Guanidine-Containing Polyhydroxyl Macrolides: Chemistry, Biology, and Structure-Activity Relationship
Abstract
:1. Introduction
2. Structural Diversity
3. Spectroscopic Characterization
4. Bioactivity
5. Acute Toxicity
6. Antimicrobial Mechanisms
6.1. Antibacterial Mechanisms
6.2. Antifungal Mechanism
7. Antimicrobial Structure-Activity Relationship
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Laxminarayan, R.; Sridhar, D.; Blaser, M.; Wang, M.; Woolhouse, M. Achieving global targets for antimicrobial resistance. Science 2016, 353, 874–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Infectious Diseases Society of America. The 10 × 20 initiative: Pursuing a global commitment to develop 10 new antibacterial drugs by 2020. Clin. Infect. Dis. 2010, 50, 1081–1083. [Google Scholar] [CrossRef]
- Cassir, N.; Rolain, J.; Brouqui, P. A new strategy to fight antimicrobial resistance: The revival of old antibiotics. Front. Microbiol. 2014, 5, 551. [Google Scholar] [CrossRef]
- Bush, K. Improving known classes of antibiotics: An optimistic approach for the future. Curr. Opin. Pharmacol. 2012, 12, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xu, L.; Yuan, G.; Wang, Y.; Qu, Y.; Zhou, M. Synergistic combination of two antimicrobial agents closing each other’s mutant selection windows to prevent antimicrobial resistance. Sci. Rep. 2018, 8, 7237. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Li, P.; Qin, H.; Xu, X.; Song, X.; Cao, S.; He, S.; Lai, S. Opinions and regularity conclusions on drug combination to prevent resistance. In Proceedings of the 3rd International Caparica Congress in Antibiotic Resistance 2019 (IC2AR 2019), Caparica, Portugal, 10–13 June 2019. [Google Scholar]
- Xu, X.; Wu, X.; Yuan, G.; Xu, L.; Wang, Y. Mutant selection windows of azalomycin F5a in combination with vitamin K3 against methicillin-resistant Staphylococcus aureus. J. Biosci. Med. 2016, 4, 162–174. [Google Scholar]
- Xu, W.; Zhai, G.; Liu, Y.; Li, Y.; Shi, Y.; Hong, K.; Hong, H.; Leadlay, P.F.; Deng, Z.; Sun, Y. An iterative module in the azalomycin F polyketide synthase contains a switchable enoylreductase domain. Angew. Chem. Int. Ed. 2017, 56, 5503–5506. [Google Scholar] [CrossRef]
- Hong, H.; Fill, T.; Leadlay, P.F. A common origin for guanidinobutanoate starter units in antifungal natural products. Angew. Chem. Int. Ed. 2013, 52, 13096–13099. [Google Scholar] [CrossRef]
- Hong, H.; Sun, Y.; Zhou, Y.; Stephens, E.; Samborskyy, M.; Leadlay, P.F. Evidence for an iterative module in chain elongation on the azalomycin polyketide synthase. Beilstein J. Org. Chem. 2016, 12, 2164–2172. [Google Scholar] [CrossRef] [Green Version]
- Arai, M. Azalomycins B and F, two new antibiotics. I. Production and isolation. J. Antibiot. Ser. A 1960, 13, 46–50. [Google Scholar]
- Arai, M.; Hamano, K. Isolation of three main components, F3, F4 and F5, from azalomycin F-complex. J. Antibiot. 1970, 23, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, S.; Namikoshi, M.; Sasaki, K.; Fukushima, K.; Okuda, S. Studies on macrocyclic lactone antibotics.V.1) The structures of azalomycins F3a and F5a. Chem. Pharm. Bull. 1982, 30, 4006–4014. [Google Scholar] [CrossRef]
- Namikoshi, M.; Sasaki, K.; Koiso, Y.; Fukushima, K.; Iwasaki, S.; Nozoe, S.; Okuda, S. Studies on macrocyclic lactone antibiotics. I. Physicochemical properties of azalomycin F4a. Chem. Pharm. Bull. 1982, 30, 1653–1657. [Google Scholar] [CrossRef]
- Namikoshi, M.; Iwasaki, S.; Sasaki, K.; Yano, M.; Fukushima, K.; Nozoe, S.; Okuda, S. Studies on macrocyclic lactone antibotics. II. 1) Partial structure of azalomycin F4a. Chem. Pharm. Bull. 1982, 30, 1658–1668. [Google Scholar] [CrossRef]
- Iwasaki, S.; Namikoshi, M.; Sasaki, K.; Yano, M.; Fukushima, K.; Nozoe, S.; Okuda, S. Studies on macrocyclic lactone antibiotics. III. (1) Skeletal structure of azalomycin F4a. Chem. Pharm. Bull. 1982, 30, 1669–1673. [Google Scholar] [CrossRef]
- Chandra, A.; Nair, M.G. Azalomycin F complex from Streptomyces hygroscopicus, MSU/MN-4-75B. J. Antibiot. 1995, 48, 896–898. [Google Scholar] [CrossRef]
- Yuan, G.; Lin, H.; Wang, C.; Hong, K.; Liu, Y.; Li, J. 1H and 13C assignments of two new macrocyclic lactones isolated from Streptomyces sp. 211726 and revised assignments of azalomycins F3a, F4a and F5a. Magn. Reson. Chem. 2011, 49, 30–37. [Google Scholar] [CrossRef]
- Samain, D.; Carter Cook Jr., J.; Rinehart, K.L., Jr. Structure of scopafungin, a potent nonpolyene antifungal antibiotic. J. Am. Chem. Soc. 1982, 104, 4129–4141. [Google Scholar] [CrossRef]
- Takesako, K.; Beppu, T. Studies on new antifungal antibiotics, guanidylfungins A and B. I. Taxonomy, fermentation, isolation and characterization. J. Antibiot. 1984, 37, 1161–1169. [Google Scholar] [CrossRef]
- Grabley, S.; Hammann, P.; Raether, W.; Wink, J.; Zeeck, A. Secondary metabolites by chemical screening: II. Amycins A and B two novel niphimycin analogs isolated from a high producer strain of elaiophylin and nigericin. J. Antibiot. 1990, 43, 639–647. [Google Scholar] [CrossRef]
- Ubukata, M.; Morita, T.; Osada, H. RS-22A, B and C: New macrolide antibiotics from Streptomyces violaceusniger. II. Physico-chemical properties and structure elucidation. J. Antibiot. 1995, 48, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Kumazawa, S.; Asami, Y.; Awane, K.; Ohtani, H.; Fukuchi, C.; Mikawa, T.; Hayase, T. Structural studies of new macrolide antibiotics, shurimycins A and B. J. Antibiot. 1994, 47, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Li, P.; Pan, W.; Pang, H.; Chen, S. The relative configurations of azalomycins F5a, F4a and F3a. J. Mol. Struct. 2013, 1035, 31–37. [Google Scholar] [CrossRef]
