Effects of the Essential Oil from Pistacia lentiscus Var. chia on the Lateral Line System and the Gene Expression Profile of Zebrafish (Danio rerio)
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Zebrafish Maintenance
3.2. Extraction and Analysis of Mastic Essential Oil
3.3. Exposure of Zebrafish Larvae to Mastic Oil and In Vivo Staining of Lateral Line Neuromasts
3.4. Preparation of Mastic Oil Supplemented Fish Food
3.5. Whole Mount Immunofluorescence
3.6. H&E Staining
3.7. Immunohistochemistry
3.8. RNA Preparation and cDNA Synthesis
3.9. Microarray Analysis
3.10. Quantitative Real-Time PCR (qPCR)
3.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Takahashi, K.; Fukazawa, M.; Motohira, H.; Ochiai, K.; Nishikawa, H.; Miyata, T. A pilot study on antiplaque effects of mastic chewing gum in the oral cavity. J. Periodontol. 2003, 74, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, A.; Duran, N.; Koksal, F. In vitro and in vivo antimicrobial effects of mastic chewing gum against Streptococcus mutans and mutans streptococci. Arch. Oral. Biol. 2006, 51, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllou, A.; Chaviaras, N.; Sergentanis, T.N.; Protopapa, E.; Tsaknis, J. Chios mastic gum modulates serum biochemical parameters in a human population. J. Ethnopharmacol. 2007, 111, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Sakagami, H.; Kishino, K.; Kobayashi, M.; Hashimoto, K.; Iida, S.; Shimetani, A.; Nakamura, Y.; Takahashi, K.; Ikarashi, T.; Fukamachi, H.; et al. Selective antibacterial and apoptosis-modulating activities of mastic. In Vivo 2009, 23, 215–223. [Google Scholar]
- Huwez, F.U.; Thirlwell, D.; Cockayne, A.; Ala’Aldeen, D.A. Mastic gum kills Helicobacter pylori. N. Engl. J. Med. 1998, 339, 1946. [Google Scholar] [CrossRef]
- Marone, P.; Bono, L.; Leone, E.; Bona, S.; Carretto, E.; Perversi, L. Bactericidal activity of Pistacia lentiscus mastic gum against Helicobacter pylori. J. Chemother. 2001, 13, 611–614. [Google Scholar] [CrossRef]
- Paraschos, S.; Magiatis, P.; Mitakou, S.; Petraki, K.; Kalliaropoulos, A.; Maragkoudakis, P.; Mentis, A.; Sgouras, D.; Skaltsounis, A.L. In vitro and in vivo activities of Chios mastic gum extracts and constituents against Helicobacter pylori. Antimicrob. Agents Chemother. 2007, 51, 551–559. [Google Scholar] [CrossRef]
- Miyamoto, T.; Okimoto, T.; Kuwano, M. Chemical Composition of the Essential Oil of Mastic Gum and their Antibacterial Activity Against Drug-Resistant Helicobacter pylori. Nat. Prod. Bioprospect. 2014, 4, 227–231. [Google Scholar] [CrossRef] [Green Version]
- Andrikopoulos, N.K.; Kaliora, A.C.; Assimopoulou, A.N.; Papapeorgiou, V.P. Biological activity of some naturally occurring resins, gums and pigments against in vitro LDL oxidation. Phytother. Res. 2003, 17, 501–507. [Google Scholar] [CrossRef]
- Loizou, S.; Paraschos, S.; Mitakou, S.; Chrousos, G.P.; Lekakis, I.; Moutsatsou, P. Chios mastic gum extract and isolated phytosterol tirucallol exhibit anti-inflammatory activity in human aortic endothelial cells. Exp. Biol. Med. 2009, 234, 553–561. [Google Scholar] [CrossRef]
