Potent Antitrypanosomal Activities of 3-Aminosteroids against African Trypanosomes: Investigation of Cellular Effects and of Cross-Resistance with Existing Drugs
Abstract
:1. Introduction
2. Results
2.1. Pentacyclic 3-Aminosteroids Are More Active against Tc-IL3000 Than Tbb 427WT
2.2. Cross Resistance of 3-Aminosteroids with Standard Trypanocides
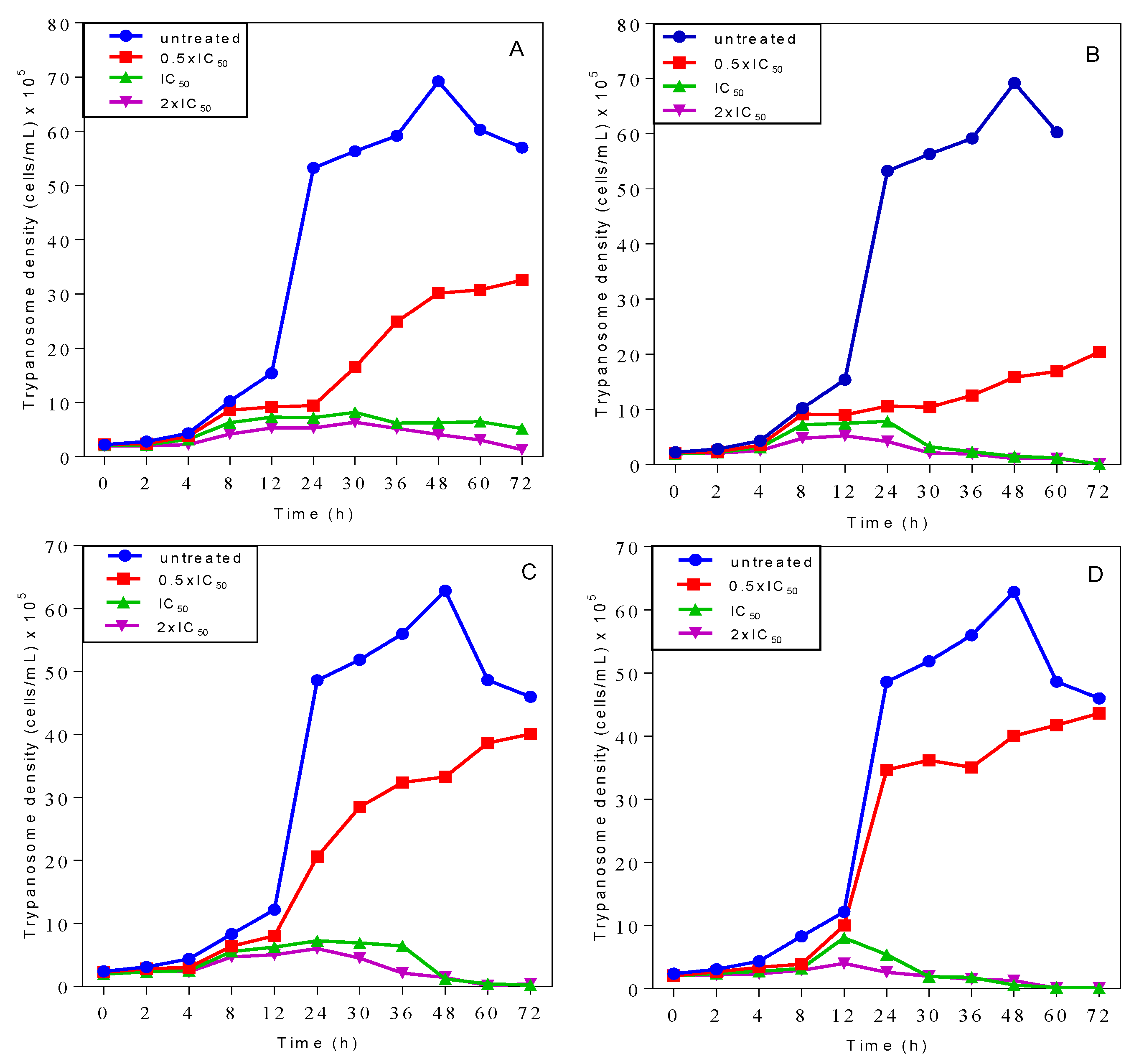
2.3. Effects on Growth Patterns of T. b. brucei Cells after Short and Long Duration of Exposure
2.4. Propidium Iodide (PI) Assay of Cellular Integrity
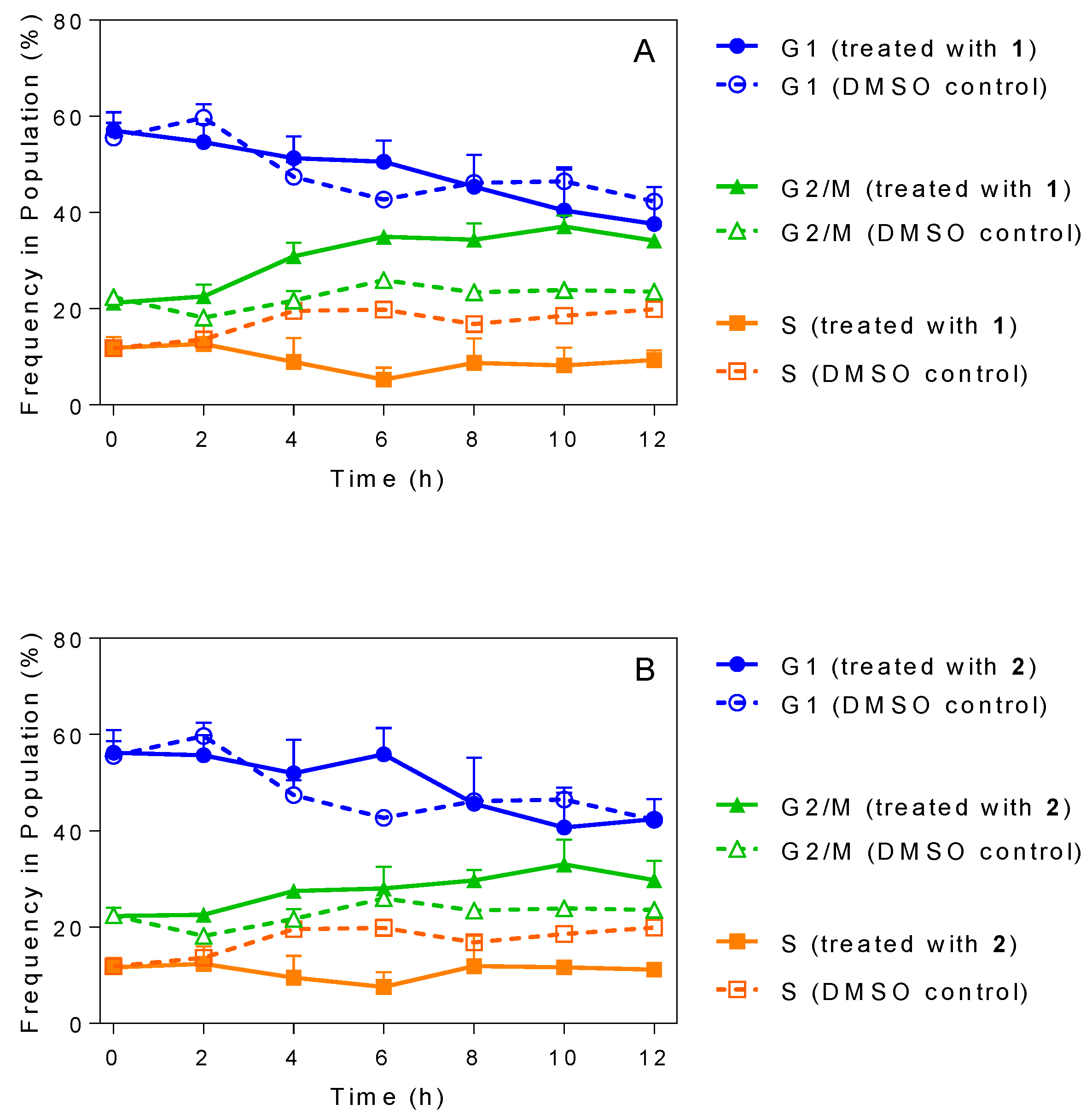
2.5. Influence of 3-Aminosteroids on DNA Content/Cell Cycle of Wild-Type T. b. brucei
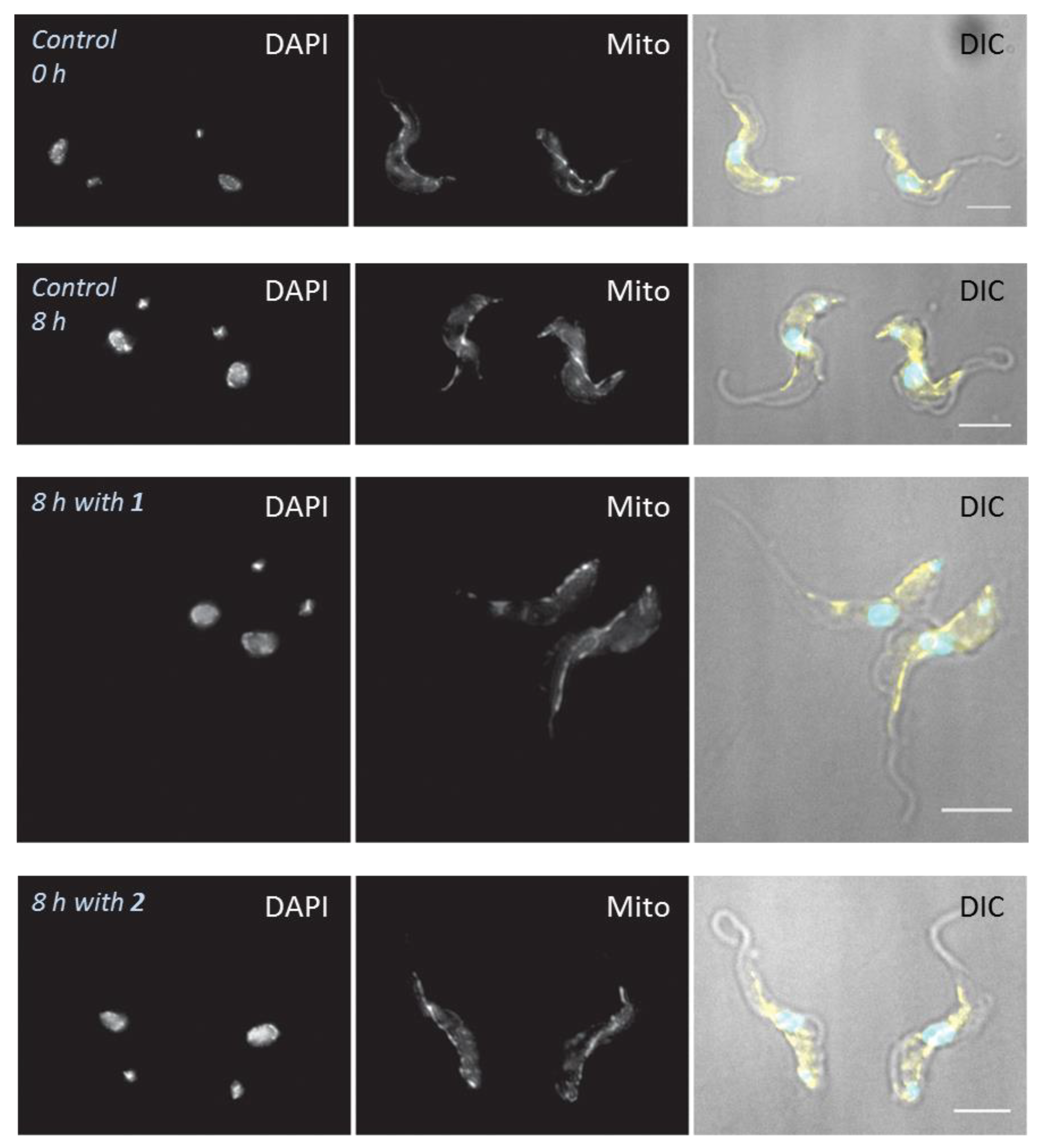
2.6. Influence of 3-Aminosteroids on Mitochondrial Membrane Potential (MMP)
2.7. Influence of 3-Aminosteroids on Intracellular ATP Levels
3. Discussion
4. Materials and Methods
4.1. Parasites, Cell Lines and Cultures
4.2. Determination of In Vitro Anti-Trypanosomal Activity of Compounds
4.3. Effect of Treatments on Growth Patterns of T. b. brucei after Short- and Long-Term Exposure
4.4. Determination of Speed of Action by Propidium Iodide (PI) Assay
4.5. Determination of Intracellular ATP Level
4.6. Effects of Treatments on Cell Morphology
4.7. Determination of Mitochondrial Membrane Potential
4.8. Determination of Cell Cycle and DNA Content Assay
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Giordani, F.; Morrison, L.J.; Rowan, T.G.; De Koning, H.P.; Barrett, M.P. The animal trypanosomiases and their chemotherapy: A review. Parasitology 2016, 143, 1862–1889. [Google Scholar] [CrossRef]
- PATTEC. A continental plan of action for the eradication of Tsetse and trypanosomosis. In The OAU Pathway for the PATTEC; Pan African Tsetse and Trypanosomosis Global Veterinaria, Eradication Campaign: Khartoum, Sudan, 2000. [Google Scholar]
- Auty, H.; Torr, S.J.; Michoel, T.; Jayaraman, S.; Morrison, L.J. Cattle trypanosomosis: The diversity of trypanosomes and implications for disease epidemiology and control. Rev. Sci. Tech. 2015, 34, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Holt, H.R.; Selby, R.; Mumba, C.; Napier, G.B.; Guitian, J. Assessment of animal African trypanosomiasis (AAT) vulnerability in cattle-owning communities of sub-Saharan Africa. Parasites Vectors 2016, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- CFSPH. African animal trypanosomiasis. In The Fact Sheet; The Centre for Food Security and Public Health: Victoria, BC, Canada, 2009. [Google Scholar]