- Yuan, G.; Hong, K.; Lin, H.; She, Z.; Li, J. New azalomycin F analogs from mangrove Streptomyces sp. 211726 with activity against microbes and cancer cells. Mar. Drugs 2013, 11, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xu, X.; Yuan, G.; Wang, Y.; Qu, Y.; Liu, E. Mechanism of azalomycin F5a against methicillin-resistant Staphylococcus aureus. Biomed. Res. Int. 2018, 2018, 6942452. [Google Scholar]
- Cheng, J.; Yang, S.H.; Palaniyandi, S.A.; Han, J.S.; Yoon, T.; Kim, T.; Suh, J. Azalomycin F complex is an antifungal substance produced by Streptomyces malaysiensis MJM1968 isolated from agricultural soil. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 545–552. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, J.D.; Hong, J.S.; Ham, J.H.; Kim, B.S. Identification of antifungal niphimycin from Streptomyces sp. KP6107 by screening based on adenylate kinase assay. J. Basic. Microbiol. 2013, 53, 581–589. [Google Scholar] [CrossRef]
- Nakayama, K.; Yamaguchi, T.; Doi, T.; Usuki, Y.; Taniguchi, M.; Tanaka, T. Synergistic combination of direct plasma membrane damage and oxidative stress as a cause of antifungal activity of polyol macrolide antibiotic niphimycin. J. Biosci. Bioeng. 2002, 94, 207–211. [Google Scholar] [CrossRef]
- Yuan, G.; Xu, L.; Xu, X.; Li, P.; Zhong, Q.; Xia, H.; Hu, Y.; Li, P.; Song, X.; Li, J.; et al. Azalomycin F5a, a polyhydroxy macrolide binding to the polar head of phospholipid and targeting to lipoteichoic acid to kill methicillin-resistant Staphylococcus aureus. Biomed. Pharmacother. 2019, 109, 1940–1950. [Google Scholar] [CrossRef]
- Berlinck, R.G.S. Natural guanidine derivatives. Nat. Prod. Rep. 1999, 16, 339–365. [Google Scholar] [CrossRef]
- Berlinck, R.G.S.; Romminger, S. The chemistry and biology of guanidine natural products. Nat. Prod. Rep. 2016, 33, 456–490. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2015, 32, 116–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magara, M.; Amino, E.; Ito, H.; Takase, Z.; Nakamura, J.; Senda, C.; Kato, T. Laboratory and clinical studies on azalomycin F. Antibiot. Chemother. 1962, 12, 554–558. [Google Scholar]
- Koshino, H.; Kobinata, K.; Uzawa, J.; Uramoto, M.; Isono, K.; Osada, H. Structure of malolactomycins A and B, novel 40-membered macrolide antibiotics. Tetrahedron 1993, 49, 8827–8836. [Google Scholar] [CrossRef]
- Fréchet, D.; Danzer, M.; Debu, F.; Monegier du Sorbier, B.; Reisdorf, D.; Snozzi, C.; Vuilhorgne, M. Structure elucidation of RP 63834 a new macrocyclic lactone antibiotic. Tetrahedron 1991, 47, 61–70. [Google Scholar] [CrossRef]
- Ye, L.; Zhao, S.; Li, Y.; Jiang, S.; Zhao, Y.; Li, J.; Yan, K.; Wang, X.; Xiang, W.; Liu, C. Streptomyces lasiicapitis sp. nov., an actinomycete that produces kanchanamycin, isolated from the head of an ant (Lasius fuliginosus L.). Int. J. Syst. Evol. Microbiol. 2017, 67, 1529–1534. [Google Scholar] [PubMed]
- Fiedler, H.P.; Nega, M.; Pfefferle, C.; Groth, I.; Kempter, C.; Stephan, H.; Metzger, J.W. Kanchanamycins, new polyol macrolide antibiotics produced by Streptomyces olivaceus Tü 4018. I. Taxonomy, fermentation, isolation and biological activities. J. Antibiot. 1996, 49, 758–764. [Google Scholar] [CrossRef]
- Fiedler, H.P.; Wörner, W.; Zähner, H.; Kaiser, H.P.; Keller-Schierlein, W.; Müller, A. Metabolic products of microorganisms. 200 Isolation and characterization of niphithricins A, B, and elaiophylin, antibiotics produced by Streptomyces violaceoniger. J. Antibiot. 1981, 34, 1107–1118. [Google Scholar] [CrossRef]
- Ubukata, M.; Shiraishi, N.; Kobinata, K.; Kudo, T.; Yamaguchi, I.; Osada, H. RS-22A, B and C: New macrolide antibiotics from Streptomyces violaceusniger I. Taxonomy, fermentation, isolation and biological activities. J. Antibiot. 1995, 48, 289–292. [Google Scholar] [CrossRef]
- Mukhopadhyay, T.; Vljayakumar, E.K.S.; Nadkarni, S.R.; Sawant, S.N.; Kenia, J.; Sachse, B. DemethylazaIomycins F4a and F5a, two new antifungal metabolites from Actinomycete sp. HIL Y-9120362. J. Antibiot. 1995, 48, 1350–1352. [Google Scholar] [CrossRef]
- Ko, H.; Lee, H.; Oh, W.; Ahn, S.; Kim, B.; Kang, D.; Mheen, T.; Ahn, J. Isolation and characterization of MT2617-2B, a phospholipase C inhibitor produced by an actinomycetes isolate. Kor. J. Appl. Microbiol. Biotechnol. 1996, 24, 19–26. [Google Scholar]
- Saitou, N.; Nei, M. The Neighbor-Joining Method: A new method for reconstructing phylogenetic trees. Mol. Bio. Evol. 1987, 4, 406–425. [Google Scholar]
- Aiyar, A. The use of CLUSTAL W and CLUSTAL X for multiple sequence alignment. Methods Mol. Biol. 2000, 132, 221–241. [Google Scholar] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Kuroda, S.; Ohara, H.; Katoh, Y.; Kaji, H. Copiamycin, a new antifungal antibiotic derived from S. Hygroscopicus var. chrystallogenes. J. Antibiot. Ser. A 1965, 18, 63–67. [Google Scholar]
- Hamagishi, Y.; Kawano, K.; Kamei, H.; Oki, T. Inhibitory effects of copiamycin A, a macrocyclic lactone antibiotic, on gastric H+, K+-ATPase, acid secretion and ulcer formation. Jpn. J. Pharmacol. 1991, 55, 283–286. [Google Scholar] [CrossRef]
- Fukai, T.; Kuroda, J.; Nomura, T.; Uno, J.; Akao, M. Skeletal structure of neocopiamycin B from Streptomyces hygroscopicus var. crystallogenes. J. Antibiot. 1999, 52, 340–344. [Google Scholar] [CrossRef]
- Arai, T.; Uno, J.; Horimi, I.; Fukushima, K. Isolation of neocopiamycin A from Streptomyces hygroscopicus var. crystallogenes, the copiamycin source. J. Antibiot. 1984, 37, 103–109. [Google Scholar] [CrossRef]
- Fukai, T.; Takahashi, C.; Nomura, T.; Uno, J.; Arai, T. Guanidolide A, a novel antibiotic produced by Streptomyces hygroscopicus var. crystallogenes, the copiamycin source. Heterocycles 1988, 27, 2333–2340. [Google Scholar]
