Application of Diethylzinc/Propyl Gallate Catalytic System for Ring-Opening Copolymerization of rac-Lactide and ε-Caprolactone
Abstract
1. Introduction
2. Results and Discussion
2.1. Polymers Characterization
2.2. Toxicity Studies
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Synthesis of the Catalytic System
3.2.2. Polymerization Procedure
3.2.3. Measurements
3.2.4. Spectroscopic Data
3.3. Toxicity Studies
3.3.1. Microtox and Spirotox Tests
3.3.2. Umu—Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dobrzyński, P.; Kasperczyk, J.; Jelonek, K.; Ryba, M.; Walski, M.; Bero, M. Application of the lithium and magnesium initiators for the synthesis of glycolide, lactide, and epsilon-caprolactone copolymers biocompatible with brain tissue. J. Biomed. Mater. Res. A 2006, 79, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Dobrzynski, P.; Kasperczyk, J. Synthesis of biodegradable copolymers with low-toxicity zirconium compounds. IV. Copolymerization of glycolide with trimethylene carbonate and 2,2-dimethyltrimethylene carbonate: Microstructure analysis of copolymer chains by high-resolution nuclear magnetic resonance spectroscopy. J. Polym. Sci. Part Polym. Chem. 2006, 44, 98–114. [Google Scholar]
- Seyednejad, H.; Ghassemi, A.H.; van Nostrum, C.F.; Vermonden, T.; Hennink, W.E. Functional aliphatic polyesters for biomedical and pharmaceutical applications. J. Control. Release 2011, 152, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Kenar, H.; Ozdogan, C.Y.; Dumlu, C.; Doger, E.; Kose, G.T.; Hasirci, V. Microfibrous scaffolds from poly(l-lactide-co-ε-caprolactone) blended with xeno-free collagen/hyaluronic acid for improvement of vascularization in tissue engineering applications. Mater. Sci. Eng. C 2019, 97, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Varshney, S.K. Polylactides—Chemistry, Properties and Green Packaging Technology: A Review. Int. J. Food Prop. 2011, 14, 37–58. [Google Scholar] [CrossRef]
- Orchel, A.; Jelonek, K.; Kasperczyk, J.; Dobrzynski, P.; Marcinkowski, A.; Pamula, E.; Orchel, J.; Bielecki, I.; Kulczycka, A. The Influence of Chain Microstructure of Biodegradable Copolyesters Obtained with Low-Toxic Zirconium Initiator to In Vitro Biocompatibility. BioMed. Res. Int. 2013, 2013, 12. [Google Scholar] [CrossRef]
- Dechy-Cabaret, O.; Martin-Vaca, B.; Bourissou, D. Controlled Ring-Opening Polymerization of Lactide and Glycolide. Chem. Rev. 2004, 104, 6147–6176. [Google Scholar] [CrossRef]
- Dobrzyński, P.; Kasperczyk, J.; Bero, M. Application of Calcium Acetylacetonate to the Polymerization of Glycolide and Copolymerization of Glycolide with ε-Caprolactone and l-Lactide. Macromolecules 1999, 32, 4735–4737. [Google Scholar] [CrossRef]
- De Mattos, J.; Dantas, F.; Bezerra, R.; Bernardo-Filho, M.; Cabral-Neto, J.; Lage, C.; Leitão, A.; Caldeira-de-Araújo, A. Damage induced by stannous chloride in plasmid DNA. Toxicol. Lett. 2000, 116, 159–163. [Google Scholar] [CrossRef]
- Salánki, Y.; D’eri, Y.; Platokhin, A.; Sh.-Rózsa, K. The neurotoxicity of environmental pollutants: The effects of tin (Sn2+) on acetylcholine-induced currents in greater pond snail neurons. Neurosci. Behav. Physiol. 2000, 30, 63–73. [Google Scholar]
- Lewis, R.J. Sax’s Dangerous Properties of Industrial Materials, 8th ed.; Van Nostrand Reinhold: New York, NY, USA, 1992. [Google Scholar]
- Kricheldorf, H.R.; Kreiser-Saunders, I.; Damrau, D.-O. Resorbable initiators for polymerizations of lactones. Macromol. Symp. 1999, 144, 269–276. [Google Scholar] [CrossRef]
- Guillerm, B.; Lemaur, V.; Cornil, J.; Lazzaroni, R.; Dubois, P.; Coulembier, O. Ammonium betaines: Efficient ionic nucleophilic catalysts for the ring-opening polymerization of L-lactide and cyclic carbonates. Chem. Commun. 2014, 50, 10098–10101. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, M.H. Concerning the ring-opening polymerization of lactide and cyclic esters by coordination metal catalysts. Pure Appl. Chem. 2010, 82, 1647–1662. [Google Scholar] [CrossRef]
- Piotrowska, U.; Sobczak, M. Enzymatic Polymerization of Cyclic Monomers in Ionic Liquids as a Prospective Synthesis Method for Polyesters Used in Drug Delivery Systems. Molecules 2014, 20, 1–23. [Google Scholar] [CrossRef]
- Pastusiak, M.; Dobrzynski, P.; Kaczmarczyk, B.; Kasperczyk, J. Polymerization mechanism of trimethylene carbonate carried out with zinc(II) acetylacetonate monohydrate. J. Polym. Sci. Part Polym. Chem. 2011, 49, 2504–2512. [Google Scholar] [CrossRef]
- Dobrzynski, P. Synthesis of biodegradable copolymers with low-toxicity zirconium compounds. II. Copolymerization of glycolide with ϵ-caprolactone initiated by zirconium(IV) acetylacetonate and zirconium(IV) chloride. J. Polym. Sci. Part Polym. Chem. 2002, 40, 1379–1394. [Google Scholar] [CrossRef]
- