Effects of Forchlorfenuron on the Morphology, Metabolite Accumulation, and Transcriptional Responses of Siraitia grosvenorii Fruit
Abstract
:1. Introduction
2. Results
2.1. Morphological and Cytological Observations
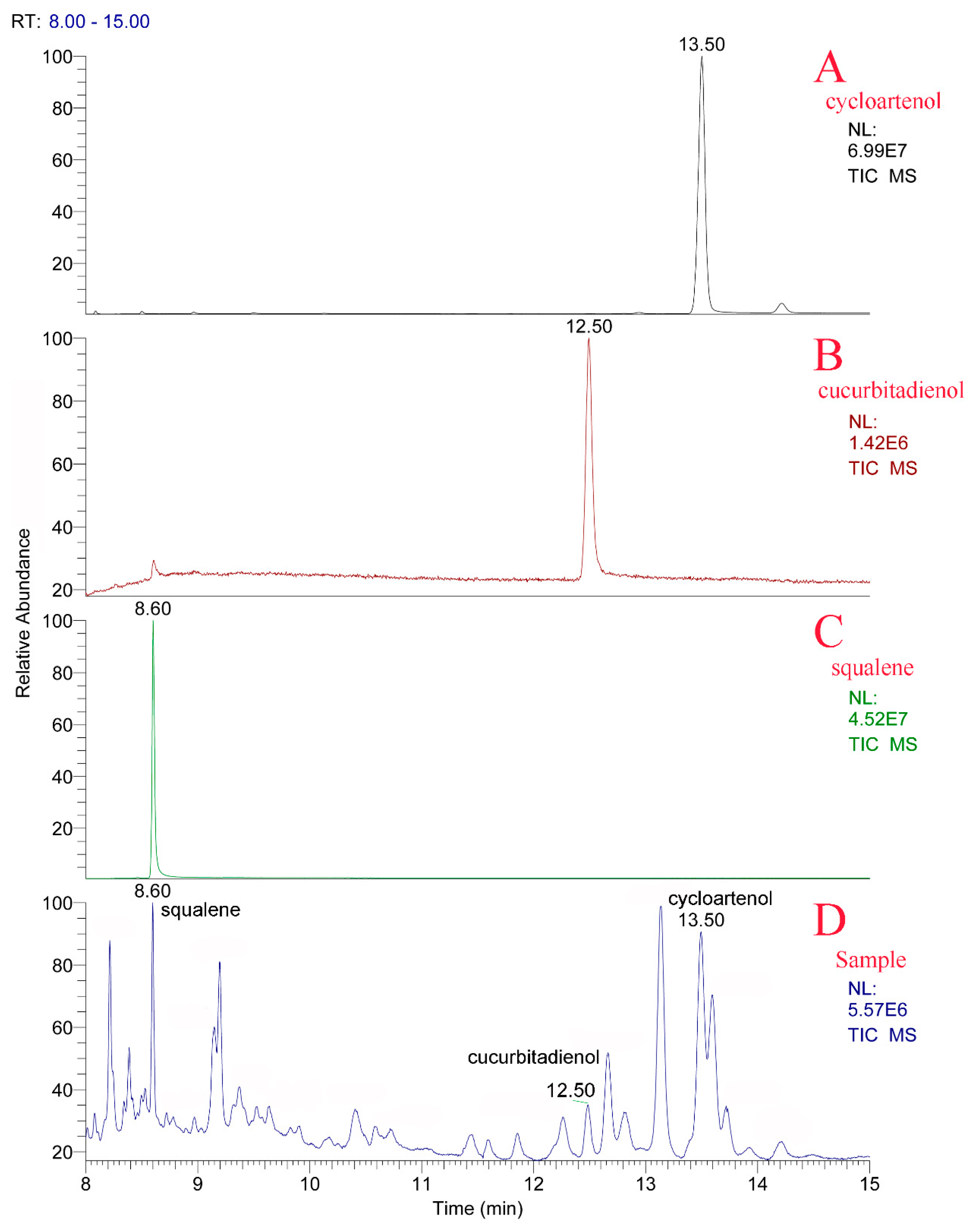
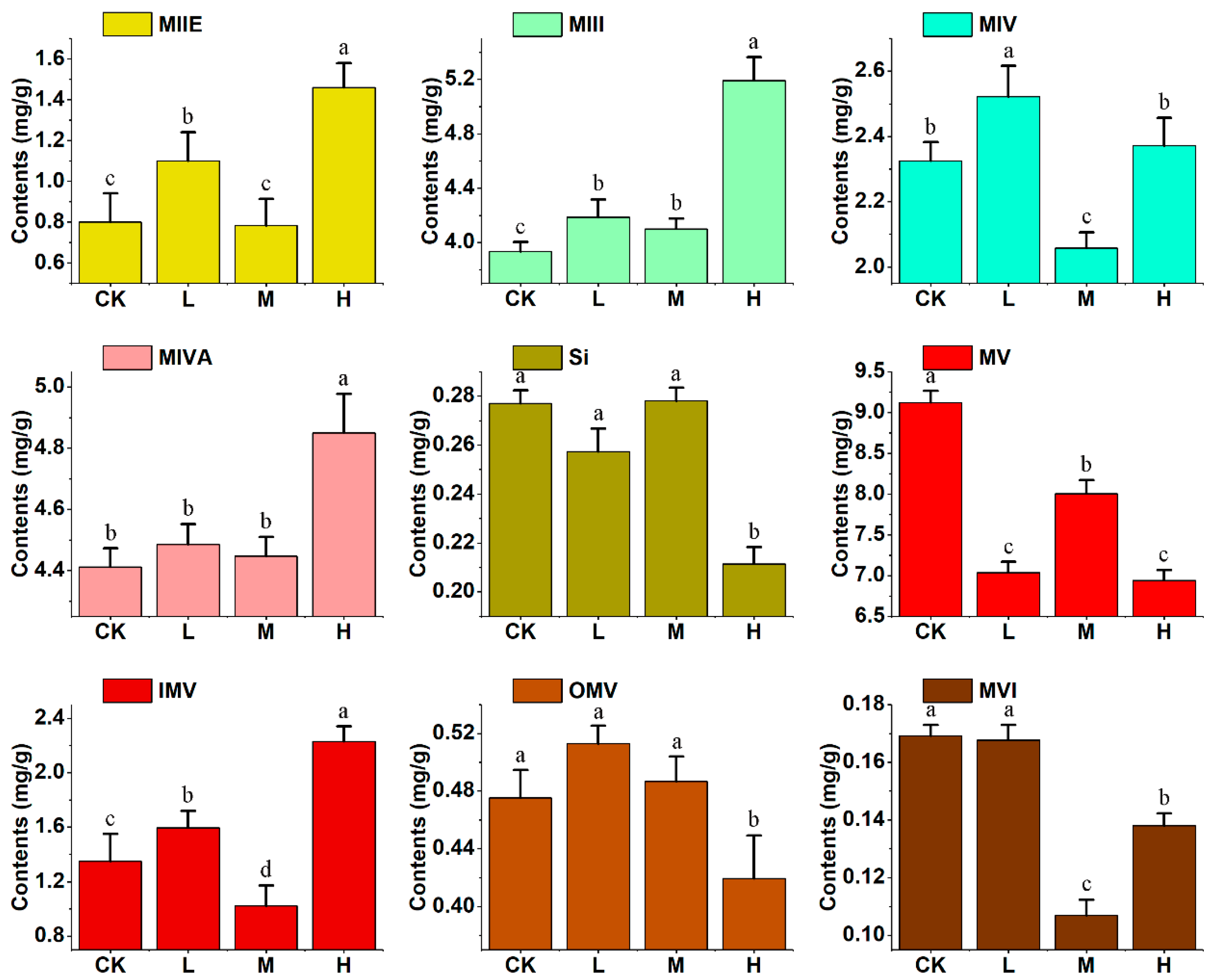
2.2. The Determination of Three Intermediates and Nine Mogrosides in S. grosvenorii Fruit
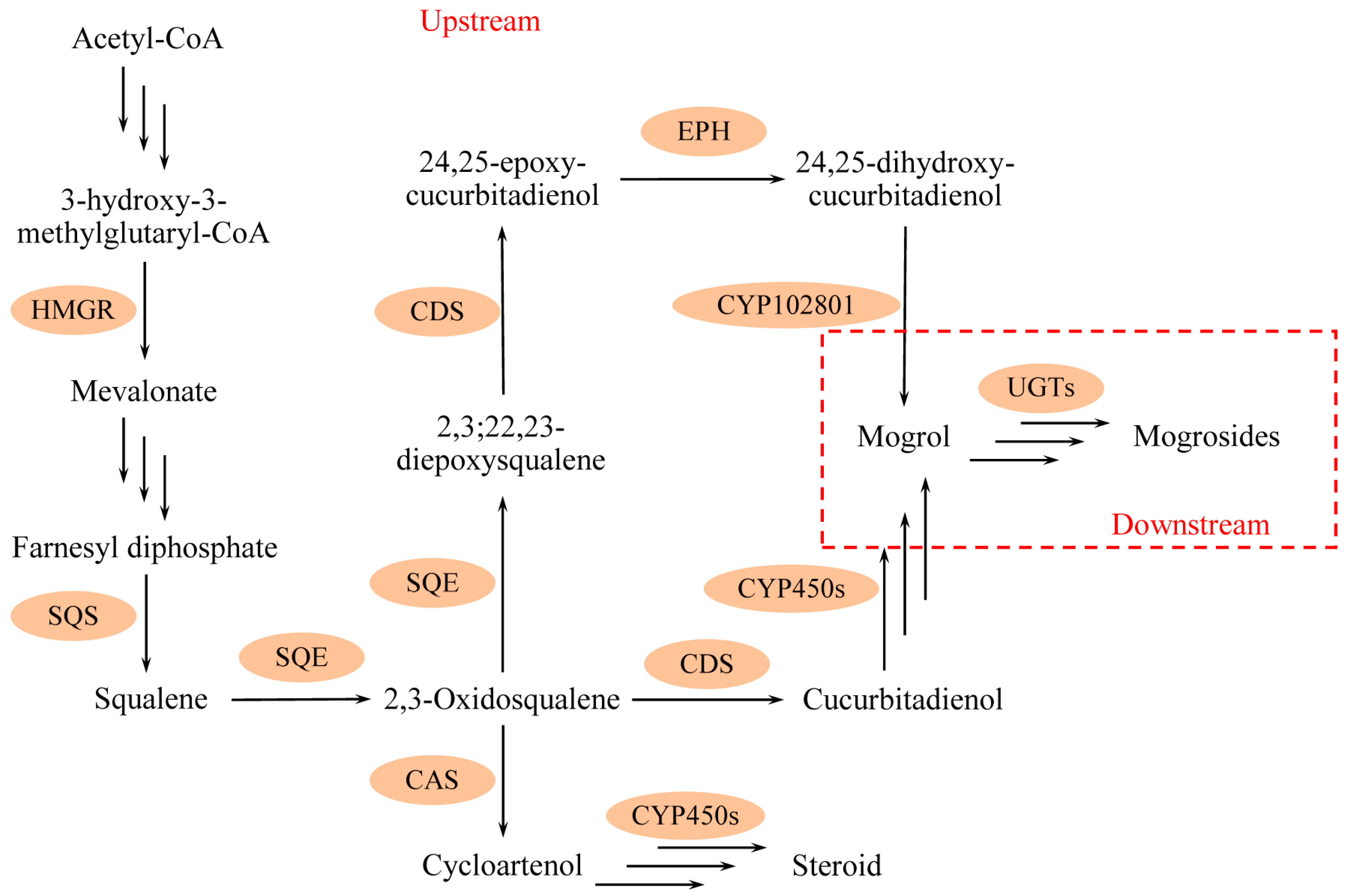
2.3. Gene Expression Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Materials, CPPU Treatments, and Sample Preparation
4.3. Morphological and Cytological Observations
4.4. GC-MS Analysis of Squalene, Cucurbitadienol, and Cycloartenol
4.5. LC-MS/MS Analysis of Nine Mogrosides
4.6. Quantitative Real-Time PCR (qRT-PCR) Analysis of Gene Expression
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| LHG | Luo-Han-Guo |
| TCM | Traditional Chinese Medicine |
| CPPU | Forchlorfenuron |
| DAT | Days-after-treatment |
| HMGR | 3-hydroxy-3-methyl glutaryl coenzyme A reductase |
| SQS | squalene synthase |
| SQE | squalene epoxidase |
| CDS | cucurbitadienol synthase |
| EPH | epoxide hydrolase |
| CYP450 | cytochrome P450 |
| UGT | UDP-glycosyltransferases |
| PGR | plant growth regulators |
Appendix A
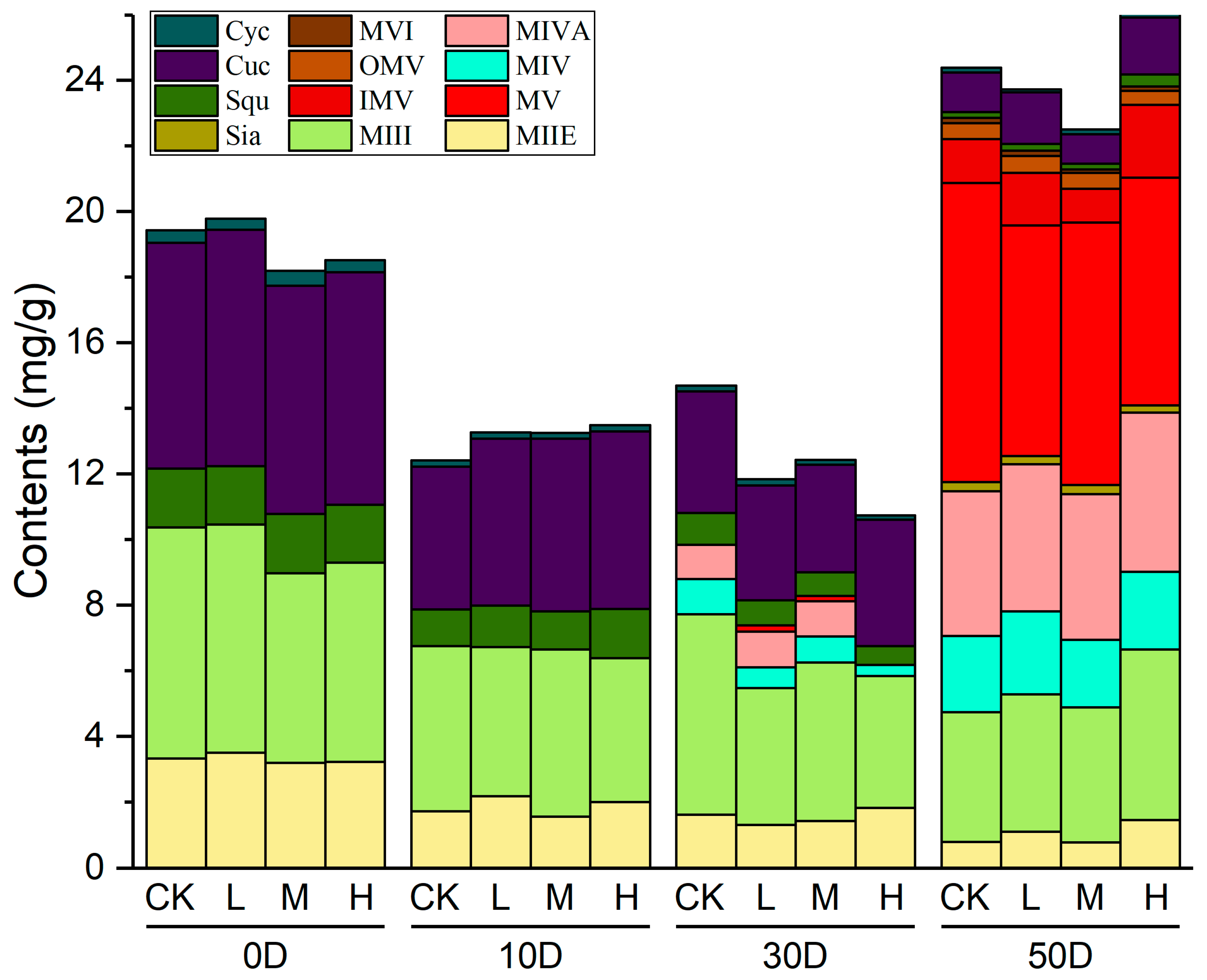

| Compounds | CK (Control) | |||
| 0D | 10D | 30D | 50D | |
| Squalene | 1.80 ± 0.15 | 1.12 ± 0.13 | 0.96 ± 0.11 | 0.17 ± 0.04 |
| Cucurbitadienol | 6.87 ± 0.22 | 4.36 ± 0.49 | 3.71 ± 0.65 | 1.21 ± 0.09 |
| Cycloartenol | 0.39 ± 0.03 | 0.19 ± 0.01 | 0.18 ± 0.02 | 0.14 ± 0.03 |
| MIIE | 3.33 ± 0.07 | 1.73 ± 0.06 | 1.63 ± 0.16 | 0.80 ± 0.14 |
| MIII | 7.05 ± 0.20 | 5.02 ± 0.09 | 6.09 ± 0.21 | 3.93 ± 0.07 |
| MIV | ND a | ND | 1.09 ± 0.01 | 2.33 ± 0.06 |
| MIVA | ND | ND | 1.04 ± 0.04 | 4.41 ± 0.06 |
| Si | ND | ND | ND | 0.28 ± 0.01 |
| MV | ND | ND | ND | 9.12 ± 0.15 |
| IMV | ND | ND | ND | 1.35 ± 0.20 |
| OMV | ND | ND | ND | 0.48 ± 0.02 |
| MVI | ND | ND | ND | 0.17 ± 0.00 |
| Compounds | Low Concentration Treatment | |||
| 0D | 10D | 30D | 50D | |
| Squalene | 1.78 ± 0.18 | 1.26 ± 0.22 | 0.77 ± 0.08 | 0.21 ± 0.01 |
| Cucurbitadienol | 7.21 ± 0.35 | 5.09 ± 0.35 | 3.51 ± 0.51 | 1.56 ± 0.12 |