- Kohno, J.; Nishio, M.; Kawano, K.; Suzuki, S.; Komatsubara, S. TMC-34, a new macrolide antifungal antibiotic. J. Antibiot. 1995, 48, 1173–1175. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, M.; Wu, C.; Tan, Y.; Li, J.; Hao, X.; Duan, Y.; Guan, Y.; Shang, X.; Wang, Y.; et al. Identification and proposed relative and absolute configurations of niphimycins C-E from the marine-derived Streptomyces sp. IMB7-145 by genomic analysis. J. Nat. Prod. 2018, 81, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Blinov, N.O.; Golovkina, L.M.; Khokhlova, I.M.; Khlebarova, E.I.; Kleĭner, E.M.; Georgieva, I.K.; Sheĭkova, G.N.; Koreniako, A.I. Study of the antibiotics of the endomycin group. Antibiotiki 1967, 12, 867–874. [Google Scholar] [PubMed]
- Stefanelli, S.; Corti, E.; Montanini, N.; Denaro, M.; Sarubbi, E. Inhibitors of type-I interleukin-1 receptor from microbial metabolites. J. Antibiot. 1997, 50, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Reusser, F. Scopafungin, an inhibitor of oxidative phosphorylation in mitochondria. Biochem. Pharmacol. 1972, 21, 1031–1038. [Google Scholar] [CrossRef]
- Bergy, M.E.; Hoeksema, H. Scopafungin, a crystalline endomycin component. J. Antibiot. 1972, 25, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Takesako, K.; Nakamura, T.; Obayashi, A.; Iwasaki, S.; Namikoshi, M.; Okuda, S.; Beppu, T. Demalonyl derivatives of azalomycin F4 and scopafungin. J. Antibiot. 1986, 39, 713–716. [Google Scholar] [CrossRef]
- Johnson, L.E.; Dietz, A. Scopafungin, a crystalline antibiotic produced by Streptomyces hygroscopicus var. enhygrus var. nova. Appl. Microbiol. 1971, 22, 303–308. [Google Scholar]
- Ivanova, V.; Schlegel, R.; Dornberger, K. N’-Methylniphimycin, a novel minor congener of niphimycin from Streptomyces spec. 57-13. J. Basic Microbiol. 1998, 38, 415–419. [Google Scholar] [CrossRef]
- Takesako, K.; Beppu, T. Studies on new antifungal antibiotics, guanidylfungins A and B. II. Structure elucidation and biosynthesis. J. Antibiot. 1984, 37, 1170–1186. [Google Scholar] [CrossRef]
- Stephan, H.; Kempter, C.; Metzger, J.W.; Jung, G.; Potterat, O.; Pfefferle, C.; Fiedler, H.P. Kanchanamycins, new polyol macrolide antibiotics produced by Streptomyces olivaceus Tü 4018. II. Structure elucidation. J. Antibiot. 1996, 49, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, V.; Kolarova, M.; Aleksieva, K. Malonyl-4,5-dihydroniphimycin: A new polyol macrolide antibiotic, produced by Streptomyces hygroscopicus. Z. Naturforsch. B 2007, 62b, 1187–1192. [Google Scholar] [CrossRef]
- Ivanova, V.; Gesheva, V.; Kolarova, M. Dihydroniphimycin: New polyol macrolide antibiotic produced by Streptomyces hygroscopicus 15 isolation and structure elucidation. J. Antibiot. 2000, 53, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Jin, W.Z. Structure determination of new antifungal antibiotics, polaramycins A and B. Acta Pharm. Sin. 1997, 32, 352–356. [Google Scholar]
- Kobinata, K.; Koshino, H.; Kusakabe, H.; Kobayashi, Y.; Yamaguchi, I.; Isono, K.; Osada, H. Isolation and characterization of a new antibiotic, malolactomycin A. J. Antibiot. 1993, 46, 1912–1915. [Google Scholar] [CrossRef]
- Tanaka, Y.; Yoshida, H.; Enomoto, Y.; Shiomi, K.; Shinose, M.; Takahashi, Y.; Liu, J.R.; Omura, S. Malolactomycins C and D, new 40-membered macrolides active against Botrytis. J. Antibiot. 1997, 50, 194–200. [Google Scholar] [CrossRef]
- Mazzeo, G.; Santoro, E.; Andolfi, A.; Cimmino, A.; Troselj, P.; Petrovic, A.G.; Superchi, S.; Evidente, A.; Berova, N. Absolute configurations of fungal and plant metabolites by chiroptical methods. ORD, ECD, and VCD studies on phyllostin, scytolide, and oxysporone. J. Nat. Prod. 2013, 76, 588–599. [Google Scholar] [CrossRef]
- Takesako, K.; Beppu, T.; Nakamura, T.; Obayashi, A. Demalonyl derivatives of guanidylfungin A and copiamycin: Their synthesis and antifungal activity. J. Antibiot. 1985, 38, 1363–1370. [Google Scholar] [CrossRef]
- Yuan, G.; Li, P.; Yang, J.; Pang, H.; Pei, Y. Anti-methicillin-resistant Staphylococcus aureus assay of azalomycin F5a and its derivatives. Chin. J. Nat. Med. 2014, 12, 309–313. [Google Scholar] [CrossRef]
- Wu, X.; Xu, L.; Li, P.; Wang, Y.; Yuan, G. The anti-methicillin-resistant Staphylococcus aureus activities of azalomycin F derivatives and those of them combined with vitamin K3. Chin. J. Antibiot. 2016, 41, 584–589. [Google Scholar]
- Pretsch, E.; Bühlmann, P.; Afforlter, C. Structure Determination of Organic Compounds Tables of Spectral Data; Springer: New York, NY, USA, 2000; p. 250. [Google Scholar]
- Arai, M. Azalomycin F, an antibiotic against fungi and Trichomonas. Arzneimittel-Forschung 1968, 18, 1396–1399. [Google Scholar] [PubMed]
- Futamura, M.; Kamiya, S.; Tsukamoto, M.; Hirano, A.; Monden, Y.; Arakawa, H.; Nishimura, S. Malolactomycin D, a potent inhibitor of transcription controlled by the Ras responsive element, inhibits Ras-mediated transformation activity with suppression of MMP-1 and MMP-9 in NIH3T3 cells. Oncogene 2001, 20, 6724–6730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugawara, S. Effect of azalomycin F on bacteria. J. Antibiot. 1968, 21, 83–84. [Google Scholar] [CrossRef] [PubMed]
- Seiga, K.; Yamaji, K. Microbiological study of copiamycin. Appl. Microbiol. 1971, 21, 986–989. [Google Scholar] [PubMed]
- Uno, J.; Shigematsu, M.L.; Arai, T. Novel synergism of two antifungal agents, copiamycin and imidazole. Antimicrob. Agents Chemother. 1983, 24, 552–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, G.; Li, P.; Chen, S.; Zhu, X.; Zhang, Q. Anti-methicillin-resistant Staphylococcus aureus activities of three main components of azalomycin F. Chin. Pharm. J. 2014, 49, 644–648. [Google Scholar]
- Againa, S.; Kalushkova, M.; Georgieva, I. Niphimycin III. Chromatographic separation of the components of the niphimycin complex. J. Chromatogr. 1975, 109, 177–180. [Google Scholar] [CrossRef]
- Jin, W.; Meng, W.; Wang, Y.; Zhu, C.; Zhang, W.; Xu, X. Polaramycins A and B, novel antifungal antibiotics from Streptomyces hygroscopicus. Isolation, properties and characterization. Chin. J. Antibiot. 1997, 22, 1–7. [Google Scholar]
- Mogi, T.; Matsushita, K.; Murase, Y.; Kawahara, K.; Miyoshi, H.; Ui, H.; Shiomi, K.; Omura, S.; Kita, K. Identification of new inhibitors for alternative NADH dehydrogenase (NDH-II). FEMS Microbiol. Lett. 2009, 291, 157–161. [Google Scholar] [CrossRef] [Green Version]
- Newberry, E.P.; Willis, D.; Latifi, T.; Boudreaux, J.M.; Towler, D.A. Fibroblast growth factor receptor signaling activates the human interstitial collagenase promoter via the bipartite Ets-AP1 element. Mol. Endocrinol. 1997, 11, 1129–1144. [Google Scholar] [CrossRef]
- Johansson, N.; Ala-aho, R.; Uitto, V.; Grénman, R.; Fusenig, N.E.; López-Otín, C.; Kähäri, V.M. Expression of collagenase-3 (MMP-13) and collagenase-1 (MMP-1) by transformed keratinocytes is dependent on the activity of p38 mitogen-activated protein kinase. J. Cell Sci. 2000, 113, 227–235. [Google Scholar] [PubMed]
- Stamenkovic, I. Matrix metalloproteinases in tumor invasion and metastasis. Semin. Cancer Biol. 2000, 10, 415–433. [Google Scholar] [CrossRef] [PubMed]
- Curran, S.; Murray, G.I. Matrix metalloproteinases in tumour invasion and metastasis. J. Pathol. 1999, 189, 300–308. [Google Scholar] [CrossRef]
- Benziger, D.P.; Edelson, J. Absorption from the vagina. Drug Metab. Rev. 1983, 14, 137–168. [Google Scholar]
- Dumova, A.M.; Volynskaia, S.L.; Slonitskaia, N.N.; Mikhaĭlets, G.A. Effect of imbrimycin on the body of animals. Antibiotiki 1975, 20, 303–307. [Google Scholar]
- Arai, M. Azalomycins B and F, two new antibiotics. II. Properties of azalomycins B and F. J. Antibiot. Ser. A 1960, 13, 51–56. [Google Scholar]
- Kuroda, S.; Uno, J.; Arai, T. Target substances of some antifungal agents in the cell membrane. Antimicrob. Agents. Chemother. 1978, 13, 454–459. [Google Scholar] [CrossRef] [Green Version]
- Mishra, N.N.; Bayer, A.S. Correlation of cell membrane lipid profiles with daptomycin resistance in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chem. 2013, 57, 1082–1085. [Google Scholar] [CrossRef]
- Short, S.A.; White, D.C. Metabolism of phosphatidylglycerol, lysylphosphatidylglycerol, and cardiolipin of Staphylococcus aureus. J. Bacteriol. 1971, 108, 219–226. [Google Scholar]
- Vickery, C.R.; Wood, B.M.; Morris, H.G.; Losick, R.; Walker, S. Reconstitution of Staphylococcus aureus lipoteichoic acid synthase activity identifies Congo red as a selective inhibitor. J. Am. Chem. Soc. 2018, 140, 876–879. [Google Scholar] [CrossRef]
- Richter, S.G.; Elli, D.; Kim, H.K.; Hendrickx, A.P.A.; Sorg, J.A.; Schneewind, O.; Missiakas, D. Small molecule inhibitor of lipoteichoic acid synthesis is an antibiotic for Gram-positive bacteria. Proc. Natl. Acad. Sci. USA 2013, 110, 3531–3536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugawara, S. Mode of action of azalomycin F. Effect of azalomycin F on Candida albicans. J. Antibiot. 1967, 20, 93–108. [Google Scholar] [PubMed]
- Ogita, A.; Matsumoto, K.; Fujita, K.; Usuki, Y.; Hatanaka, Y.; Tanaka, T. Synergistic fungicidal activities of amphotericin B and N-Methyl-N”-dodecylguanidine: A constituent of polyol macrolide antibiotic niphimycin. J. Antibiot. 2007, 60, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Usuki, Y.; Matsumoto, K.; Inoue, T.; Yoshioka, K.; Iio, H.; Tanaka, T. Structure-activity relationship studies on niphimycin, a guanidylpolyol macrolide antibiotic. Part 1: The role of the N-methyl-N′-alkylguanidinium moiety. Bioorg. Med. Chem. Lett. 2006, 16, 1553–1556. [Google Scholar] [CrossRef]
- Ivanova, V.; Gushterova, A. Malonylniphimycin: Macrolide antibiotic from Streptomyces hygroscopicus B-7: Physico-chemical properties and structure elucidation. J. Antibiot. 1997, 50, 965–969. [Google Scholar] [CrossRef]
- Komiyama, K.; Edanami, K.; Tanoh, A.; Yamamoto, H.; Umezawa, I. Studies on the biological activity of stubomycin. J. Antibiot. 1983, 36, 301–311. [Google Scholar] [CrossRef]
- Quiros, L.M.; Aguirrezabalaga, I.; Olano, G.; Mendez, C.; Salas, J.A. Two glycosyltransferases and a glycosidase are involved in oleandomycin modification during its biosynthesis by Streptomyces antibioticus. Mol. Microbiol. 1998, 28, 1177–1185. [Google Scholar] [CrossRef]
- Xia, G.; Kohler, T.; Peschel, A. The wall teichoic acid and lipoteichoic acid polymers of Staphylococcus aureus. Int. J. Med. Microbiol. 2010, 300, 148–154. [Google Scholar] [CrossRef]
- Percy, M.G.; Gründling, A. Lipoteichoic acid synthesis and function in Gram-positive bacteria. Annu. Rev. Microbiol. 2014, 68, 81–100. [Google Scholar] [CrossRef]


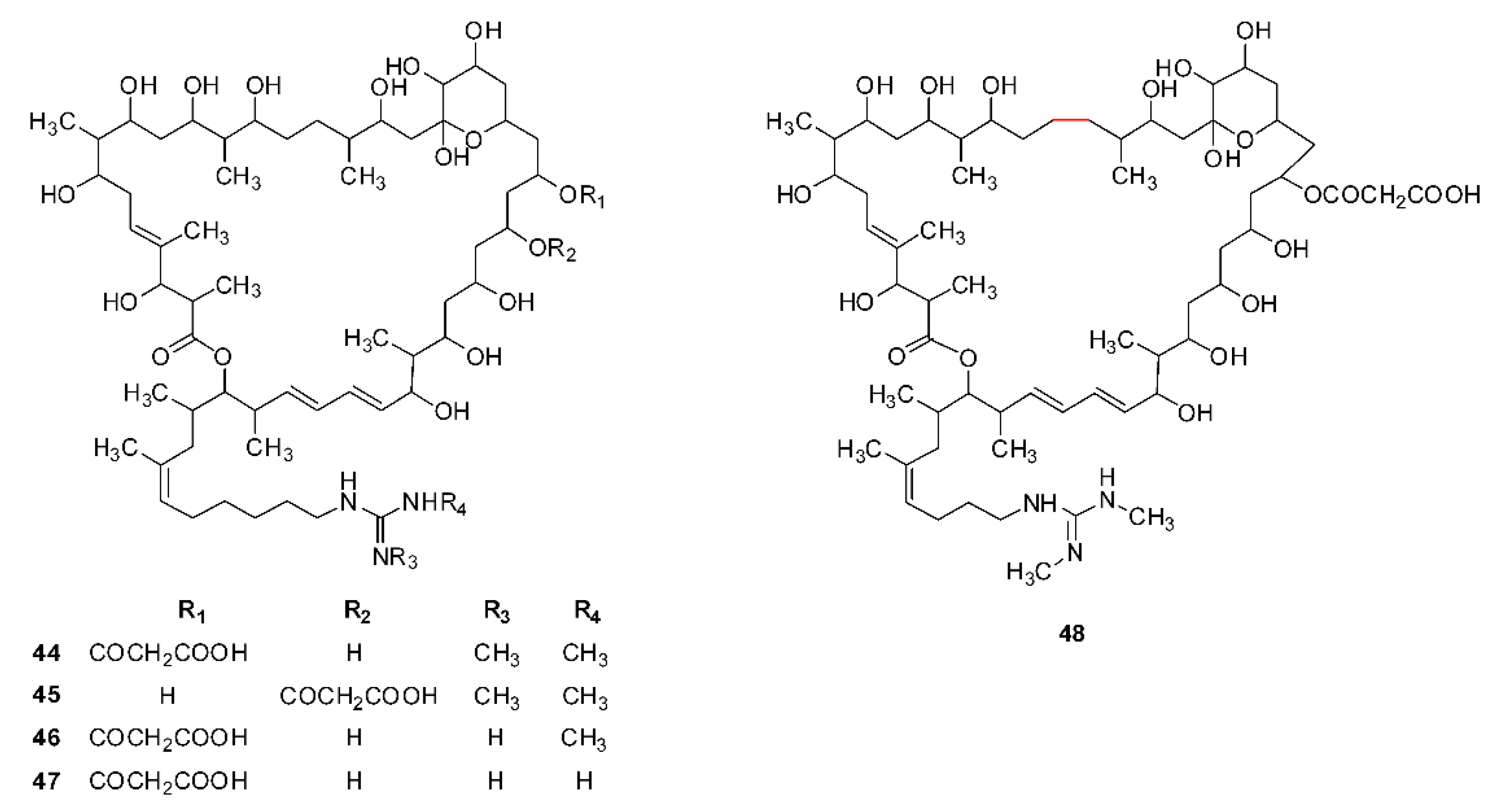
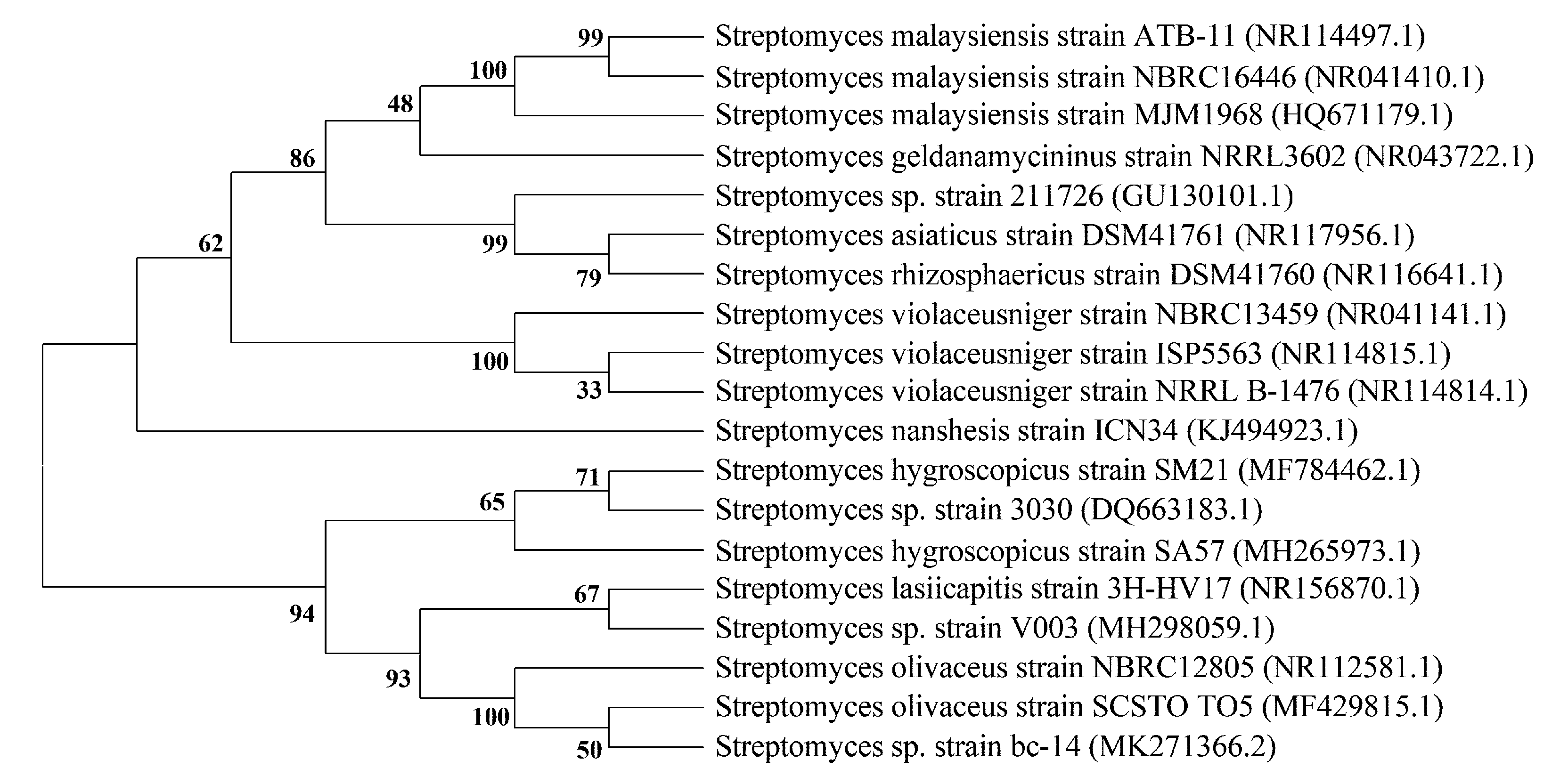
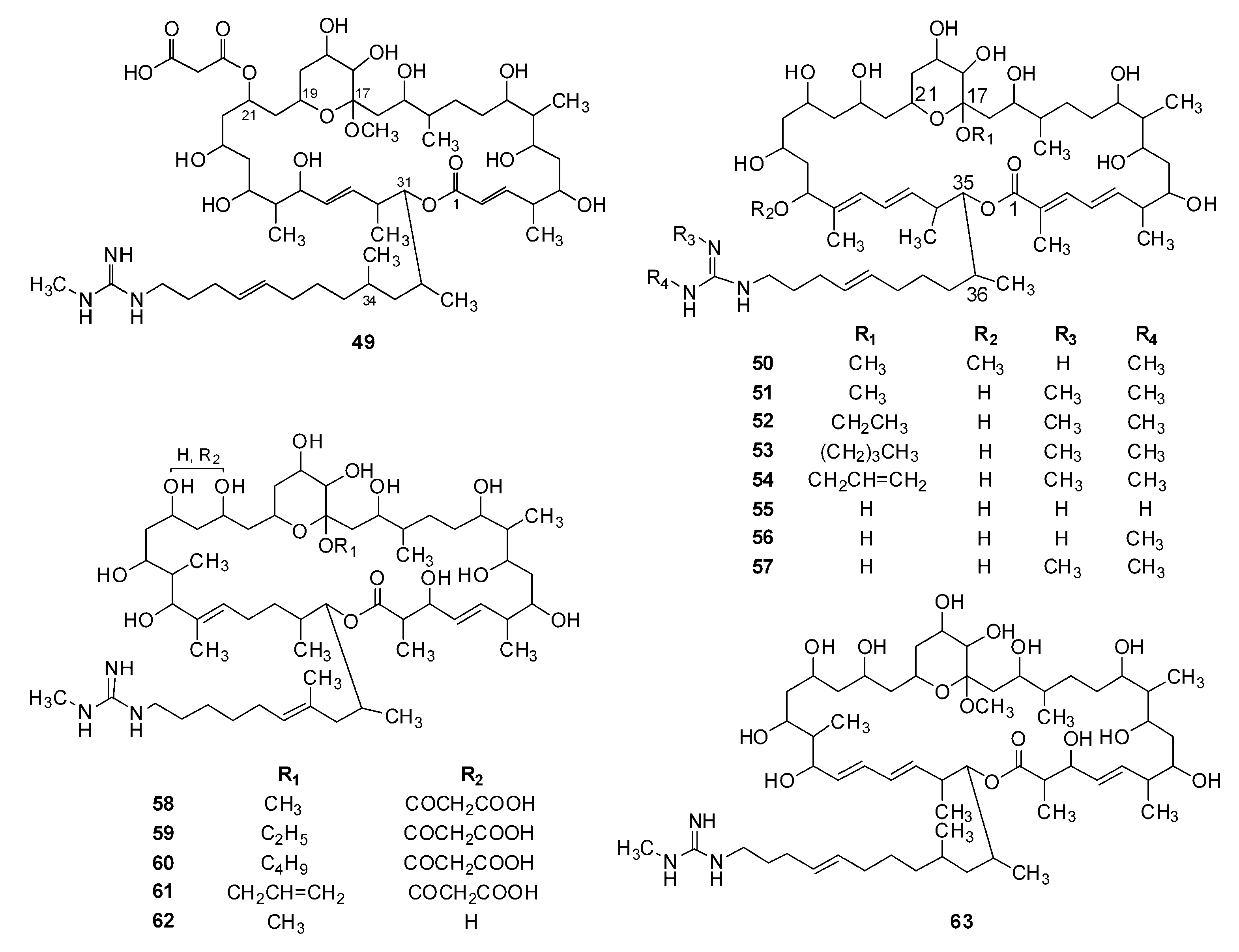


| Compounds | Name | Sources | References (Publication Time) |
|---|---|---|---|
| 1 | Copiamycin A | Streptomyces hygroscopicus var. crystallogenes ATCC 19040 | [47] (1965) |
| Streptomyces violaceoniger TÜ 905 | [39] (1981) | ||
| Streptomyces hygroscopicus sp. M931-1 | [48] (1991) | ||
| Actinomycete MT2617-2 | [40] (1996) | ||
| Streptomyces hygroscopicus var. crystallogenes IFM 1136 | [49] (1999) | ||
| 2 | Neocopiamycin A | Streptomyces hygroscopicus var. crystallogenes | [50] (1984) |
| Streptomyces hygroscopicus var. crystallogenes IFM 1136 | [49] (1999) | ||
| 3 | Neocopiamycin B | Streptomyces hygroscopicus var. crystallogenes IFM 1136 | [49] (1999) |