- Qiao, J.; Li, A.; Jin, X.; Wang, J. Mastic alleviates allergic inflammation in asthmatic model mice by inhibiting recruitment of eosinophils. Am. J. Respir. Cell Mol. Biol. 2011, 45, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Ebrahimzadeh, M.A.; Nabavi, S.F.; Hafezi, S.; Nabavi, S.M.; Eslami, S. Antiinflammatory and antioxidant activities of gum mastic. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 765–769. [Google Scholar] [PubMed]
- Magiatis, P.; Melliou, E.; Skaltsounis, A.L.; Chinou, I.B.; Mitaku, S. Chemical composition and antimicrobial activity of the essential oils of Pistacia lentiscus var. chia. Planta Med. 1999, 65, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Paraschos, S.; Magiatis, P.; Gikas, E.; Smyrnioudis, I.; Skaltsounis, A.L. Quality profile determination of Chios mastic gum essential oil and detection of adulteration in mastic oil products with the application of chiral and non-chiral GC-MS analysis. Fitoterapia 2016, 114, 12–17. [Google Scholar] [CrossRef]
- Xynos, N.; Termentzi, A.; Fokialakis, N.; Skaltsounis, A.L.; Aligiannis, N. Supercritical CO2 extraction of mastic gum and chemical characterization of bioactive fractions using LC-HRMS/MS and GC–MS. J. Supercrit. Fluid. 2018, 133, 349–356. [Google Scholar] [CrossRef]
- Koutsoudaki, C.; Krsek, M.; Rodger, A. Chemical composition and antibacterial activity of the essential oil and the gum of Pistacia lentiscus Var. chia. J. Agric. Food. Chem. 2005, 53, 7681–7685. [Google Scholar] [CrossRef]
- Balan, K.V.; Prince, J.; Han, Z.; Dimas, K.; Cladaras, M.; Wyche, J.H.; Sitaras, N.M.; Pantazis, P. Antiproliferative activity and induction of apoptosis in human colon cancer cells treated in vitro with constituents of a product derived from Pistacia lentiscus L. var. chia. Phytomedicine 2007, 14, 263–272. [Google Scholar] [CrossRef]
- Loutrari, H.; Magkouta, S.; Pyriochou, A.; Koika, V.; Kolisis, F.N.; Papapetropoulos, A.; Roussos, C. Mastic oil from Pistacia lentiscus var. chia inhibits growth and survival of human K562 leukemia cells and attenuates angiogenesis. Nutr. Cancer. 2006, 55, 86–93. [Google Scholar] [CrossRef]
- He, M.L.; Li, A.; Xu, C.S.; Wang, S.L.; Zhang, M.J.; Gu, H.; Yang, Y.Q.; Tao, H.H. Mechanisms of antiprostate cancer by gum mastic: NF-kappaB signal as target. Acta Pharmacol. Sin. 2007, 28, 446–452. [Google Scholar] [CrossRef]
- Dimas, K.; Hatziantoniou, S.; Wyche, J.H.; Pantazis, P. A mastic gum extract induces suppression of growth of human colorectal tumor xenografts in immunodeficient mice. In Vivo 2009, 23, 63–68. [Google Scholar]
- Moulos, P.; Papadodima, O.; Chatziioannou, A.; Loutrari, H.; Roussos, C.; Kolisis, F.N. A transcriptomic computational analysis of mastic oil-treated Lewis lung carcinomas reveals molecular mechanisms targeting tumor cell growth and survival. BMC Med. Genomics 2009, 2, 68. [Google Scholar] [CrossRef] [PubMed]
- Buriani, A.; Fortinguerra, S.; Sorrenti, V.; Dall’Acqua, S.; Innocenti, G.; Montopoli, M.; Daniela Gabbia, D.; Carrara, M. Human Adenocarcinoma Cell Line Sensitivity to Essential Oil Phytocomplexes from Pistacia Species: A Multivariate Approach. Molecules 2017, 22, 1336. [Google Scholar] [CrossRef] [PubMed]
- Kanther, M.; Rawls, J.F. Host-microbe interactions in the developing zebrafish. Curr. Opin. Immunol. 2010, 22, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Falcinelli, S.; Picchietti, S.; Rodiles, A.; Cossignani, L.; Merrifield, D.L.; Taddei, A.R.; Maradonna, F.; Olivotto, I.; Gioacchini, G.; Carnevali, O. Lactobacillus rhamnosus lowers zebrafish lipid content by changing gut microbiota and host transcription of genes involved in lipid metabolism. Sci. Rep. 2015, 5, 9336. [Google Scholar] [CrossRef] [PubMed]