| Cycloartenol | 0.34 ± 0.02 | 0.19 ± 0.02 | 0.18 ± 0.01 | 0.09 ± 0.01 |
| MIIE | 3.40 ± 0.11 | 2.18 ± 0.01 | 1.31 ± 0.02 | 1.10 ± 0.14 |
| MIII | 6.96 ± 0.25 | 4.54 ± 0.11 | 4.16 ± 0.27 | 4.18 ± 0.13 |
| MIV | ND | ND | 0.63 ± 0.03 | 2.52 ± 0.09 |
| MIVA | ND | ND | 1.09 ± 0.08 | 4.49 ± 0.07 |
| Si | ND | ND | ND | 0.26 ±0.01 |
| MV | ND | ND | 0.19 ± 0.03 | 7.03 ± 0.14 |
| IMV | ND | ND | ND | 1.60 ± 0.12 |
| OMV | ND | ND | ND | 0.51 ± 0.01 |
| MVI | ND | ND | ND | 0.17 ± 0.01 |
| Compounds | Medium Concentration Treatment | |||
| 0D | 10D | 30D | 50D | |
| Squalene | 1.81 ± 0.14 | 1.16 ± 0.08 | 0.72 ± 0.19 | 0.18 ± 0.05 |
| Cucurbitadienol | 6.95 ± 0.21 | 5.26 ± 0.37 | 3.29 ± 0.55 | 0.89 ± 0.07 |
| Cycloartenol | 0.45 ± 0.02 | 0.18 ± 0.02 | 0.15 ± 0.01 | 0.15 ± 0.02 |
| MIIE | 3.20 ± 0.19 | 1.56 ± 0.12 | 1.42 ± 0.10 | 0.78 ± 0.13 |
| MIII | 5.78 ± 0.54 | 5.10 ± 0.01 | 4.83 ± 0.25 | 4.10 ± 0.08 |
| MIV | ND | ND | 0.79 ± 0.05 | 2.06 ± 0.05 |
| MIVA | ND | ND | 1.07 ± 0.05 | 4.45 ± 0.06 |
| Si | ND | ND | ND | 0.28 ± 0.01 |
| MV | ND | ND | 0.15 ± 0.01 | 8.00 ± 0.16 |
| IMV | ND | ND | ND | 1.02 ± 0.15 |
| OMV | ND | ND | ND | 0.49 ± 0.02 |
| MVI | ND | ND | ND | 0.11 ± 0.01 |
| Compounds | High Concentration Treatment | |||
| 0D | 10D | 30D | 50D | |
| Squalene | 1.76 ± 0.17 | 1.49 ± 0.11 | 0.58 ± 0.14 | 0.36 ± 0.07 |
| Cucurbitadienol | 7.09 ± 0.44 | 5.42 ± 0.47 | 3.85 ± 0.50 | 1.75 ± 0.10 |
| Cycloartenol | 0.37 ± 0.01 | 0.19 ± 0.01 | 0.13 ± 0.02 | 0.13 ± 0.04 |
| MIIE | 3.22 ± 0.07 | 2.00 ± 0.04 | 1.83 ± 0.05 | 1.46 ± 0.12 |
| MIII | 6.08 ± 0.31 | 4.38 ± 0.05 | 4.00 ± 0.05 | 5.19 ± 0.17 |
| MIV | ND | ND | 0.34 ± 0.03 | 2.37 ± 0.08 |
| MIVA | ND | ND | ND | 4.85 ± 0.13 |
| Si | ND | ND | ND | 0.21 ± 0.01 |
| MV | ND | ND | ND | 6.94 ± 0.13 |
| IMV | ND | ND | ND | 2.23 ± 0.11 |
| OMV | ND | ND | ND | 0.42 ± 0.03 |
| MVI | ND | ND | ND | 0.14 ± 0.00 |
| Analytes | Molecular Formula | tR (min) | m/z Precursor | m/z Productions | DP (V) | CE (eV) |
|---|---|---|---|---|---|---|
| MIV | C66H112O34 | 1.16 | 1447.6 | 1285.4 a/1123.5 | −230 | −105 |
| OMV | C60H100O29 | 2.62 | 1283.7 | 1121.6 a/959.5 | −220 | −90 |