- Shaw, A.P.M.; Cecchi, G.; Wint, G.R.W.; Mattioli, R.C.; Robinson, T.P. Mapping the economic benefits to livestock keepers from intervening against bovine trypanosomosis in Eastern Africa. Prev. Vet. Med. 2014, 113, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.C.; Feldmann, U.; Hendrickx, G.; Wint, W.; Jannin, J.; Slingenbergh, J. Tsetse and trypanosomiasis intervention policies supporting sustainable animal-agricultural development. J. Food Agric. Environ. 2004, 2, 310–314. [Google Scholar]
- Hursey, B.S. The programme against African trypanosomiasis: Aims, objectives and achievements. Trends Parasitol. 2001, 17, 2–3. [Google Scholar] [CrossRef]
- Delespaux, V.; De Koning, H.P. Drugs and drug resistance in African trypanosomiasis. Drug Resist. Updat. 2007, 10, 30–50. [Google Scholar] [CrossRef]
- Assefa, A.; Shibeshi, W. Drug resistance in African animal trypanosomes: A review. Afr. J. Microbiol. Res. 2018, 12, 380–386. [Google Scholar]
- Holmes, P. First WHO meeting of stakeholders on elimination of gambiense Human African Trypanosomiasis. PLoS Negl. Trop. Dis. 2014, 8, e3244. [Google Scholar] [CrossRef]
- Holmes, P. On the road to elimination of rhodesiense human African trypanosomiasis: First WHO meeting of stakeholders. PLoS Negl. Trop. Dis. 2015, 9, e0003571. [Google Scholar] [CrossRef]
- Morrison, L.J.; Vezza, L.; Rowan, T.; Hope, J.C. Animal African trypanosomiasis: Time to increase focus on clinically relevant parasite and host species. Trends Parasitol. 2016, 32, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Nnadi, C.O.; Nwodo, N.J.; Kaiser, M.; Brun, R.; Schmidt, T.J. Steroid Alkaloids from Holarrhena africana with Strong Activity against Trypanosoma brucei rhodesiense. Molecules 2017, 22, 1129. [Google Scholar] [CrossRef] [PubMed]
- Moretti, C.; Sauvain, M.; Lavaud, C.; Massiot, G.; Bravo, J.A.; Munoz, V. A novel antiprotozoal aminosteroid from Saracha punctata. J. Nat. Prod. 1998, 61, 1390–1393. [Google Scholar] [CrossRef] [PubMed]
- Nnadi, C.O.; Althaus, J.B.; Nwodo, N.J.; Schmidt, T.J. A 3D-QSAR study on the antitrypanosomal and cytotoxic activities of steroid alkaloids by comparative molecular field analysis. Molecules 2018, 23, 1113. [Google Scholar] [CrossRef]
- Schenone, M.; Dančík, V.; Wagner, B.K.; Clemons, P.A. Target identification and mechanism of action in chemical biology and drug discovery. Nat. Chem. Biol. 2013, 9, 232. [Google Scholar] [CrossRef]
- McNamara, C.; Winzeler, E.A. Target identification and validation of novel antimalarials. Future Microbiol. 2011, 6, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Xie, L.; Bourne, P.E. Structure-based systems biology for analyzing off-target binding. Curr. Opin. Struct. Biol. 2011, 21, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Apsel, B.; Blair, J.A.; Gonzalez, B.; Nazif, T.M.; Feldman, M.E.; Aizenstein, B.; Hoffman, R.; Williams, R.L.; Shokat, K.M.; Knight, Z.A. Targeted polypharmacology: Discovery of dual inhibitors of tyrosine and phosphoinositide kinases. Nat. Chem. Biol. 2008, 4, 691. [Google Scholar] [CrossRef]