- Polednik, K.M.; Koch, A.C.; Felzien, L.K. Effects of Essential Oil from Thymus vulgaris on Viability and Inflammation in Zebrafish Embryos. Zebrafish 2018, 15, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.S.; Keita, H.; Ortiz, B.L.S.; Dos Santos Sampaio, T.I.; Ferreira, I.M.; Lima, E.S.; de Jesus Amazonas da Silva, M.; Fernandes, C.P.; de Faria Mota Oliveira, A.E.M.; da Conceicao, E.C.; et al. Anti-inflammatory activity of nanoemulsions of essential oil from Rosmarinus officinalis L.: In vitro and in zebrafish studies. Inflammopharmacology 2018, 26, 1057–1080. [Google Scholar] [CrossRef]
- Nayak, S.; Khozin-Goldberg, I.; Cohen, G.; Zilberg, D. Dietary Supplementation With omega6 LC-PUFA-Rich Algae Modulates Zebrafish Immune Function and Improves Resistance to Streptococcal Infection. Front. Immunol. 2018, 9, 1960. [Google Scholar] [CrossRef]
- Li, J.M.; Li, L.Y.; Qin, X.; Ning, L.J.; Lu, D.L.; Li, D.L.; Zhang, M.L.; Wang, X.; Du, Z.Y. Systemic regulation of L-carnitine in nutritional metabolism in zebrafish, Danio rerio. Sci. Rep. 2017, 7, 40815. [Google Scholar] [CrossRef] [Green Version]
- Ma, E.Y.; Raible, D.W. Signaling pathways regulating zebrafish lateral line development. Curr. Biol. 2009, 19, R381–R386. [Google Scholar] [CrossRef]
- Coffin, A.B.; Ou, H.; Owens, K.N.; Santos, F.; Simon, J.A.; Rubel, E.W.; Raible, D.W. Chemical screening for hair cell loss and protection in the zebrafish lateral line. Zebrafish 2010, 7, 3–11. [Google Scholar] [CrossRef]
- Paraschos, S.; Mitakou, S.; Skaltsounis, A.L. Chios gum mastic: A review of its biological activities. Curr. Med. Chem. 2012, 19, 2292–2302. [Google Scholar] [CrossRef] [PubMed]
- Corbi, G.; Conti, V.; Davinelli, S.; Scapagnini, G.; Filippelli, A.; Ferrara, N. Dietary Phytochemicals in Neuroimmunoaging: A New Therapeutic Possibility for Humans? Front. Pharmacol. 2016, 7, 364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spyridopoulou, K.; Tiptiri-Kourpeti, A.; Lampri, E.; Fitsiou, E.; Vasileiadis, S.; Vamvakias, M.; Bardouki, H.; Goussia, A.; Malamou-Mitsi, V.; Panayiotidis, M.I.; et al. Dietary mastic oil extracted from Pistacia lentiscus var. chia suppresses tumor growth in experimental colon cancer models. Sci. Rep. 2017, 7, 3782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlastos, D.; Drosopoulou, E.; Efthimiou, I.; Gavriilidis, M.; Panagaki, D.; Mpatziou, K.; Kalamara, P.; Mademtzoglou, D.; Mavragani-Tsipidou, P. Genotoxic and Antigenotoxic Assessment of Chios Mastic Oil by the In Vitro Micronucleus Test on Human Lymphocytes and the In Vivo Wing Somatic Test on Drosophila. PLoS ONE 2015, 10, e0130498. [Google Scholar] [CrossRef] [Green Version]
- Steiner, A.B.; Kim, T.; Cabot, V.; Hudspeth, A.J. Dynamic gene expression by putative hair-cell progenitors during regeneration in the zebrafish lateral line. Proc. Natl. Acad. Sci. USA 2014, 111, E1393–E1401. [Google Scholar] [CrossRef] [Green Version]
- Wolman, M.; Granato, M. Behavioral genetics in larval zebrafish: Learning from the young. Dev. Neurobiol. 2012, 72, 366–372. [Google Scholar] [CrossRef]