| MV | C60H102O29 | 3.78 | 1285.4 | 1123.5 a/961.4 | −220 | −85 |
| IMV | C60H102O29 | 4.64 | 1285.4 | 1123.5 a/961.4 | −210 | −85 |
| Si | C54H92O24 | 5.35 | 1123.4 | 961.6 a/799.2 | −220 | −75 |
| MIVA | C54H92O24 | 5.91 | 1123.4 | 961.6 a/799.2 | −220 | −75 |
| MIV | C54H92O24 | 7.01 | 1123.4 | 961.6 a/799.2 | −220 | −75 |
| MIII | C48H82O19 | 7.82 | 961.4 | 799.5 a/637.2 | −170 | −70 |
| MIIE | C42H72O14 | 8.11 | 799.5 | 637.6 a/475.5 | −170 | −65 |
| Source temperature (°C) | 500 | |||||
| Ionization voltage (V) | −4500 | |||||
| GS1 (psi) | 55 | |||||
| GS2 (psi) | 55 | |||||
| CUR (psi) | 20 | |||||
| CAD | high | |||||
| Dwell time (ms) | 400 | |||||
| EP (V) | −10 | |||||
| CXP (V) | −10 | |||||
| Gene Name | The Forward Primer Sequence (5′-3′) | The Reverse primer Sequence (5′-3′) |
|---|---|---|
| SgHMGR | TAGGCTCCAAAGTATCCG | CAGTTTACAGCAGCAGGTT |
| SgSQS | CTGAGACACCCA GATGACT | GAGGGCTCGCAGAACAAGA |
| SgSQE1 | GCTTCGACCATCAACACATTG | TTCCTCCAAGGCTCAAGTAATC |
| SgSQE2 | GCCCACTCTCAGCAAGTATC | GAAGATTCCTCCGAGGCTTAAA |
| SgCDS | GTTGGGTTGAAGATCCCTACTC | CCACAACTGGCTCCCATTAT |
| SgCAS | CAAATACAACATGCTCACC | TAGCCCTTCTTAGAGTGCC |
| SgCPR1 | CGGCAGTCGGAAAGTTAAGA | GTCTGTGTACCGAAGAAGATTGA |
| SgCPR2 | TGCGTTGTGGTCCTTGTT | GGCTCTGGCTCTTTGACTATC |
| SgCYP102801 | GACGATGTCTACGAAGCGATAC | GTGGGTGACATTGAAGGAAGA |
| SgEPH1 | CCGATGATACCGAAAGAGAAGG | GAACTTGCCGGCGAAATAATC |
| SgEPH2 | GTGACCCACAAGCTCCATATT | CGGCGTAAACGTCGATATCTT |
| SgEPH3 | CTTGGGATCGAGAAGGTGTTT | CACCAGCGCCTTGATTCTAT |
| SgEPH4 | ACTATCGCGCTATTGACCTAAC | CAAATCGAGATCGCCGACTATAA |
| SgUGT73-251-5 | TATGAGTCCGATGCTCCAATTC | GAGAGAAGAAGAGGGAGGAAGA |
| SgUGT73-327-2 | AGAGAGTGAGAGAGAGCAAGAG | CAGCCACAGTGAGTGACAAA |
| SgUGT73-348-2 | GAAGAAAGGACGAAGGGAAGAG | GCCGCAGTGGGTTAGAAA |
| SgUGT73-251-6 | GAAAGTGAGGGAAGCCATAGAA | TATAGCTCTCTTCGCCGTCT |
| SgUGT74-345-2 | CGACCATTCCATCGGCATATT | GCCTAGTGTTCTTCCCATCTTC |
| SgUGT75-281-2 | GACTCGTCGGTCGTTTACATATC | ACGTGTGGCCACTGTTTAATA |
| SgUGT85-269-1 | CCGATTGAAGTAGCGGAAGAA | CCTCAACGAGCTTCGGTATAAA |
| SgUGT85-269-4 | CTCAATCCTCCGGTCTCATTC | GGGTCCAACGGTGTAGATTT |
| SgUGT94-289-1 | CTGTTAACCTCGACGCCATTA | GCTGATCAGGAGAAGATGGAAG |