- Das, A.; Li, H.; Liu, T.; Bellofatto, V. Biochemical characterization of Trypanosoma brucei RNA polymerase II. Mol. Biochem. Parasitol. 2006, 150, 201–210. [Google Scholar] [CrossRef]
- Matovu, E.; Stewart, M.L.; Geiser, F.; Brun, R.; Mäser, P.; Wallace, L.J.; Seebeck, T. Mechanisms of arsenical and diamidine uptake and resistance in Trypanosoma brucei. Eukaryot. Cell 2003, 2, 1003–1008. [Google Scholar] [CrossRef]
- Bridges, D.J.; Gould, M.K.; Nerima, B.; Mäser, P.; Burchmore, R.J.; De Koning, H.P. Loss of the high-affinity pentamidine transporter is responsible for high levels of cross-resistance between arsenical and diamidine drugs in African trypanosomes. Mol. Pharmacol. 2007, 71, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Eze, A.A.; Gould, M.K.; Munday, J.C.; Tagoe, D.N.; Stelmanis, V.; Schnaufer, A.; De Koning, H.P. Reduced Mitochondrial Membrane Potential Is a Late Adaptation of Trypanosoma brucei brucei to Isometamidium Preceded by Mutations in the γ Subunit of the F1Fo-ATPase. PLoS Negl. Trop. Dis. 2016, 10, e0004791. [Google Scholar] [CrossRef] [PubMed]
- Gould, M.K.; Bachmaier, S.; Ali, J.A.; Alsford, S.; Tagoe, D.N.; Munday, J.C.; Schnaufer, D.H.; Boshart, M.; de Koning, H.P. Cyclic AMP effectors in African trypanosomes revealed by genome-scale RNA interference library screening for resistance to the phosphodiesterase inhibitor CpdA. Antimicrob. Agents Chemother. 2013, 57, 4882–4893. [Google Scholar] [CrossRef]
- Baker, N.; Glover, L.; Munday, J.C.; Andrés, D.A.; Barrett, M.P.; De Koning, H.P.; Horn, D. Aquaglyceroporin 2 controls susceptibility to melarsoprol and pentamidine in African trypanosomes. Proc. Natl Acad. Sci. USA 2012, 109, 10996–11001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebiloma, G.U.; Ayuga, T.D.; Balogun, E.O.; Gil, L.A.; Donachie, A.; Kaiser, M.; Herraiz, T.; Inaoka, D.K.; Shiba, T.; Harada, S.; et al. Inhibition of trypanosome alternative oxidase without its N-terminal mitochondrial targeting signal (ΔMTS-TAO) by cationic and non-cationic 4-hydroxybenzoate and 4-alkoxybenzaldehyde derivatives active against T. brucei and T. congolense. Eur. J. Med. Chem. 2018, 150, 385–402. [Google Scholar] [CrossRef] [PubMed]
- Jeacock, L.; Baker, N.; Wiedemar, N.; Mäser, P.; Horn, D. Aquaglyceroporin-null trypanosomes display glycerol transport defects and respiratory-inhibitor sensitivity. PLoS Pathog. 2017, 13, e1006307. [Google Scholar] [CrossRef] [PubMed]
