Nitrogen-Doped Hierarchical Meso/Microporous Carbon from Bamboo Fungus for Symmetric Supercapacitor Applications
Abstract
:1. Introduction
2. Results and Discussion
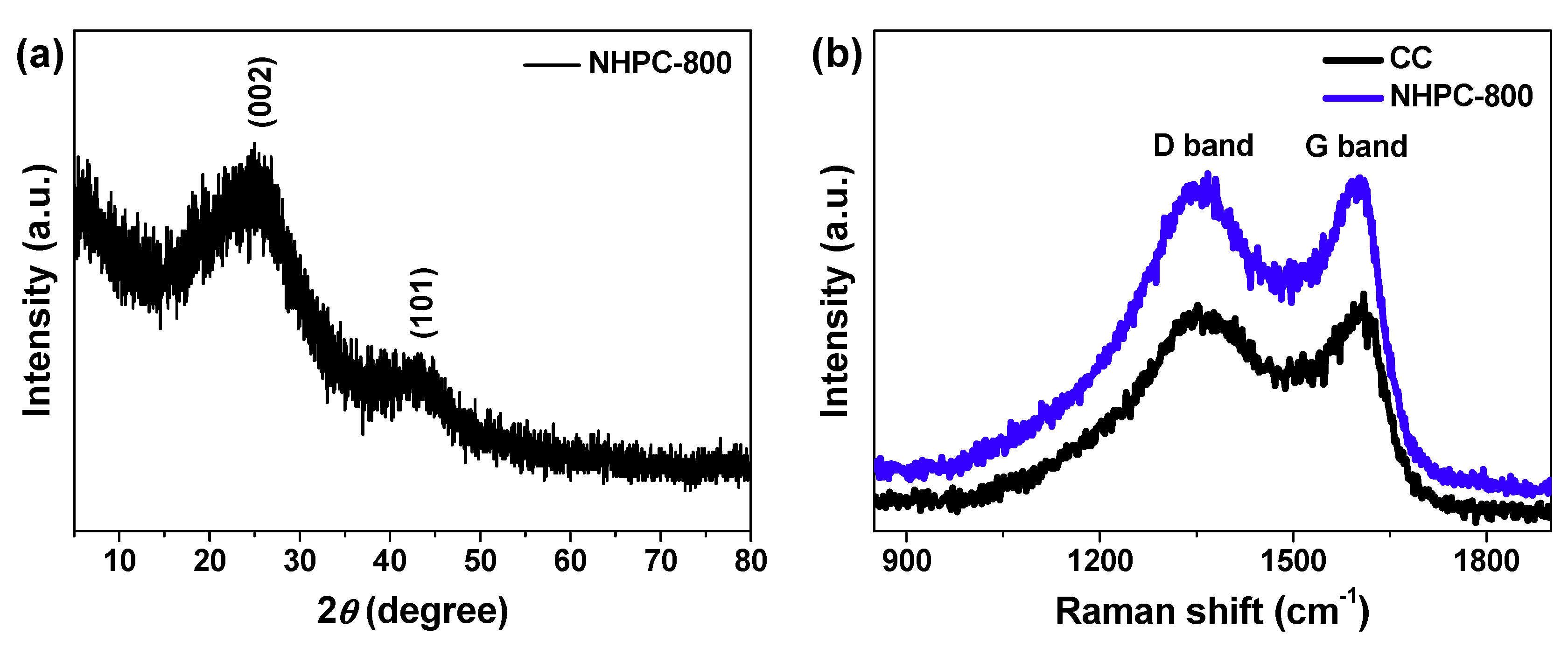
2.1. Characterizations of Bamboo Fungus-Derived NHPC-800
2.2. Electrochemical Properties
3. Materials and Methods
3.1. Reagents
3.2. Synthesis of Bamboo Fungus-Derived NHPC
3.3. Characterizations
3.4. Electrochemical Measurements and Corresponding Calculations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Titirici, M.-M.; White, R.J.; Brun, N.; Budarin, V.L.; Su, D.S.; del Monte, F.; Clark, J.H.; MacLachlan, M.J. Sustainable carbon materials. Chem. Soc. Rev. 2015, 44, 250–290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; He, L.; Shi, T.; Zha, R. Nanocasting and direct synthesis strategies for mesoporous carbons as supercapacitor electrodes. Chem. Mater. 2018, 30, 7391–7412. [Google Scholar] [CrossRef]
- Yang, J.; Hu, J.; Zhu, M.; Zhao, Y.; Chen, H.; Pan, F. Ultrahigh surface area meso/microporous carbon formed with self-template for high-voltage aqueous supercapacitors. J. Power Sources 2017, 365, 362–371. [Google Scholar] [CrossRef]
- Yu, P.; Liang, Y.; Dong, H.; Hu, H.; Liu, S.; Peng, L.; Zheng, M.; Xiao, Y.; Liu, Y. Rational synthesis of highly porous carbon from waste bagasse for advanced supercapacitor application. ACS Sustain. Chem. Eng. 2018, 6, 15325–15332. [Google Scholar] [CrossRef]
- Liu, W.; Mei, J.; Liu, G.; Kou, Q.; Yi, T.; Xiao, S. Nitrogen-doped hierarchical porous carbon from wheat straw for supercapacitors. ACS Sustain. Chem. Eng. 2018, 6, 11595–11605. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, W.; Zhou, W.; Chen, S.; Zhang, Y. Hierarchical porous carbon derived from Sichuan pepper for high-performance symmetric supercapacitor with decent rate capability and cycling stability. Nanomaterials 2019, 9, 553. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ren, J.; Xia, L.; Wu, H.; Xie, F.; Zheng, Q.; Xu, C.; Lin, D. Nitrogen-doped hierarchical porous carbon framework derived from waste pig nails for high performance supercapacitors. ChemElectroChem 2017, 4, 3184–3187. [Google Scholar] [CrossRef]
- Ma, H.; Li, C.; Zhang, M.; Hong, J.-D.; Shi, G. Graphene oxide induced hydrothermal carbonization of egg white proteins for high-performance supercapacitors. J. Mater. Chem. A 2017, 5, 17040–17047. [Google Scholar] [CrossRef]
- Genovese, M.; Jiang, J.; Lian, K.; Holm, N. High capacitive performance of exfoliated biocharnanosheets from biomass waste corn cob. J. Mater. Chem. A 2015, 3, 2903–2913. [Google Scholar] [CrossRef]
- Yao, Q.; Wang, H.; Wang, C.; Jin, C.; Sun, Q. One step construction of nitrogen–carbon derived from Bradyrhizobiumjaponicum for supercapacitor applications with a soybean leaf as a separator. ACS Sustain. Chem. Eng. 2018, 6, 4695–4704. [Google Scholar] [CrossRef]
- Liu, X.; Ma, C.; Li, J.; Zielinska, B.; Kalenczuk, R.J.; Chen, X.; Chu, P.K.; Tang, T.; Mijowska, E. Biomass-derived robust three-dimensional porous carbon for high volumetric performance supercapacitors. J. Power Sources 2019, 412, 1–9. [Google Scholar] [CrossRef]