| SgUGT94-289-2 | CCAAACACGGACCTACCTATT | GACCAACCGTCAGTGTAGTT |
| SgUGT94-289-3 | CAGAGAAGATGTGCGGAAGAA | TCAGCGACCATCTCATCAAAC |
| SgUBQ | ATAAAAGACCCAGCACCACATTC | CCCTTGCCGACTACAACATCC |
References
- Xia, Y.; Rivero-Huguet, M.E.; Hughes, B.H.; Marshall, W.D. Isolation of the sweet components from Siraitia grosvenorii. Food Chem. 2008, 107, 1022–1028. [Google Scholar] [CrossRef]
- Li, C.; Lin, L.; Sui, F.; Wang, Z.; Huo, H.; Dai, L.; Jiang, T. Chemistry and pharmacology of Siraitia grosvenorii: Areview. Chin. J. Nat. Med. 2014, 12, 89–102. [Google Scholar] [PubMed]
- Philippe, R.N.; De, M.M.; Anderson, J.; Ajikumar, P.K. Biotechnological production of natural zero-calorie sweeteners. Curr. Opin. Biotechnol. 2014, 26, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Feng, Q.; Zhang, K.; Qin, X.; Guo, Y.; Shi, H.; Zhang, L.; Zhang, Z.; Ma, X. In vitro AMPK activating effect and in vivo pharmacokinetics of mogroside V, a cucurbitane-type triterpenoid from Siraitia grosvenorii fruits. Rsc Adv. 2016, 6, 7034–7041. [Google Scholar] [CrossRef]
- Suzuki, Y.A.; Yuji, M.; Hiroshi, I.; Masaki, S.; Yoshihisa, N. Triterpene glycosides of Siraitia grosvenori inhibit rat intestinal maltase and suppress the rise in blood glucose level after a single oral administration of maltose in rats. J. Agric. Food Chem. 2005, 53, 2941–2946. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, M.; Konoshima, T.; Murata, Y.; Sugiura, M.; Nishino, H.; Tokuda, H.; Matsumoto, K.; Kasai, R.; Yamasaki, K. Anticarcinogenic activity of natural sweeteners, cucurbitane glycosides, from Momordica grosvenori. Cancer Lett. 2003, 198, 37–42. [Google Scholar] [CrossRef]
- Itkin, M.; Davidovichrikanati, R.; Cohen, S.; Portnoy, V.; Doronfaigenboim, A.; Oren, E.; Freilich, S.; Tzuri, G.; Baranes, N.; Shen, S. The biosynthetic pathway of the nonsugar, high-intensity sweetener mogroside V from Siraitia grosvenorii. Proc. Natl. Acad. Sci. Usa. 2016, 113, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Ma, X.; Mo, C.; Wilson, I.W.; Song, C.; Zhao, H.; Yang, Y.; Fu, W.; Qiu, D. An efficient approach to finding Siraitia grosvenorii triterpene biosynthetic genes by RNA-seq and digital gene expression analysis. Bmc Genom. 