- De Koning, H.P.; Jarvis, S.M. Adenosine transporters in bloodstream forms of T. brucei: Substrate recognition motifs and affinity for trypanocidal drugs. Mol. Pharmacol. 1999, 56, 1162–1170. [Google Scholar] [CrossRef]
- Munday, J.C.; Tagoe, D.N.A.; Eze, A.A.; Krezdorn, J.A.; Rojas López, K.E.; Alkhaldi, A.A.M.; McDonald, F.; Still, J.; Alzahrani, K.J.; Settimo, L.; et al. Functional analysis of drug resistance-1 associated mutations in the Trypanosoma brucei Adenosine Transporter 1 (TbAT1) and the proposal of a structural model for the protein. Mol. Microbiol. 2015, 96, 887–900. [Google Scholar] [CrossRef]
- Collar, C.J.; Al-Salabi, M.I.; Stewart, M.L.; Barrett, M.P.; Wilson, W.D.; De Koning, H.P. Predictive computational models of substrate binding by a nucleoside transporter. J. Biol. Chem. 2009, 284, 34028–34035. [Google Scholar] [CrossRef]
- De Koning, H.P. The ever-increasing complexities of arsenical-diamidine cross-resistance in African trypanosomes. Trends Parasitol. 2008, 24, 345–349. [Google Scholar] [CrossRef]
- Utz, S.; Roditi, I.; Renggli, C.K.; Almeida, I.C.; Acosta-Serrano, A.; Bütikofer, P. Trypanosoma congolense procyclins: Unmasking cryptic major surface glycoproteins in procyclic forms. Eukaryot. Cell 2006, 5, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- De Koning, H.P. Drug resistance in protozoan parasites. Emerg. Top. Life Sci. 2017, 1, 627–632. [Google Scholar] [CrossRef] [Green Version]
- Alkhaldi, A.A.; Creek, D.J.; Ibrahim, H.; Kim, D.H.; Quashie, N.B.; Burgess, K.E.; Changtam, C.; Barrett, M.P.; Suksamrarn, A.; de Koning, H.P. Potent trypanocidal curcumin analogs bearing a monoenone linker motif act on Trypanosoma brucei by forming an adduct with trypanothione. Mol. Pharmacol. 2015, 87, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Luscher, A.; De Koning, H.P.; Maser, P. Chemotherapeutic strategies against Trypanosoma brucei: Drug targets vs. drug targeting. Curr. Pharm. Des. 2007, 13, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Munday, J.C.; Settimo, L.; De Koning, H.P. Transport proteins determine drug sensitivity and resistance in a protozoan parasite, Trypanosoma brucei. Front. Pharmacol. 2015, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Babokhov, P.; Sanyaolu, A.O.; Oyibo, W.A.; Fagbenro-Beyioku, A.F.; Iriemenam, N.C. A current analysis of chemotherapy strategies for the treatment of human African trypanosomiasis. Pathog. Glob. Health 2013, 107, 242–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graf, F.E.; Ludin, P.; Wenzler, T.; Kaiser, M.; Brun, R.; Pyana, P.P.; Mäser, P. Aquaporin 2 mutations in Trypanosoma brucei gambiense field isolates correlate with decreased susceptibility to pentamidine and melarsoprol. PLoS Negl. Trop. Dis. 2013, 7, e2475. [Google Scholar] [CrossRef]