| 4 | Demalonylcopiamycin | Streptomyces hygroscopicus var. crystallogenes IFM 1136 | [49] (1999) |
| 5 | Demalonylmethylcopiamycin | Streptomyces hygroscopicus var. crystallogenes IFM 1136 | [49] (1999) |
| 6 | Guanidolide A | Streptomyces hygroscopicus var. crystallogenes IFM 1136 Streptomyces | [49] (1999) |
| hygroscopicus var. crystallogenes | [51] (1988) | ||
| 7 | TMC-34 | Streptomyces. A-3030 | [52] (1995) |
| 8 | Azalomycin F3a | Streptomyces hygroscopicus var. azalomyceticus | [11] (1960) |
| Streptomyces hygroscopicus MSU/MN-4-75B | [17] (1995) | ||
| Streptomyces malaysiensis MJM1968 | [36] (2010) | ||
| Streptomyces sp. 211726 | [18] (2011) | ||
| 9 | Azalomycin F4a | Streptomyces hygroscopicus var. azalomyceticus, | [11] (1960) |
| Streptomyces hygroscopicus MSU/MN-4-75B | [17] (1995) | ||
| Streptomyces malaysiensis MJM1968, | [36] (2010) | ||
| Streptomyces sp. 211726 | [18] (2011) | ||
| 10 | Azalomycin F5a | Streptomyces hygroscopicus var. azalomyceticus, | [11] (1960) |
| Streptomyces hygroscopicus MSU/MN-4-75B | [17] (1995) | ||
| Streptomyces malaysiensis MJM1968, | [36] (2010) | ||
| Streptomyces sp. 211726 | [18] (2011) | ||
| 11 | 2-Demethyl azalomycin F4a | Actinomycete sp. HIL Y-9120362 | [39] (1995) |
| 12 | 2-Demethyl azalomycin F5a | Actinomycete sp. HIL Y-9120362 | [39] (1995) |
| 13 | 25-Malonyl demalonyl azalomycin F5a monoester | Streptomyces sp. 211726, | [25] (2013) |
| 14 | 23-Valine demalonyl azalomycin F5a ester | Streptomyces sp. 211726, | [25] (2013) |
| 15 | 23-(6-Methyl) heptanoic acid demalonylazalomycin F3a ester | Streptomyces sp. 211726, | [25] (2013) |
| 16 | 23-(6-Methyl) heptanoic acid demalonylazalomycin F4a ester | Streptomyces sp. 211726, | [25] (2013) |
| 17 | 23-(6-Methyl) heptanoic acid demalonylazalomycin F5a ester | Streptomyces sp. 211726, | [25] (2013) |
| 18 | 23-(9-Methyl) decanoic acid demalonylazalomycin F4a ester | Streptomyces sp. 211726, | [25] (2013) |
| 19 | 23-(10-Methyl) undecanoic acid demalonylazalomycin F4a ester | Streptomyces sp. 211726, | [25] (2013) |
| 20 | RS-22A | Streptomyces violaceusniger RS-22 | [22,38] (1995) |
| 21 | RS-22B | Streptomyces violaceusniger RS-22 | [22,38] (1995) |
| 22 | RS-22C | Streptomyces violaceusniger RS-22 | [22,38] (1995) |
| 23 | Azalomycin F4a 2-ethylpentyl ester | Streptomyces sp.211726 | [18] (2011) |
| 24 | Azalomycin F5a 2-ethylpentyl ester | Streptomyces sp.211726 | [18] (2011) |
| 25 | Shurimycin A | Streptomyces hygroscopicus A1491 | [23] (1992) |
| 26 | Shurimycin B | Streptomyces hygroscopicus A1491 | [23] (1992) |
| 27 | Amycin B | Streptomyces sp. DSM 3816, | [21] (1990) |
| Streptomyces sp. IMB7-145 | [53] (2018) | ||
| 28 | Niphimycin (scopafungin) | Streptomyces hygroscopicus B-255 | [54] (1967) |
| Streptomyces sp. DSM 3816, | [21] (1990) | ||
| Streptomyces sp.KP6107, | [28] (2013) | ||
| Streptomyces sp. GE48009 | [55] (1997) | ||
| Streptomyces hygroscopicus var. enhygrus | [56,57] (1972) | ||
| Streptomyces hygroscopicus var. enhygrus NRRL 3664 | [58] (1986) | ||
| Streptomyces hygroscopicus var. enhygrus var. nova UC-2397 | [59] (1971) | ||
| Streptomyces sp. IMB7-145 | [53] (2018) | ||
| 29 | Amycin A | Streptomyces sp. DSM 3816 | [21] (1990) [53] (2018) |
| 30 | 25-Malonyl Demalonylniphimycin | Streptomyces sp. IMB7-145 | [53] (2018) |
| 31 | 19,25-Malony Demalonylniphimycin | Streptomyces sp. IMB7-145 | [53] (2018) |
| 32 | 15-Malonyl Niphimycin | Streptomyces sp. IMB7-145 | [53] (2018) |
| 33 | 17-O-Methylniphimycin | Streptomyces sp. IMB7-145 | [53] (2018) |
| 34 | N′-methyniphimycin | Streptomyces spec. 57-13 | [60] (1998) |
| 35 | Guanidyfungina A | Streptomyces hygroscopicus No. 662 | [20,61] (1984) |
| 36 | Guanidyfungina B | Streptomyces hygroscopicus No. 662 | [20,61] (1984) |
| 37 | Kanchanamycin A | Streptomyces strain Tü 4018, Streptomyces lasiicapitis sp. nov. | [38,62] (1996) [35] (2017) |
| 38 | Kanchanamycin C | Streptomyces strain Tü 4018, Streptomyces lasiicapitis sp. nov. | [38,62] (1996) [35] (2017) |
| 39 | Kanchanamycin D | Streptomyces strain Tü 4018, Streptomyces lasiicapitis sp. nov. | [38,62] (1996) [35] (2017) |
| 40 | Malonyl-4,5-dihydroniphimycin | Streptomyces hygroscopicus 15 | [63] (2007) |
| 41 | Dihydroniphimycin | Streptomyces hygroscopicus 15 | [64] (2000) |
| 42 | Polaramycin A | Streptomyces hygroscopicus LP-93 | [65] (1997) |
| 43 | Polaramycin B | Streptomyces hygroscopicus LP-93 | [65] (1997) |
| 44 | Malolactomycin A | Streptomyces sp. 83-634 | [45,66] (1993) |
| 45 | Malolactomycin B | Streptomyces sp. 83-634 | [45] (1993) |
| 46 | Malolactomycin C | Streptomyces KP-3144 | [67] (1997) |
| 47 | Malolactomycin D | Streptomyces KP-3144 | [67] (1997) |
| 48 | RP 63834 | Streptomyces strain n* S-13361 | [36] (1991) |
| Compounds | Derivatives Name | Raw Materials | References (Publication Time) |
|---|---|---|---|
| 49 | 17-Methyl copiamycin | Copiamycin | [69] (1985) |
| 50 | 17,29-Dimethyl demalonylazalomycin F4a | Azalomycin F4a | [58] (1986) |
| 51 | 17-Methyl demalonylazalomycin F5a | Azalomycin F5a | [70] (2014) |
| 52 | 17-Ethyl demalonylazalomycin F5a | Azalomycin F5a | [70] (2014) |
| 53 | 17-Butyl demalonylazalomycin F5a | Azalomycin F5a | [70] (2014) |
| 54 | 17-Allyl demalonylazalomycin F5a | Azalomycin F5a | [70] (2014) |
| 55 | Demalonylazalomycin F3a | Azalomycin F3a | [71] (2016) |
| 56 | Demalonylazalomycin F4a | Azalomycin F4a | [71] (2016) |
| 57 | Demalonylazalomycin F5a | Azalomycin F5a | [71] (2016) |
| 58 | 17-Methyl guanidylfungin A | Guanidylfungin A | [69] (1985) |
| 59 | 17-Ethyl guanidylfungin A | Guanidylfungin A | [69] (1985) |
| 60 | 17-Butyl guanidylfungin A | Guanidylfungin A | [69] (1985) |