2011, 12, 343. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Han, X.; He, H.; Yu, R.; Zhen, G.; Jia, X.; Cheng, B.; Deng, X.W. Improved de novo genome assembly and analysis of the Chinese cucurbit Siraitia grosvenorii, also known as monk fruit or luo-han-guo. Gigascience 2018, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Itkin, M.; Davidovich-Rikanati, R.; Cohen, S.; Portnoy, V.; Doron-Faigenboim, A.; Petreikov, M.; Shen, S.; Tadmor, Y.; Burger, Y.; Lewinsohn, E.; et al. Method of Producing Mogrosides and Compositions Comprising Same and Uses Thereof. U.S. Patent 20170283844A1, 15 October 2017. [Google Scholar]
- Qing, Z.X.; Zhao, H.; Tang, Q.; Mo, C.M.; Huang, P.; Cheng, P.; Yang, P.; Yang, X.Y.; Liu, X.B.; Zheng, Y.J. Systematic identification of flavonols, flavonol glycosides, triterpene and siraitic acid glycosides from Siraitia grosvenorii using high-performance liquid chromatography/quadrupole-time-of-flight mass spectrometry combined with a screening strategy. J. Pharm. Biomed. Anal. 2017, 138, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Xiaojun, M.; Qi, T.; Changming, M. Karyotype analysis and genetic variation of a mutant in Siraitia grosvenorii. Mol. Biol. Rep. 2012, 39, 1247–1252. [Google Scholar]
- Qiao, J.; Luo, Z.; Gu, Z.; Zhang, Y.; Zhang, X.; Ma, X. Indentification of a novel specific cucurbitadienol synthase allele in Siraitia grosvenorii correlates with high catalytic efficiency. Molecules 2019, 24, 627. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, T.; Shima, K. Change in flower morphology of Torenia fournieri Lind. induced by forchlorfenuron application. Sci. Hortic-Amst. 2006, 109, 254–261. [Google Scholar] [CrossRef]
- Reynolds, A.G.; Wardle, D.A.; Zurowski, C.; Looney, N.E. Phenylureas CPPU and thidiazuron affect yield components, fruit composition, and storage potential of four seedless grape selections. J. Am. Soc. Hortic. Sci. 1992, 117, 85–89. [Google Scholar] [CrossRef]
- Maoz, I.; Bahar, A.; Kaplunov, T.; Zutchi, Y.; Daus, A.; Luriel, S.; Lichter, A. Effect of the Cytokinin Forchlorfenuron on Tannin Content of Thompson Seedless Table Grapes. Am. J. Enol. Viticult. 2014, 65, 230–237. [Google Scholar] [CrossRef]