- De Koning, H.P. Uptake of pentamidine in Trypanosoma brucei brucei is mediated by three distinct transporters. Implications for crossresistance with arsenicals. Mol. Pharmacol. 2001, 59, 586–592. [Google Scholar] [CrossRef]
- Barrett, M.P.; Vincent, I.M.; Burchmore, R.J.; Kazibwe, A.J.; Matovu, E. Drug resistance in human African trypanosomiasis. Future Microbiol. 2011, 6, 1037–1047. [Google Scholar] [CrossRef]
- Gould, M.K.; Vu, X.L.; Seebeck, T.; de Koning, H.P. Propidium iodide-based methods for monitoring drug action in the kinetoplastidae: Comparison with the Alamar Blue assay. Anal. Biochem. 2008, 382, 87–93. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Al-Salabi, M.I.; El Sabbagh, N.; Quashie, N.B.; Alkhaldi, A.A.; Escale, R.; Smith, T.K.; Vial, H.J.; De Koning, H.P. Symmetrical choline-derived dications display strong anti-kinetoplastid activity. J. Antimicrob. Chemother. 2011, 66, 111–125. [Google Scholar] [CrossRef]
- Ebiloma, G.U.; Katsoulis, E.; Igoli, J.O.; Gray, A.I.; De Koning, H.P. Multi-target mode of action of an ursane-type terpenoid from Nigerian medicinal plants targeting the African trypanosomes. Sci. Rep. 2018, 8, 4613. [Google Scholar] [CrossRef]
- Lemasters, J.J.; Theruvath, T.P.; Zhong, Z.; Nieminen, A.L. Mitochondrial calcium and the permeability transition in cell death. Biochim. Biophys. Acta 2009, 1787, 1395–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, K.R. The developmental cell biology of Trypanosoma brucei. J. Cell Sci. 2005, 118, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Lacomble, S.; Vaughan, S.; Gadelha, C.; Morphew, M.K.; Shaw, M.K.; McIntosh, J.R.; Gull, K. Basal body movements orchestrate membrane organelle division and cell morphogenesis in Trypanosoma brucei. J. Cell Sci. 2010, 123, 2884–2891. [Google Scholar] [CrossRef] [PubMed]
- Ogbadoyi, E.O.; Robinson, D.R.; Gull, K. A high-order trans-membrane structural linkage is responsible for mitochondrial genome positioning and segregation by flagellar basal bodies in trypanosomes. Mol. Biol. Cell 2003, 14, 1769–1779. [Google Scholar] [CrossRef] [PubMed]
- Rodenko, B.; Wanner, M.J.; Alkhaldi, A.A.M.; Ebiloma, G.U.; Barnes, R.L.; Kaiser, M.; Brun, R.; McCulloch, R.; Koomen, G.J.; De Koning, H.P. Targeting the parasite’s DNA with methyltriazenyl purine analogs is a safe, selective and efficacious antitrypanosomal strategy. Antimicrob. Agents Chemother. 2015, 59, 6708–6716. [Google Scholar] [CrossRef]
- Hammarton, T.C. Cell cycle regulation in Trypanosoma brucei. Mol. Biochem. Parasitol. 2007, 153, 1–8. [Google Scholar] [CrossRef]
- Dardonville, C.; Alkhaldi, A.A.; De Koning, H.P. SAR studies of diphenyl cationic trypanocides: Superior activity of phosphonium over ammonium salts. ACS Med. Chem. Lett. 2014, 6, 151–155. [Google Scholar] [CrossRef]