| 61 | 17-Allyl guanidylfungin A | Guanidylfungin A | [69] (1985) |
| 62 | 17-Methyl demalonylguanidylfungin A | Guanidylfungin A | [69] (1985) |
| 63 | 17-Methyl demalonylniphimycin | Niphimycin | [58] (1986) |
| Compounds | Minimum Inhibitory Concentrations to Various Pathogenic Microorganisms (μg/mL) | References |
|---|---|---|
| 1 | Sarcina flava IFM 2242 (25), Sarcina flava IFM 2243 (3.12), 3 isolates of Sarcina hansenii (12.5–25.0), 4 isolates of Sarcina lutea (3.12–12.5), Sarcina subflava IFM 2116 (3.12), 3 isolates of Sarcina ureae (1.56–6.25), 16 isolates of Candida albicans (0.78–100), 3 isolates of Candida guilliermondii (100), Candida tropicalis IFM 40018 (100), Candida krusei IFM 40019 (100), Candida parapsilosis IFM 40020 (100), Candida stellatoidea IFM 40021 (100), Cryptococcus neoformans IFM 40037 (1.56), C. neoformans IFM 40038 (1.56), C. neoformans IFM 40047 (6.25), Staphylococcus aureus FDA 209P (12.5), Geotrichum candidum IFM 40068 (1.56), Epidermophyton floccosum IFM 40747 (0.39), Microsporum canis (3.13), Microsporum gypseum IFM 40727 (6.25), Mucor hiemalis (3.13), Mucor Racemosus (3.13), Trichophyton mentagrophytes Kamiyama (3.13), Trichophyton rubrum IFM 40732 (3.13), Sporothrix schenckill IFM 40750 (hyphal phase) (50), Fonsecaea pedrosoi IFM 40756 (100), Histoplasnma capsulatum IFM 40752 (hyphal phase) (0.78), 4 clinical isolates of Trichophyton glabrata (0.78~3.13), 14 clinical isolates of Trichophyton vaginalis (12.5 to >100) | [39,50,51,69,76,77] |
| 2 | 8 isolates of C. albicans (1.56–6.25), C. guilliermondii IFM 40017 (3.13), C. tropicalis IFM 40018 (3.13), C. krusei IFM 40019 (3.13), C. parapsilosis IFM 40020 (3.13), C. tropicalis (3.13), C. stellatoidea (IFM 40021) (3.13), Candida utillis IFM 40099 (12.5), C. neoformans IFM 40037 (<0.78), C. neoformans IFM 40038 (<0.78), C. neoformans IFM 40047 (1.56), G. candidum IFM 40068 (1.56), Torulopsis glabrata IFM 40065 (6.25), Trichophyton cutaneum IFM 40066 (6.25), Saccharomyces cerevisiae sake IFM 40025 (12.5), S. schenckill IFM 40751 (yeast phase) (3.13), Aspergillus flavus 23 (6.25), Aspergillus fumigatus 25 (25), Aspergillus nidulans 21 (6.25), Aspergillus niger 22 (6.25), Aspergillus oryzae IFM 40607 (6.25), Aspergillus versicolor 26 (12.5), Penicillium expansum IFM 40619 (3.13), E. floccosum IFM 40747 (0.78), M. canis (1.56), M. gypseum IFM 40727 (1.56), T. mentagrophytes IFM 40737 (1.56), T. mentagrophytes Kamiyama (1.56), T. rubrum IFM 40732 (0.39), S. schenckill IFM 40750 (hyphal phase) (6.25), F. pedrosoi IFM 40756 (3.13), H. capsulatum IFM 40752 (hyphal phase) (0.78) | [50] |
| 3 | A. flavus 23 (6.25), A. fumigatus 25 (12.5), A. nidulans 21 (6.25), A. niger 22 (6.25), A. oryzae IFM-40607 (6.25), A. versicolor 26 (6.25), Penicillium glaucum 3-1 (12.5), E. floccosum IFM 40747 (<0.78), M. canis (3.12), M. gypseum IFM 40727 (3.12), T. mentagrophytes IFM 40737 (1.56), T. rubrum IFM 40732 (1.56), Sporothrix schenckii IFM 40750 (6.25), S. schenckii IFM 40751 (yeast phase) (6.25), F. pedrosoi IFM 40756 (3.12), 8 isolates of C. albicans (0.78~12.5), C. guilliermondii IFM 40017 (6.25), C. tropicalis IFM 40018 (6.25), C. krusei IFM 40019 (6.25), Candida prarsilosis IFM 40020 (6.25), C. stellatoidea IFM 40021 (3.12), C. neoformans IFM 40037 (<0.78), C. neoformans IFM 40038 (<0.78), C. neoformans IFM 40047 (<0.78), C. utillis IFM 40099 (6.25), T. glabrata IFM 40065 (<0.78) | [49] |
| 4 | S. aureus FDA 209P (12.5), Bacillus subtillis NIHJ PCI 219 (12.5), Cochiobolus miyabeanus IFO 5277 (3.2), Alternaria mali IFO 8984 (12.5), Botryotinia fuckeliana IFO 5363 (1.6), Colletorichum lagenarium IFO 7513 (1.6), Pellicularia filamentosa sp. Sasakill IFO 6258 (1.6), Pyricularia oryzae IFO 5994 (6.25), A. oryzae IFO 5239 (25), T. mentagrophytes IFO 6202 (6.25), T. glabrata IFM 40065 (<0.78), S. cerevisiae IFO 0304 (1.6), C. albicans IFO 1594 (1.6), C. krusei IFM 40019 (6.25), C. prarsilosis IFM 40020 (6.25), C. stellatoidea IFM 40021 (3.12), C. neoformans IFM 40037 (<0.78), C. neoformans IFM 40038 (<0.78), C. neoformans IFM 40047 (<0.78), C. utillis IFM 40099 (6.25) | [51] |
| 5 | S. aureus FDA 209P (3.12), S. cerevisiae IAM 4020 (6.25), C. albicans, A. nidulans 21 (12.5), P. expansum IFM 40619 (6.25), T. mentagrophytes IFM 40734 (0.78), M. gypseum IFM 40727 (0.78), E. floccosum IFM 40747 (0.78) | [51,69] |
| 6 | A. nidulans 21 (50), Paecilomyces expansum (100), T. mentagrophytes IFM 40734 (12.5), M. gypseum IFM 40727 (25), E. floccosum IFM 40747 (12.5) | [51] |
| 7 | C. albicans ATCC 48130 (3.1), C. neoformans 145 A (1.6), A. fumigatus TUKUBA 48130 (3.1), T. mentagrophytes (3.1), T. rubrum (1.6) | [52] |
| 8 | Ten isolates of S. aureus (4–8), B. subtilis PCI 219 (12.5), S. aureus 209 P (6.25–12.5), S. lutea (6.25), Corynebacterium xerosis (6.25), Mycobacterium smegmatis ATCC607 (25), C. albicans Yu 1200 (1.56), S. cerevisiae (1.56–3.12), Torula utilis (1.56–3.12), C. neoformans (1.56), Kloeckera africana (1.56), Trichophyton asteroids (3.12), Trichophyton interdigitale (1.56), A. oryzae (6.25), A. niger (6.25~12.5), Penicillium notatum (1.56–3.12), P. oryzae (0.78–1.56), Ophioborus miyabeanus (0.78), Alternaria kikuchiana (1.56), Sclerotinia libertiana (0.78–1.56), Fusarium lycopersici (3.12), Fusarium lini (1.56~3.12), Ceratostomella fimbriata (1.56), Trichomonas vaginalis (12.5) | [12,78] |
| 9 | Ten isolates of S. aureus (4~12.5), C. albicans (12.5), A. fumigatus IAM 2046 (25), A. fumigatus IAM 2046 (MCC, >200) | [58,78] |
| 10 | Ten isolates of S. aureus ATCC 33592 (4~8) | [78] |