- Blank, R.H.; Richardson, A.C.; Oshima, K.; Hampton, R.E.; Olson, M.H.; Dawson, T.E. Effect of a forchlorfenuron dip on kiwifruit fruit size. New Zeal. J. Crop. Hort. 1992, 20, 73–78. [Google Scholar] [CrossRef]
- Iwahori, S.; Tominaga, S.; Yamasaki, T. Stimulation of fruit growth of kiwifruit, Actinidia chinensis Planch., by N-(2-chloro-4-pyridyl)-N’-phenylurea, a diphenylurea-derivative cytokinin. Sci. Hortic-Amst. 1988, 35, 109–115. [Google Scholar] [CrossRef]
- Nickell, L.G. Effects of N-(2-chloro-4-pyridyl)-N’-phenylurea on grapes and other crops. P. annu meeting-Plant Growth Reg. Soc. of Am. (USA) 1986, 13, 236–241. [Google Scholar]
- Zabadal, T.J.; Bukovac, M.J. Effect of CPPU on fuit development of selected seedless and seeded grape cultivars. Hortscience 2006, 41, 154–157. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, X.D.; Guo, L.J.; Zeng, D.Q.; Xiao, D.; He, L.F.; Wang, A.Q. Effects of forchlorfenuron on yield and quality of kudzu root. J. South. Agr. 2017, 48, 1581–1586. [Google Scholar]
- Chen, M.X.; Li, J.J.; Li, M.J.; Li, X.W.; Bai, Y.H.; Zhang, X.L. Effects of combined application of paclobutrazol and forchlorfenuron on the growth and quality of virus-free Rehmannia glutinosa Libosh. Hueichingensis(Chan et Sehih) Hsiao. J. South. Agr. 2014, 45, 1947–1950. [Google Scholar]
- Song, X.; Ding, P.; Li, X.; Chen, T.; Chen, L.; Wang, B. Effect of forchlorfenuron on fruit morphology and lignans content of Schisandra chinensis. China J. Chin. Mater. Med. 2014, 39, 1579–1583. [Google Scholar]
- Bahar, A.; Kaplunov, T.; Zutahy, Y.; Daus, A.; Lurie, S.; Lichter, A. Auto-fluorescence for analysis of ripening in Thompson Seedless and colour in Crimson Seedless table grapes. Aus. J. Grape Wine R. 2012, 18, 353–359. [Google Scholar] [CrossRef]
- Zoffoli, J.P.; Latorre, B.A.; Naranjo, P. Preharvest applications of growth regulators and their effect on postharvest quality of table grapes during cold storage. Postharvest Biol. Tec. 2009, 51, 183–192. [Google Scholar] [CrossRef]