- Alkhaldi, A.A.; Martinek, J.; Panicucci, B.; Dardonville, C.; Zíková, A.; de Koning, H.P. Trypanocidal action of bisphosphonium salts through a mitochondrial target in bloodstream form Trypanosoma brucei. Int. J. Parasitol. Drugs Drug Resist. 2016, 6, 23–34. [Google Scholar] [CrossRef]
- Soeiro, M.N.; de Castro, S.L.; de Souza, E.M.; Batista, D.G.; Silva, C.F.; Boykin, D.W. Diamidine activity against trypanosomes: The state of the art. Curr. Mol. Pharmacol. 2008, 1, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Lanteri, C.A.; Tidwell, R.R.; Meshnick, S.R. The mitochondrion is a site of trypanocidal action of the aromatic diamidine DB75 in bloodstream forms of Trypanosoma brucei. Antimicrob. Agents Chemother. 2008, 52, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.L.; Krishna, S.; Burchmore, R.J.S.; Brun, R.; De Koning, H.P.; Boykin, D.W.; Tidwell, R.R.; Hall, J.E.; Barrett, M.P. Detection of arsenical drug resistance in Trypanosoma brucei using a simple fluorescence test. Lancet 2005, 366, 486–487. [Google Scholar] [CrossRef]
- Roy Chowdhury, A.; Bakshi, R.; Wang, J.; Yildirir, G.; Liu, B.; Pappas-Brown, V.; Tolun, G.; Griffith, J.D.; Shapiro, T.A.; Jensen, R.E.; et al. The killing of African trypanosomes by ethidium bromide. PLoS Pathog. 2010, 6, e1001226. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, T.A.; Englund, P.T. Selective cleavage of kinetoplast DNA minicircles promoted by antitrypanosomal drugs. Proc. Natl. Acad. Sci. USA 1990, 87, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Damper, D.; Patton, C.L. Pentamidine transport and sensitivity in brucei-group trypanosomes. J. Protozool. 1976, 23, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Beck, K.; Acestor, N.; Schulfer, A.; Anupama, A.; Carnes, J.; Panigrahi, A.K.; Stuart, K. Trypanosoma brucei Tb927. 2.6100 is an essential protein associated with kinetoplast DNA. Eukaryot. Cell 2013, 12, 970–978. [Google Scholar] [CrossRef]
- Neupert, W. Protein import into mitochondria. Ann. Rev. Biochem. 1997, 66, 863–917. [Google Scholar] [CrossRef]
- Panicucci, B.; Gahura, O.; Zíková, A. Trypanosoma brucei TbIF1 inhibits the essential F1-ATPase in the infectious form of the parasite. PLoS Negl. Trop. Dis. 2017, 11, e0005552. [Google Scholar] [CrossRef]
- Schnaufer, A.; Clark-Walker, G.D.; Steinberg, A.G.; Stuart, K. The F1-ATP synthase complex in bloodstream stage trypanosomes has an unusual and essential function. EMBO J. 2005, 24, 4029–4040. [Google Scholar] [CrossRef] [Green Version]