- Hou, J.; Jiang, K.; Wei, R.; Tahir, M.; Wu, X.; Shen, M.; Wang, X.; Cao, C. Popcorn-derived porous carbon flakes with an ultrahigh specific surface area for superior performance supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 30626–30634. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Michalkiewicz, B.; Min, J.; Ma, C.; Chen, X.; Gong, J.; Mijowska, E.; Tang, T. Selective preparation of biomass-derived porous carbon with controllable pore sizes toward highly efficient CO2 capture. Chem. Eng. J. 2019, 360, 250–259. [Google Scholar] [CrossRef]
- Wan, L.; Li, X.; Li, N.; Xie, M.; Du, C.; Zhang, Y.; Chen, J. Multi-heteroatom-doped hierarchical porous carbon derived from chestnut shell with superior performance in supercapacitors. J. Alloys Compd. 2019, 790, 760–771. [Google Scholar] [CrossRef]
- Qu, G.; Jia, S.; Wang, H.; Cao, F.; Li, L.; Qing, C.; Sun, D.; Wang, B.; Tang, Y.; Wang, J. Asymmetric supercapacitor based on porous N-doped carbon derived from pomelo peel and NiO arrays. ACS Appl. Mater. Interfaces 2016, 8, 20822–20830. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Liu, C.; Lin, C.; Ma, M. Constructed nitrogen and sulfur codoped multilevel porous carbon from lignin for high-performance supercapacitors. J. Alloys Compd. 2019, 789, 435–442. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, A.; Izquierdo, M.T.; Mathieu, S.; González-Álvarez, J.; Celzard, A.; Fierro, V. Outstanding electrochemical performance of highly N- and O-doped carbons derived from pine tannin. Green Chem. 2017, 19, 2653–2665. [Google Scholar] [CrossRef]
- Patiño, J.; López-Salas, N.; Gutiérrez, M.C.; Carriazo, D.; Ferrer, M.L.; del Monte, F. Phosphorus-doped carbon–carbon nanotube hierarchical monoliths as true three-dimensional electrodes in supercapacitor cells. J. Mater. Chem. A 2016, 4, 1251–1263. [Google Scholar] [CrossRef]
- Mehare, R.S.; Ranganath, S.P.; Chaturvedi, V.; Badiger, M.V.; Shelke, M.V. In Situ synthesis of nitrogen- and sulphur-enriched hierarchical porous carbon for high-performance supercapacitor. Energy Fuels 2018, 32, 908–915. [Google Scholar] [CrossRef]
- Kim, D.K.; Bong, S.; Jin, X.; Seong, K.-D.; Hwang, M.; Kim, N.D.; You, N.-H.; Piao, Y. Facile in situ synthesis of multiple-heteroatom-doped carbons derived from polyimide precursors for flexible all-solid-state supercapacitors. ACS Appl. Mater. Interfaces 2019, 11, 1996–2005. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Ding, B.; Wu, X. A novel way to synthesize nitrogen doped porous carbon materials with high rate performance and energy density for supercapacitors. J. Alloys Compd. 2019, 785, 110–116. [Google Scholar] [CrossRef]
- Deng, Y.; Xie, Y.; Zou, K.; Ji, X. Review on recent advances in nitrogen-doped carbons: Preparations and applications in supercapacitors. J. Mater. Chem. A 2016, 4, 1144–1173. [Google Scholar] [CrossRef]
- Gao, S.; Fan, H.; Zhang, S. Nitrogen-enriched carbon from bamboo fungus with superior oxygen reduction reaction activity. J. Mater. Chem. A 2014, 2, 18263–18270. [Google Scholar] [CrossRef]