- Li, C.Z.; Wang, J.M.; Xu, J.; He, L.F.; He, H.W. Effects of forchlorfenuron and paclobutrazol combination on the physiological characteristics of yam leaves. J. South. Agr. 2012, 43, 1952–1957. [Google Scholar]
- Wang, J.M.; He, L.F.; Mo, K.; Li, C.Z. Fertilizing forchlorfenuron and MET improve quality of yam (Dioscorea opposita Thunb.). Genom. Appl. Biol. 2010, 29, 344–348. [Google Scholar]
- Xiaojun, M.; Changming, M.; Longhua, B.; Shixin, F. A New Siraitia grosvenorii Cultivar ‘Yongqing 1’. Acta Hortic. Sin. 2008, 35, 1855. [Google Scholar]
- Liangchang, F.; Xiaojun, M.; Hualong, B.; Xin, Z. Progress on research of tissue culture of Siraitia grosvenorii. China J. Chin. Mater. Med. 2005, 30, 325–328. [Google Scholar]
- Liang, Z.S.; Zhang, Y.J.; Liu, Y.; Liu, F.H. Roles of reactive oxygen species in methyl jasmonate and nitric oxide-induced tanshinone production in Salvia miltiorrhiza hairy roots. Plant. Cell Rep. 2012, 31, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Luo, Z.; Li, Y.; Ren, G.; Liu, C.; Ma, X. Effect of abscisic acid on accumulation of five active components in root of Glycyrrhiza uralensis. Molecules 2017, 22, 1982. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rojo, S.; Cerda-García-Rojas, C.M.; Esparza-García, F.; Plasencia, J.; Poggi-Varaldo, H.M.; Ponce-Noyola, T.; Ramos-Valdivia, A.C. Long-term response on growth, antioxidant enzymes, and secondary metabolites in salicylic acid pre-treated Uncaria tomentosa microplants. Biotechnol. Lett. 2015, 37, 2489–2496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Luo, Z.; Guo, Y.; Mo, C.; Tu, D.; Ma, X.; Bai, L. Methyl jasmonate-induced accumulation of metabolites and transcriptional responses involved in triterpene biosynthesis in Siraitia grosvenorii fruit at different growing stages. Acta Soc. Bot. Pol. 2016, 85, 1–12. [Google Scholar] [CrossRef]
- Tu, D.; Luo, Z.; Wu, B.; Ma, X.; Shi, H.; Mo, C.; Huang, J.; Xie, W. Developmental, chemical and transcriptional characteristics of artificially pollinated and hormone-induced parthenocarpic fruits of Siraitia grosvenorii. Rsc Adv. 2017, 7, 12419–12428. [Google Scholar] [CrossRef]