- Michels, P.A.; Bringaud, F.; Herman, M.; Hannaert, V. Metabolic functions of glycosomes in trypanosomatids. Biochim. Biophys. Acta 2006, 1763, 1463–1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhuri, M.; Ott, R.D.; Hill, G.C. Trypanosome alternative oxidase: From molecule to function. Trends Parasitol. 2006, 22, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Ebiloma, G.U.; Balogun, E.O.; Cueto Díaz, E.; De Koning, H.P.; Dardonville, C. Alternative Oxidase inhibitors: Development and efficient mitochondrion-targeting as a strategy for new drugs against pathogenic parasites and fungi. Med. Res. Rev. 2019, in press. [Google Scholar]
- Miller, P.G.; Klein, R.A. Effects of oligomycin on glucose utilization and calcium transport in African trypanosomes. J. Gener. Microbiol. 1980, 116, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Nolan, D.P.; Voorheis, H.P. The mitochondrion in bloodstream forms of Trypanosoma brucei is energized by the electrogenic pumping of protons catalysed by the F1F0-ATPase. Eur. J. Biochem. 1992, 209, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Munday, J.C.; Eze, A.A.; Baker, N.; Glover, L.; Clucas, C.; Aguinaga Andrés, D.; Natto, M.J.; Teka, I.A.; McDonald, J.; Lee, R.S.; et al. Trypanosoma brucei Aquaglyceroporin 2 is a high affinity transporter for pentamidine and melaminophenyl arsenic drugs and is the main genetic determinant of resistance to these drugs. J. Antimicrob. Chemother. 2014, 69, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |




| Compounds | Tbb 427WT | Tc-IL3000 | |||
|---|---|---|---|---|---|
| IC50, µM | S. I. | IC50, µM | S. I. | ||
| 1 |  | 4.53 ± 0.16 | 1.1 | 20.8 ± 0.84 | 0.3 |
| 2 | 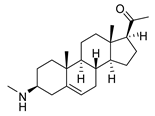 | 1.75 ± 0.17 | 1.4 | 3.78 ± 1.96 | 0.7 |
| 3 | 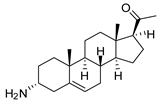 | 3.41 ± 0.17 | 4.6 | 9.49 ±0.30 | 1.7 |
| 4 | 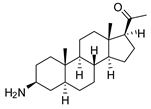 | 4.00 ± 0.24 | 4.3 | 2.93 ± 0.33 | 5.9 |
| 5 |  | 1.62 ± 0.09 | 10.5 | 6.08 ± 4.97 | 2.8 |
| 6 | 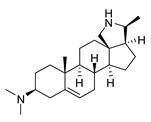 | >100 | <0.5 | 1.07 ± 0.32 | 47.2 |
| 7 |  | 3.42 ± 0.21 | 8.0 | 0.22 ± 0.35 | 123.5 |
| 8 | 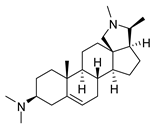 | 3.51 ± 0.10 | 17.4 | 1.65 ± 0.92 | 37.2 |
| 9 | 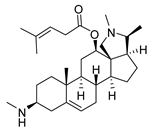 | 2.20 ± 0.08 | 6.5 | 0.22 ± 0.13 | 65.9 |
| 10 |  | 4.79 ± 0.81 | 20.4 | 0.045 ± 0.03 | 2130 |
| Pentamidine | 0.004 ± 0.001 | - | 0.448 ± 0.28 | - | |
| Diminazene | 0.066 ± 0.002 | - | 0.093 ± 0.01 | - | |
| Compound | TbAT1-KO | AQP1-3-KO | AQP2/3-KO | ISMR | B48 | R0.8 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 (µM) | RF | IC50 (µM) | RF | IC50 (µM) | RF | IC50 (µM) | RF | IC50 (µM) | RF | IC50 (µM) | RF | |
| 1 | 3.2 ± 0.6 | 0.7 | 3.6 ± 0.2 | 0.8 | 6.6 ± 0.5 | 1.4 | 7.2 ± 0.8 | 1.6 | 4.4 ± 0.2 | 0.9 | 2.6 ± 0.3 | 0.6 |