- Domadia, P.; Swarup, S.; Bhunia, A.; Sivaraman, J.; Dasgupta, D. Inhibition of bacterial cell division protein FtsZ by cinnamaldehyde. Biochem. Pharmacol. 2007, 74, 831–840. [Google Scholar] [CrossRef]
- Bulfon, C.; Volpatti, D.; Galeotti, M. Current research on the use of plant-derived products in farmed fish. Aquacult. Res. 2015, 46, 513–551. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food. Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhang, S.; Wang, H.; Piao, X. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: A review. J. Anim. Sci. Biotechnol. 2015, 6, 7. [Google Scholar] [CrossRef]
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Samuel, C.E. Antiviral actions of interferon. Interferon-regulated cellular proteins and their surprisingly selective antiviral activities. Virology 1991, 183, 1–11. [Google Scholar] [CrossRef]
- Platanias, L.C. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 2005, 5, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Dong, C.; Qi, L.; Chen, W.; Huang, L.; Li, Z.; Xia, Q.; Liu, D.; Huang, M.; Weng, S.; et al. Characteristics of the interferon regulatory factor pairs zfIRF5/7 and their stimulation expression by ISKNV Infection in zebrafish (Danio rerio). Dev. Comp. Immunol. 2010, 34, 1263–1273. [Google Scholar] [CrossRef]
- Feng, H.; Zhang, Y.B.; Zhang, Q.M.; Li, Z.; Zhang, Q.Y.; Gui, J.F. Zebrafish IRF1 regulates IFN antiviral response through binding to IFNvarphi1 and IFNvarphi3 promoters downstream of MyD88 signaling. J. Immunol. 2015, 194, 1225–1238. [Google Scholar] [CrossRef]
- Varela, M.; Diaz-Rosales, P.; Pereiro, P.; Forn-Cuni, G.; Costa, M.M.; Dios, S.; Romero, A.; Figueras, A.; Novoa, B. Interferon-induced genes of the expanded IFIT family show conserved antiviral activities in non-mammalian species. PLoS ONE 2014, 9, e100015. [Google Scholar] [CrossRef]
- Klamp, T.; Boehm, U.; Schenk, D.; Pfeffer, K.; Howard, J.C. A giant GTPase, very large inducible GTPase-1, is inducible by IFNs. J. Immunol. 2003, 171, 1255–1265. [Google Scholar] [CrossRef]
- Verhelst, J.; Hulpiau, P.; Saelens, X. Mx proteins: Antiviral gatekeepers that restrain the uninvited. Microbiol. Mol. Biol. Rev. 2013, 77, 551–566. [Google Scholar] [CrossRef]
- Trinchieri, G. Type I interferon: Friend or foe? J. Exp. Med. 2010, 207, 2053–2063. [Google Scholar] [CrossRef]
- Michelucci, A.; Cordes, T.; Ghelfi, J.; Pailot, A.; Reiling, N.; Goldmann, O.; Binz, T.; Wegner, A.; Tallam, A.; Rausell, A.; et al. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc. Natl. Acad. Sci. USA 2013, 110, 7820–7825. [Google Scholar] [CrossRef] [Green Version]
- Mills, E.L.; Kelly, B.; O’Neill, L.A.J. Mitochondria are the powerhouses of immunity. Nat. Immunol. 2017, 18, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Corfield, A.P.; Carroll, D.; Myerscough, N.; Probert, C.S. Mucins in the gastrointestinal tract in health and disease. Front. Biosci. 2001, 6, D1321–D1357. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.; Bell, A.; Mantle, M.; Pearson, J.P. The structure and physiology of gastrointestinal mucus. Adv. Exp. Med. Biol. 1982, 144, 115–133. [Google Scholar] [PubMed]
- Gomez, G.D.; Balcazar, J.L. A review on the interactions between gut microbiota and innate immunity of fish. FEMS Immunol. Med. Microbiol. 2008, 52, 145–154. [Google Scholar] [CrossRef]