- Matsuo, S.; Kikuchi, K.; Fukuda, M.; Honda, I.; Imanishi, S. Roles and regulation of cytokinins in tomato fruit development. J. Exp. Bot. 2012, 63, 5569–5579. [Google Scholar] [CrossRef] [PubMed]
- Serrani, J.C.; Atares, F.A.; Garcia-Martmez, J.L. Effect of gibberellin and auxin on parthenocarpic fruit growth induction in the cv Micro-Tom of Tomato. J. Plant. Growth Regul. 2007, 26, 211–221. [Google Scholar] [CrossRef]
- Pharmacopoeia of the People’s of Republic China, 2015 ed.; Commission, C.P. (Ed.) China Medical Science Press: Beijing, China, 2015. [Google Scholar]
- Zuliang, L.; Hongwu, S.; Kailun, Z.; Xijun, Q.; Yuhua, G.; Xiaojun, M. Liquid chromatography with tandem mass spectrometry method for the simultaneous determination of multiple sweet mogrosides in the fruits of Siraitia grosvenorii and its marketed sweeteners. J. Sep. Sci. 2016, 2016, 4124–4135. [Google Scholar]
- Zhao, H.; Wang, J.; Tang, Q.; Mo, C.; Guo, J.; Chen, T.; Lin, H.; Tang, J.; Guo, L.; Huang, L.; et al. Functional expression of two NADPH-cytochrome P450 reductases from Siraitia grosvenorii. Int. J. Biol. Macromol. 2018, 120, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Ma, X.; Bai, L.; Pan, L.; Feng, S.; Mo, C. Screening of reference genes in Siraitia grosvenorii and spatio-temporal expression analysis of 3-hydroxy-3-methylglutaryl coenzyme A. Chin. Tradit. Herb. Drugs 2014, 45, 2224–2229. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, H.; Liao, J.; Cui, S.; Luo, Z.; Ma, X. Effects of Forchlorfenuron on the Morphology, Metabolite Accumulation, and Transcriptional Responses of Siraitia grosvenorii Fruit. Molecules 2019, 24, 4076. https://doi.org/10.3390/molecules24224076
Shi H, Liao J, Cui S, Luo Z, Ma X. Effects of Forchlorfenuron on the Morphology, Metabolite Accumulation, and Transcriptional Responses of Siraitia grosvenorii Fruit. Molecules. 2019; 24(22):4076. https://doi.org/10.3390/molecules24224076
Chicago/Turabian StyleShi, Hongwu, Jingjing Liao, Shengrong Cui, Zuliang Luo, and Xiaojun Ma. 2019. "Effects of Forchlorfenuron on the Morphology, Metabolite Accumulation, and Transcriptional Responses of Siraitia grosvenorii Fruit" Molecules 24, no. 22: 4076. https://doi.org/10.3390/molecules24224076