| 2 | 1.6 ± 0.2 | 0.9 | 1.5 ± 0.2 | 0.9 | 2.7 ± 0.2 | 1.5 | 2.9 ± 0.9 | 1.7 | 2.3 ± 0.4 | 1.3 | 1.1 ± 0.1 | 0.6 |
| 3 | 19 ± 0.3 *** | 5.6 | 4.7 ± 0.2 | 1.4 | 9.1 ± 1.0 ** | 2.7 | 11.0 ± 0.5 *** | 3.2 | 17.4 ± 0.8 *** | 5.1 | 5.3 ± 0.6 | 1.6 |
| 4 | 5.5 ± 0.4 | 1.4 | 2.7 ± 0.3 | 0.7 | 5.8 ± 0.4 | 1.4 | 8.0 ± 0.5 * | 2.0 | 7.8 ± 0.7 | 1.9 | 5.9 ± 0.6 | 1.5 |
| 5 | 1.7 ± 0.1 | 1.0 | 1.4 ± 0.1 | 0.9 | 2.6 ± 0.4 | 1.6 | 2.9 ± 0.3 | 1.8 | 3.5 ± 0.05 *** | 2.1 | 1.7 ± 0.6 | 1.1 |
| 6 | >100 | - | >100 | - | >100 | - | >100 | - | >100 | - | >100 | - |
| 7 | 8.2 ± 0.2 *** | 2.4 | 1.7 ± 0.2 | 0.5 | 3.4 ± 0.2 | 1.0 | 10 ± 0.4 *** | 2.9 | 8.2 ± 0.1 *** | 2.4 | 6.0 ± 0.9* | 1.8 |
| 8 | 4.2 ± 0.1 | 1.2 | 4.6 ± 0.2 | 1.3 | 4.1 ± 0.1 | 1.2 | 2.7 ± 0.1 | 0.8 | 3.5 ± 0.4 | 1.0 | 3.4 ± 0.2 | 1.0 |
| 9 | 2.1 ± 0.1 | 0.9 | 1.8 ± 0.2 | 0.8 | 3.2 ± 0.3 | 1.4 | 3.7 ± 1.0 | 1.7 | 3.4 ± 0.2 | 1.6 | 2.6 ± 0.2 | 1.2 |
| 10 | 3.3 ± 0.2 | 0.7 | 1.4 ± 0.2 | 0.3 | 3.3 ± 0.1 | 0.7 | 6.2 ± 0.8 | 1.3 | 4.6 ± 0.1 | 0.9 | 2.6 ± 0.1 | 0.5 |
| PMD a | 0.0077 ± 0.0002 ** | 2.0 | 0.058 ± 0.003 *** | 15 | 0.046 ± 0.002 *** | 12 | 0.0065 ± 0.0010 * | 1.7 | 0.55 ± 0.07 ** | 143 | 0.0072 ± 0.0004 ** | 1.9 |
| DA b | 0.47 ± 0.02 *** | 7.0 | ND | - | ND | - | 1.1 ± 0.1 *** | 17 | 0.74 ± 0.04 *** | 11 | ND | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nnadi, C.O.; Ebiloma, G.U.; Black, J.A.; Nwodo, N.J.; Lemgruber, L.; Schmidt, T.J.; de Koning, H.P. Potent Antitrypanosomal Activities of 3-Aminosteroids against African Trypanosomes: Investigation of Cellular Effects and of Cross-Resistance with Existing Drugs. Molecules 2019, 24, 268. https://doi.org/10.3390/molecules24020268
Nnadi CO, Ebiloma GU, Black JA, Nwodo NJ, Lemgruber L, Schmidt TJ, de Koning HP. Potent Antitrypanosomal Activities of 3-Aminosteroids against African Trypanosomes: Investigation of Cellular Effects and of Cross-Resistance with Existing Drugs. Molecules. 2019; 24(2):268. https://doi.org/10.3390/molecules24020268
Chicago/Turabian StyleNnadi, Charles O., Godwin U. Ebiloma, Jennifer A. Black, Ngozi J. Nwodo, Leandro Lemgruber, Thomas J. Schmidt, and Harry P. de Koning. 2019. "Potent Antitrypanosomal Activities of 3-Aminosteroids against African Trypanosomes: Investigation of Cellular Effects and of Cross-Resistance with Existing Drugs" Molecules 24, no. 2: 268. https://doi.org/10.3390/molecules24020268
APA StyleNnadi, C. O., Ebiloma, G. U., Black, J. A., Nwodo, N. J., Lemgruber, L., Schmidt, T. J., & de Koning, H. P. (2019). Potent Antitrypanosomal Activities of 3-Aminosteroids against African Trypanosomes: Investigation of Cellular Effects and of Cross-Resistance with Existing Drugs. Molecules, 24(2), 268. https://doi.org/10.3390/molecules24020268