- Zhao, W.; Zhu, Y.; Zhang, L.; Xie, Y.; Ye, X. Facile synthesis of three-dimensional porous carbon for high-performance supercapacitors. J. Alloys Compd. 2019, 787, 1–8. [Google Scholar] [CrossRef]
- Zheng, K.; Li, Y.; Zhu, M.; Yu, X.; Zhang, M.; Shi, L.; Cheng, J. Three-dimensional interconnected porous graphitic carbon derived from rice straw for high performance supercapacitors. J. Power Sources 2017, 366, 270–277. [Google Scholar] [CrossRef]
- Guo, N.; Li, M.; Sun, X.; Wang, F.; Yang, R. Enzymatic hydrolysis lignin derived hierarchical porous carbon for supercapacitors in ionic liquids with high power and energy densities. Green Chem. 2017, 19, 2595–2602. [Google Scholar] [CrossRef]
- Chen, Z.; Cao, R.; Ge, Y.; Tu, Y.; Xia, Y.; Yang, X. N- and O-doped hollow carbonaceous spheres with hierarchical porous structure for potential application in high-performance capacitance. J. Power Sources 2017, 363, 356–364. [Google Scholar] [CrossRef]
- Xia, Y.; Fang, R.; Xiao, Z.; Huang, H.; Gan, Y.; Yan, R.; Lu, X.; Liang, C.; Zhang, J.; Tao, X.; et al. Confining sulfur in N-doped porous carbon microspheres derived from microalgaes for advanced lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2017, 9, 23782–23791. [Google Scholar] [CrossRef]
- Yang, M.; Long, X.; Li, H.; Chen, H.; Liu, P. Porous organic-polymer-derived nitrogen-doped porous carbon nanoparticles for efficient oxygen reduction electrocatalysis and supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 2236–2244. [Google Scholar] [CrossRef]
- Yan, J.; Wang, Q.; Lin, C.; Wei, T.; Fan, Z. Interconnected frameworks with a sandwiched porous carbon layer/graphenehybrids for supercapacitors with high gravimetric and volumetric performances. Adv. Energy Mater. 2014, 4, 1400500. [Google Scholar] [CrossRef]
- Qin, F.; Tian, X.; Guo, Z.; Shen, W. Asphaltene-based porous carbon nanosheet as electrode for supercapacitor. ACS Sustain. Chem. Eng. 2018, 6, 15708–15719. [Google Scholar] [CrossRef]
- Wei, X.; Wan, S.; Jiang, X.; Wang, Z.; Gao, S. Peanut-shell-like porous carbon from nitrogen-containing poly-N-phenylethanolamie for high-performance supercapacitor. ACS Appl. Mater. Interfaces 2015, 7, 22238–22245. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, T.; Lin, X.; Chen, H.; Chen, S.; Jiang, Z.; Chen, Y.; Liu, J.; Huang, J.; Liu, M. Self-templated synthesis of hierarchically porous N-doped carbon derived from biomass for supercapacitors. ACS Sustain. Chem. Eng. 2018, 6, 13932–13939. [Google Scholar] [CrossRef]
- Zou, Z.; Zhou, W.; Zhang, Y.; Yu, H.; Hu, C.; Xiao, W. High-performance flexible all-solid-state supercapacitor constructed by free-standing cellulose/reduced grapheneoxide/silver nanoparticles composite film. Chem. Eng. J. 2019, 357, 45–55. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, Z.; Gao, Y.; An, W.; Cao, Z.; Liu, J. Biomass-swelling assisted synthesis of hierarchical porous carbon fibers for supercapacitor electrodes. ACS Appl. Mater. Interfaces 2016, 8, 28283–28290. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, G.-P.; Song, H.D.; Park, S.; Yi, J. Preparation of energy storage material derived from a used cigarette filter for a supercapacitor electrode. Nanotechnology 2014, 25, 345601. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Hou, B.-H.; Lü, H.-Y.; Wan, F.; Wang, J.; Wu, X.-L. Porous N-doped carbon material derived from prolific chitosan biomass as a high-performance electrode for energy storage. RSC Adv. 2015, 5, 97427–97434. [Google Scholar] [CrossRef]