- Stephens, W.Z.; Burns, A.R.; Stagaman, K.; Wong, S.; Rawls, J.F.; Guillemin, K.; Bohannan, B.J. The composition of the zebrafish intestinal microbial community varies across development. ISME J. 2016, 10, 644–654. [Google Scholar] [CrossRef]
- Laparra, J.M.; Sanz, Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacol. Res. 2010, 61, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Serifi, I.; Tzima, E.; Soupsana, K.; Karetsou, Z.; Beis, D.; Papamarcaki, T. The zebrafish homologs of SET/I2PP2A oncoprotein: Expression patterns and insights into their physiological roles during development. Biochem. J. 2016, 473, 4609–4627. [Google Scholar] [CrossRef]
- Tzima, E.; Serifi, I.; Tsikari, I.; Alzualde, A.; Leonardos, I.; Papamarcaki, T. Transcriptional and Behavioral Responses of Zebrafish Larvae to Microcystin-LR Exposure. Int. J. Mol. Sci. 2017, 18, 365. [Google Scholar] [CrossRef]
Sample Availability: Samples of the mastic essential oil and α-pinene are available from the authors. |



| Compounds | RT | Relative (%) Area | Formula | Structure | MW |
|---|---|---|---|---|---|
| α-pinene | 9.24 | 67.71 | C10H16 |  | 136.24 |
| camphene | 9.73 | 0.70 | C10H16 | 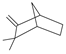 | 136.24 |
| verbenene | 9.87 | 0.07 | C10H14 |  | 134.22 |
| β-pinene | 10.93 | 3.05 | C10H16 |  | 136.24 |
| myrcene | 11.79 | 18.81 | C10H16 |  | 136.24 |
| limonene | 13.44 | 0.89 | C10H16 |  | 136.24 |
| linalol | 16.67 | 0.73 | C10H18O | 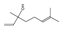 | 154.25 |
| α-campholenic ald | 17.60 | 0.26 | C10H16O | 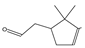 | 152.23 |
| pinocarveol | 18.61 | 0.32 | C10H16O |  | 152.23 |
| trans-verbenol | 18.78 | 0.07 | C10H16O | 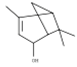 | 152.23 |
| cis-verbenol | 18.97 | 0.69 | C10H16O | 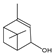 | 152.23 |
| verbenone | 21.59 | 0.32 | C10H14O | 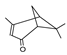 | 150.22 |
| caryophyllene | 33.61 | 0.50 | C15H24 | 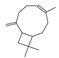 | 204.36 |
| Gene Symbol | Gene Description | NCBI Gene ID | p-Value | FC (abs) |
|---|---|---|---|---|
| muc5.2 | mucin 5.2 | 572172 | 9.37 × 10−3 | 2.24 |
| irg1l | immunoresponsive gene 1, like | 562007 | 1.56 × 10−2 | 1.87 |
| LOC100001880 | interferon-induced very large GTPase 1-like, si:dkey-202l22.6 | 100001880 | 1.50 × 10−3 | 1.92 |
| LOC556554 | skin mucus antibacterial l-amino acid oxidase-like//orthologous to human interleukin 4 induced 1 | 556554//337166 | 1.03 × 10−4 | −1.96 |
| irf7 | interferon regulatory factor 7 | 393650 | 2.14 × 10−4 | −2.80 |
| irf1b | interferon regulatory factor 1b | 792160 | 4.02 × 10−4 | −1.77 |
| si:dkey-79f11.7 | si:dkey-79f11.7 (interferon-induced protein 44) | 100007552 | 1.70 × 10−2 | −1.88 |
| LOC795887 | interferon-induced protein 44 | 795887 | 6.45 × 10−4 | −2.10 |
| zgc:152791 | zgc:152791 (interferon stimulated gene 12) | 641326 | 3.38 × 10−5 | −2.13 |
| LOC558511 | orthologous to human interferon alpha inducible protein 27 like 2 | 558511 | 2.16 × 10−3 | −1.80 |
| LOC556241 | interferon-induced very large GTPase 1-like, si:dkey-85k7.12 | 556241 | 2.62 × 10−4 | −2.03 |
| il21r.1 | interleukin 21 receptor, tandem duplicate 1 | 100134976 | 1.17 × 10−2 | −1.76 |
| b2m | beta-2-microglobulin | 30400 | 6.20 × 10−4 | −2.21 |