| 11 | The diameter of the zone of inhibition (mm): C. albicans (11), Penicillium digitatum (15), Fusarium culmorum 100 (17), A. mali P37 (20), P. oryzae K02 (16), Leptosphaeria oryzae J02 (23), Pellicularia sasakill J03 (28), Pseudomonas herpotrichoides 008 (19), Neurospora crassa SGF-18 (11), Botrytis cinereal E02 (22), B. cinereal A06 (13), B. cinereal D01 (17), Phytophthora infestans J08 (13) | [41] |
| 12 | The diameter of the zone of inhibition (mm): C. albicans (slight), P. digitatum (14), F. culmorum 100 (16), A. mali P37 (18), P. oryzae K02 (20), L. oryzae J02 (27), P. sasakill J03 (25), P. herpotrichoides 008 (16), N. crassa SGF-18 (12), B. cinereal E02 (20), B. cinereal A06 (12), B. cinereal (D01) (13), P. infestans J08 (15) | [41] |
| 13 | C. albicans ATCC 10231 (3.13), S. aureus S014 (0.39), B. subtilis S028 (0.20), Esherichia coli S002 (3.13) | [25] |
| 14 | C. albicans ATCC 10231 (6.25), S. aureus S014 (1.56), B. subtilis S028 (0.39), E. coli S002 (6.25) | [25] |
| 15 | C. albicans ATCC 10231 (3.13), S. aureus S014 (0.78), B. subtilis S028 (0.39), E. coli S002 (3.13) | [25] |
| 16 | B. subtilis S028 (0.20), C. albicans ATCC 10231 (1.56), E. coli S002 (6.25), S. aureus S014 (1.56) | [25] |
| 17 | B. subtilis S028 (0.78), C. albicans ATCC 10231 (1.56), E. coli S002 (12.5), S. aureus S014 (0.78) | [25] |
| 18 | B. subtilis S028 (0.39), C. albicans ATCC 10231 (3.13), E. coli S002 (25), S. aureus S014 (0.39) | [25] |
| 19 | B. subtilis S028 (0.39), C. albicans ATCC 10231 (3.13), E. coli S002 (3.13), S. aureus S014 (0.39) | [25] |
| 20 | S. aureus (6.25~12.5), Streptococcus pyogenes Cook (25), C. neoformans KC-201 (3.13), C. albicans KC-07 (3.13), C. tropicalis KC-104 (1.56), C. prarsilosis KC-110 (6.25), C. glabrata KC-308 (6.25), A. flavus KA-06 (12.5), A. fumigatus KA-01 (12.5), T. mentagrophytes KD-114 (12.5), T. rubrum KD-114 (12.5), M. canis KD-305 (12.5), M. gypseum KD-318 (12.5) | [40] |
| 21 | S. aureus (6.25~12.5), S. pyogenes Cook (12.5), C. neoformans KC-201 (3.13), C. albicans KC-07 (3.13), C. tropicalis KC-104 (3.13), C. prarsilosis KC-110 (6.25), C. glabrata KC-308) (6.25), A. flavus KA-06 (12.5), A. fumigatus KA-01 (12.5), T. mentagrophytes KD-114 (12.5), T. rubrum KD-114 (12.5), M. canis KD-305 (12.5), M. gypseum KD-318 (12.5) | [40] |
| 22 | S. aureus (6.25~12.5), S. pyogenes Cook (25), C. neoformans KC-201 (3.13), C. albicans KC-07 (6.25), C. tropicalis KC-104 (3.13), C. prarsilosis KC-110 (6.25), C. glabrata KC-308 (6.25), A. flavus KA-06 (12.5), A. fumigatus KA-01 (25), T. mentagrophytes KD-114 (12.5), T. rubrum KD-114 (12.5), M. canis KD-305 (12.5), M. gypseum KD-318 (12.5) | [40] |
| 23 | C. albicans ATCC 10231 (2.34) | [18] |
| 24 | C. albicans ATCC 10231 (12.5) | [32] |
| 25 | B. subtillis (3.1), S. lutea (3.1), S. aureus 209P (1.56), Botrytis fragilis (6.2), C. neoformans (1.56), T. mentagrophytes (3.1), A. fumigatus (3.1), A. mali (3.1), Fusarium oxysporum (12.5), B. cinereal (0.78), P. oryzae (0.78), Rhizoctonia solani (0.78), C. albicans (3.1) | [23] |
| 26 | B. subtillis (3.1), S. lutea (3.1), S. aureus 209P (1.56), Bacteroides fragilis (12.5), C. neoformans (1.56), T. mentagrophytes (3.1), A. fumigatus (6.2), A. mali (3.1), F. oxysporum (12.5), B. cinereal (0.78), P. oryzae (0.78), R. solani (1.56), C. albicans (6.2) | [23] |
| 27 | T. mentagrophytes (3.91), T. rubrum (3.91), M. canis (1.95), C. albicans (3.91), A. niger (1.95), 3 isolates of S. aureus (3.13), S. pyogenes 308 (6.25), S. pyogenes 77 A (6.25), Staphylococcus. faecium D (6.25) | [21,53,60] |
| 28 | Five isolates of C. albicans (1.56~12.5), Trichophyton gypseum, (1.56–15.6), Fusarium graminarum (3.12~7.8), B. subtillis (6.25). 15.6), B. subtilis (31.25)., T. mentagrophytes (3.91–10), T. rubrum (1.95), T. asteroids UC-4775 (1), M. canis (0.48), M. canis UC-1395 (10), Cryptococcus immnzitis UC-1119 (1), C. neoformans UC-1139 (1), A. niger (3.91), A. fumigatus IAM 2046 (12.5), B. subtilis ATCC 6633 (16), B. subtilis UC-564 (4), B. dermatitidlis UC-1911 (1), F. culmorum JP 15 (25), Geotrichutim sp. UC-1207 (1), Hormodendrulmn compactum UC-1222 (1), Hormodendrulmn capsulatum UC-1220 (0.1), Nocardia asteroidles UC-2052 (10), Phialophora verrlucosai UC-1807 (1), 9 isolates of S. aureus (6.25–16), Streptococcus hemolyticus UC- 15 (31), S. pyogenes 308 (25), S. pyogenes 77 A (25), S. faecium D (50), Staphylococcus faecalis UC-3235 (31), 3 isolates of S. epidermidis (32), S. schenlckii UC-1364 (10), 7 isolates of Enterococcus faecalis (32~64), A. fumigatus IAM 2046 (MCC, >200) | [8,21,53,59,60,63,79] |
| 29 | T. mentagrophytes (7.81), T. rubrum (7.81), M. canis (1.95), C. albicans (31.2), A. niger (7.81), 3 isolates of S. aureus (50), S. pyogenes 308 (100), S. pyogenes 77 A (100), Penicillium chrysogenum (7.81), B. subtilis (50), Micrococcus luteus (6.25), | [21,53,58,59,60,63,79] |
| 30 | Three isolates of S. epidermidis (16–64), 5 isolates of S. aureus (8~16), E. faecalis (64), E. faecium (64), C. albicans ATCC 10231 (16) | [53] |
| 31 | Four isolates of S. aureus (64), C. albicans ATCC 10231 (64) | [53] |
| 32 | Five isolates of Staphylococcus epidermidis (16~32), S. epidermidis 12-8 (64), C. albicans ATCC 10231 (16) | [53] |
| 33 | Five isolates of S. epidermidis (8–32), 2 isolates of S. epidermidis (16~32), E. faecalis ATCC 29212 (32), 2 isolates of E. faecalis (64), 3 isolates of E. faecium 12-3 (64), C. albicans ATCC 10231 (8) | [53] |
| 34 | The diameter of inhibition zone (mm): C. albicans (18), F. culmorum JP 15 (18), Glomerella cingulate (13), Klyuveromyces marxianus IMET 25148 (19), P. notatum JP 36 (15), Sporobolomyces salmonicolor SBUG 549 (24) | [60] |
| 35 | S. aureus FDA 209P (12.5), C. albicans IAM 4888 (25), S. cerevisiae IAM 4020 (50), A. fumigatus IAM 2153 (50), T. mentagrophytes (12.5), Sporotrichum schenckii (25), Paecilomyces variotii IAM 5001 (6.25), C. albicans (MCC, >200), A. fumigatus (MCC, >200) | [20] |
