-SO2NH2 | NH-CH2CH(OH)CH2OH | 30 | 45.5 | 4 | 62.9 | 16 | 67.1 |
| 13 a | 1 | [N(CH2CH2)2N]COOCH3 | NH-CH2CH(OH)CH2OH | 72 | 20.2 | 8 | 46.0 | 18 | 51.4 |
| 14 a | 1 | NH-C6H4(1,4)-4-OH | NH-CH2CH(OH)CH2OH | 96 | 24.6 | 7 | 54.8 | 14 | 60.7 |
| 15 a | 1 | [N(CH2CH2)2N]CH2COOCH3 | NH-CH2CH(OH)CH2OH | 72 | 27.0 | 5 | 53.9 | 13 | 56.8 |
| 16 | 2 | [N(CH2CH2)2N]CH2CH2COOCH3 | NH-C6H4(1,4)-4-COCH3 | 16 | 26.8 | 5 | 47.0 | 9 | 49.8 |
| 17 | 2 | NH-CH2CH(OH)CH2OH | NH-C6H4(1,4)-4-COCH3 | 9 | 35.5 | 3 | 46.0 | 10 | 47.6 |
| Catalyst | t (h) | %Yield |
|---|---|---|
| supported Cu(I) a | 2 | 59.9 |
| CuI a | 75 | 28.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Havránková, E.; Csöllei, J.; Pazdera, P. New Approach for the One-Pot Synthesis of 1,3,5-Triazine Derivatives: Application of Cu(I) Supported on a Weakly Acidic Cation-Exchanger Resin in a Comparative Study. Molecules 2019, 24, 3586. https://doi.org/10.3390/molecules24193586
Havránková E, Csöllei J, Pazdera P. New Approach for the One-Pot Synthesis of 1,3,5-Triazine Derivatives: Application of Cu(I) Supported on a Weakly Acidic Cation-Exchanger Resin in a Comparative Study. Molecules. 2019; 24(19):3586. https://doi.org/10.3390/molecules24193586
Chicago/Turabian StyleHavránková, Eva, Jozef Csöllei, and Pavel Pazdera. 2019. "New Approach for the One-Pot Synthesis of 1,3,5-Triazine Derivatives: Application of Cu(I) Supported on a Weakly Acidic Cation-Exchanger Resin in a Comparative Study" Molecules 24, no. 19: 3586. https://doi.org/10.3390/molecules24193586
APA StyleHavránková, E., Csöllei, J., & Pazdera, P. (2019). New Approach for the One-Pot Synthesis of 1,3,5-Triazine Derivatives: Application of Cu(I) Supported on a Weakly Acidic Cation-Exchanger Resin in a Comparative Study. Molecules, 24(19), 3586. https://doi.org/10.3390/molecules24193586