| Fas | Fas cell surface death receptor (TNF receptor superfamily, member 6) | 768248 | 8.53 × 10−4 | −1.70 |
| hla-dpa1 | HLA-DPA1 protein, zgc:123107 | 553497//641415 | 1.41 × 10−2 | −1.83 |
| Mxa | myxovirus (influenza) resistance A | 360142 | 2.20 × 10−3 | −1.84 |
| stat1b | signal transducer and activator of transcription 1b | 368481 | 3.73 × 10−5 | −1.99 |
| urgcp, LOC100005729 | upregulator of cell proliferation | 100005729 | 7.42 × 10−3 | −1.82 |
| LOC798269 | tumor necrosis factor receptor superfamily member 5 | 798269 | 1.22 × 10−2 | −1.85 |
| lgals3bpa | lectin, galactoside-binding, soluble, 3 binding protein a | 677742 | 1.44 × 10−3 | −2.48 |
| lgals3bpb | lectin, galactoside-binding, soluble, 3 binding protein b | 405809 | 1.15 × 10−4 | −1.91 |
| lgals9l1 | lectin, galactoside-binding, soluble, 9 (galectin 9)-like 1 | 337597 | 4.33 × 10−2 | −1.98 |
| ccl38a.5| ccl38a.1 | chemokine (C-C motif) ligand 38, duplicate 5| chemokine (C-C motif) ligand 38, duplicate 1 | 563208| 794050 | 3.81 × 10−3 | −2.59 |
| ccl34b.4 | chemokine (C-C motif) ligand 34b, duplicate 4 | 556621 | 2.59 × 10−2 | −2.09 |
| LOC100334176 | GTPase IMAP family member 8 | 100334176 | 2.33 × 10−2 | −3.02 |
| LOC100150889, tapbpl | TAP binding protein- like | 100150889 | 3.27 × 10−3 | −1.74 |
| LOC100007087 | basic leucine zipper ATF transcriptional factor -like, si:dkey-23i12.7 | 100007087 | 8.18 × 10−3 | −2.19 |
| ftr19 | finTRIM family, member 19 (tripartite motif-containing protein 47) | 100301514 | 3.72 × 10−4 | −1.74 |
| si:ch211-284p22.1, trim35-19 | tripartite motif containing 35-19 | 562814 | 2.74 × 10−5 | −1.81 |
| crp3 | C-reactive protein 3 | 100141350 | 3.68 × 10−3 | −2.96 |
| LOC100332428| LOC562648 | poly [ADP-ribose] polymerase 14 | 100332428| 562648 | 1.42 × 10−3 | −1.72 |
| zgc:153893, xaf1 | zgc:153893, (XIAP-associated factor 1) | 767709 | 2.52 × 10−4 | −1.75 |
| gig2d, LOC100005232 | grass carp reovirus (GCRV)-induced gene 2d | 100005232 | 1.20 × 10−4 | −1.88 |
| Gene Symbol | Gene ID | Forward Primer | Reverse Primer |
|---|---|---|---|
| irf7 | 393650 | GTGGAAAGTGGGCAGTACGA | GCCGCTGACTATAGCCCATT |
| irg1l | 562007 | TGCACTAGATGTGGCAGAGC | AGCATACATGTGCTGGCAGT |
| mucin 5b | 572172 | GGTGTCTGTTCCGATCAATC | TCATCCTTGTCGCCATTGTA |
| mxa | 360142 | TCGAGTTTCGACCTTGGCAC | GACGCTTGCTTGCAATTGTGTA |
| stat1b | 368481 | GCTTATCCCGAGATACACTCCT | GCTGCTTTACGTGGCATTTC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serifi, I.; Tzima, E.; Bardouki, H.; Lampri, E.; Papamarcaki, T. Effects of the Essential Oil from Pistacia lentiscus Var. chia on the Lateral Line System and the Gene Expression Profile of Zebrafish (Danio rerio). Molecules 2019, 24, 3919. https://doi.org/10.3390/molecules24213919
Serifi I, Tzima E, Bardouki H, Lampri E, Papamarcaki T. Effects of the Essential Oil from Pistacia lentiscus Var. chia on the Lateral Line System and the Gene Expression Profile of Zebrafish (Danio rerio). Molecules. 2019; 24(21):3919. https://doi.org/10.3390/molecules24213919
Chicago/Turabian StyleSerifi, Iliana, Eleni Tzima, Haido Bardouki, Evangeli Lampri, and Thomais Papamarcaki. 2019. "Effects of the Essential Oil from Pistacia lentiscus Var. chia on the Lateral Line System and the Gene Expression Profile of Zebrafish (Danio rerio)" Molecules 24, no. 21: 3919. https://doi.org/10.3390/molecules24213919