| 36 | S. aureus FDA 209P (50), B. subtillis PCI 219 (50), C. albicans IAM 4888 (100), A. oryzae (25), A. fumigatus (MCC, >200), T. mentagrophytes (6.25), C. albicans (MCC, >200) | [20] |
| 37 | S. aureus ATCC 11632 (30), Pseudomonas fluorescens ATCC 13525 (3), Aspergillus viridinutans CBS 12754 (100), P. notatum Tü 136 (30) | [38] |
| 38 | Arthrobacter aurescens ATCC 13344 (3), B. subtillis ATCC 6051 (10), S. aureus ATCC 11632 (10), E. coli K12 (10), P. fluorescens ATCC 13525 (0.1), C. albicans ATCC 10231 (10), S. cerevisiae Tü 125 (10), A. viridinutans CBS 12754 (10), P. variotii 137 (30), P. notatum Tü 136 (3) | [38] |
| 39 | P. fluorescens ATCC 13525 (3), A. viridinutans CBS 12754 (100), P. notatum Tü 136 (30) | [38] |
| 40 | C. albicans (25), A. niger (6.25), P. chrysogenum (7.81), B. subtillis (50), S. aureus (50), M. luteus (6.25), M. canis (1.95), S. pyogenes (100) | [63,64] |
| 41 | C. albicans (7), A. niger (3.13), P. chrysogenum (6.25), B. subtillis (15.62), S. aureus (12.50), M. luteus (3.13), M. canis (0.98), S. pyogenes (25) | [63,64] |
| 42 43 | B. cinereal Persoon (ID50, 5), Botryosphaeria dothidea (ID50, 1.25); Diameter of inhibition zone (mm): C. neoformans 14 (22), S. cerevisiae 2399 (15), saccharomyces sake yeast (Papulacadnin B resistant strain) (20), C. albicans duke (20), C. albicans duke (Amphotericin B resistant strain) (23), C. albicans 3 (18), C. tropicalis (20), M. gypseum (20), T. mentagrophytes (25), A. niger (12) | [80] |
| 44 | S. aureus FDA 209P (12.5), B. subtillis NIHJ PCI 219 (12.5), C. miyabeanus IFO 5277 (3.2), A. mali IFO 8984 (12.5), B. fuckeliana IFO 5363 (1.6), C. lagenarium IFO 7513 (1.6), P. filamentosa sp. Sasakill IFO 6258 (1.6), P. oryzae IFO 5994 (6.25), A. oryzae IFO 5239 (25), T. mentagrophytes IFO 6202 (6.25), S. cerevisiae IFO 0304 (1.6), C. albicans IFO 1594 (1.6) | [66] |
| 46 | P. infestans (100), Cladosporium fluvum (25), B. cinereal (25), P. oryzae (25), Cercospora beticola (100) | [67] |
| 49 | S. aureus FDA 209P (25), C. albicans Yu 1200 (25) | [51,69] |
| 50 | C. albicans Yu1200 (12.5), A. fumigatus IAM 2046(12.5), A. fumigatus (IAM 2046) (MCC, 50) | [58] |
| 51 | Four isolates of S. aureus (0.50~1.00) | [70] |
| 52 | Four isolates of S. aureus (0.67~1.00) | [70] |
| 53 | Four isolates of S. aureus (0.67~0.83) | [70] |
| 54 | Four isolates of S. aureus (0.50~0.83) | [70] |
| 55 | Four isolates of S. aureus (0.25~0.50) | [71] |
| 56 | Four isolates of S. aureus (0.25) | [71] |
| 57 | Four isolates of S. aureus (0.25) | [71] |
| 58 | S. aureus FDA 209P (12.5), B. subtillis PCI 219 (25), 2 isolates of C. albicans IAM 4888 (25~50), S. cerevisiae IAM 4020 (50), A. fumigatus IAM 2153 (12.5), M. racemosus (12.5), P. variotii IAM 5001(12.5), S. schenckii (12.5), C. albicans Yu 1200 (MCC, >200) | [69] |
| 59 | S. aureus FDA 209P (6.25), B. subtillis PCI 219 (12.5), C. albicans IAM 4888 (25), S. cerevisiae IAM 4020 (50), A. fumigatus IAM 2153 (25), M. racemosus (6.25), P. variotii IAM 5001 (12.5), S. schenckii (25) | [69] |
| 60 | S. aureus FDA 209P (12.5), B. subtillis PCI 219 (25), C. albicans IAM 4888(25), S. cerevisiae IAM 4020 (50), A. fumigatus IAM 2153 (50), M. racemosus (25), P. variotii IAM 5001 (12.5), S. schenckii (25) | [69] |
| 61 | S. aureus FDA 209P (6.25), B. subtillis PCI 219 (12.5), C. albicans IAM 4888 (25), S. cerevisiae IAM 4020 (50), A. fumigatus IAM 2153 (25), M. racemosus (12.5), P. variotii IAM 5001 (12.5), S. schenckii (25) | [69] |
| 62 | S. aureus FDA 209P (0.78), B. subtillis PCI 219 (1.56), 3 isolates of C. albicans (3.12~25), S. cerevisiae IAM 4020 (6.25), A. fumigatus IAM 2153 (3.12), M. racemosus (3.12), P. variotii IAM 5001 (1.56), S. schenckii (3.12) | [69] |
| 63 | T. mentagrophytes (15.6), T. rubrum (15.6), M. canis (7.81), C. albicans (6.25~31.2), A. niger (15.6), A. fumigatus IAM 2046 (6.25), 3 isolates of S. aureus 6511 (12.5), S. pyogenes 308 (6.25), S. pyogenes 77 A (6.25), S. faecium D (50), A. fumigatus (MCC, 12.5) | [21,58,60,53] |
| Compounds | Organisms | Test Type | Administration | Dose (mg/kg) | References |
|---|---|---|---|---|---|
| Copiamycin | mouse | LD50 | Intraperitoneal | 24.8 | [76] |
| mouse | LD50 | Subcutaneous | 61.5 | [76] | |
| Neocopiamycins A and B | mouse | LD0 | Intraperitoneal | >1000 | [50] |
| mouse | LD0 | Intravenous | >30 and >25 | [50] | |
| mouse | LD0 | Oral | >1000 | [50] | |
| Azalomycin F | mouse | LD50 | Intraperitoneal | 18 or 26 | [87,88] |
| mouse | LD50 | Intravenous | 12.5 | [73] | |
| mouse | LD50 | Oral | 580 | [73] | |
| mouse | LD50 | Subcutaneous | 162 | [73] | |
| Azalomycin F a | mouse | LD50 | Intraperitoneal | 97.9 | [25] |
| Guanidylfungin A | mouse | LD50 | Intraperitoneal | 12.5 | [20] |
| Malolactomycin A | mouse | LD50 | Intraperitoneal | 6.7 | [66] |
| Malolactomycin C | mouse | LD0 | Intraperitoneal | >30 | [67] |
| Malolactomycin D | mouse | LD0 | Intraperitoneal | >30 | [67] |
| RS-22 b | mouse | LD50 | Intravenous | 25 | [38] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Yuan, G.; Li, P.; Cao, S. Guanidine-Containing Polyhydroxyl Macrolides: Chemistry, Biology, and Structure-Activity Relationship. Molecules 2019, 24, 3913. https://doi.org/10.3390/molecules24213913
Song X, Yuan G, Li P, Cao S. Guanidine-Containing Polyhydroxyl Macrolides: Chemistry, Biology, and Structure-Activity Relationship. Molecules. 2019; 24(21):3913. https://doi.org/10.3390/molecules24213913
Chicago/Turabian StyleSong, Xiaoyuan, Ganjun Yuan, Peibo Li, and Sheng Cao. 2019. "Guanidine-Containing Polyhydroxyl Macrolides: Chemistry, Biology, and Structure-Activity Relationship" Molecules 24, no. 21: 3913. https://doi.org/10.3390/molecules24213913