- Hasegawa, G.; Aoki, M.; Kanamori, K.; Nakanishi, K.; Hanada, T.; Tadanaga, K. Monolithic electrode for electric double-layer capacitors based on macro/meso/microporous S-containing activated carbon with high surface area. J. Mater. Chem. 2011, 21, 2060–2063. [Google Scholar] [CrossRef]
- Chen, M.; Kang, X.; Wumaier, T.; Dou, J.; Gao, B.; Han, Y.; Xu, G.; Liu, Z.; Zhang, L. Preparation of activated carbon from cotton stalk and its application in supercapacitor. J. Solid State Electrochem. 2013, 17, 1005–1012. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, S.; Pan, N.; Lo Hsieh, Y. High energy density supercapacitors from lignin derived submicron activated carbon fibers in aqueous electrolytes. J. Power Sources 2014, 270, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Zhao, Y.; Cheng, H.; Hu, C.; Hu, Y.; Meng, Y.; Shao, H.; Zhang, Z.; Qu, L. An all-cotton-derived, arbitrarily foldable, high-rate, electrochemical supercapacitor. Phys. Chem. Chem. Phys. 2013, 15, 8042–8045. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Geng, C.; Lv, S.; Shao, G.; Ma, S.; Wu, M. Nitrogen, oxygen and phosphorus decorated porous carbons derived from shrimp shells for supercapacitors. Electrochim. Acta 2015, 176, 982–988. [Google Scholar] [CrossRef]
- Zhou, L.; Cao, H.; Zhu, S.; Hou, L.; Yuan, C. Hierarchical micro-/mesoporous N- and O-enriched carbon derived from disposable cashmere: A competitive cost-effective material for high-performance electrochemical capacitors. Green Chem. 2015, 17, 2373–2382. [Google Scholar] [CrossRef]
- Li, Z.; Li, L.; Li, Z.; Liao, H.; Zhang, H. Ultrathin carbon gauze for high-rate supercapacitor. Electrochim. Acta 2016, 222, 990–998. [Google Scholar] [CrossRef]
- He, X.; Ling, P.; Yu, M.; Wang, X.; Zhang, X.; Zheng, M. Rice husk-derived porous carbons with high capacitance by ZnCl2 activation for supercapacitors. Electrochim. Acta 2013, 105, 635–641. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds reported in this article are available from the authors. |








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, Z.; Lei, Y.; Li, Y.; Zhang, Y.; Xiao, W. Nitrogen-Doped Hierarchical Meso/Microporous Carbon from Bamboo Fungus for Symmetric Supercapacitor Applications. Molecules 2019, 24, 3677. https://doi.org/10.3390/molecules24203677
Zou Z, Lei Y, Li Y, Zhang Y, Xiao W. Nitrogen-Doped Hierarchical Meso/Microporous Carbon from Bamboo Fungus for Symmetric Supercapacitor Applications. Molecules. 2019; 24(20):3677. https://doi.org/10.3390/molecules24203677
Chicago/Turabian StyleZou, Zhanghua, Yu Lei, Yingming Li, Yanhua Zhang, and Wei Xiao. 2019. "Nitrogen-Doped Hierarchical Meso/Microporous Carbon from Bamboo Fungus for Symmetric Supercapacitor Applications" Molecules 24, no. 20: 3677. https://doi.org/10.3390/molecules24203677





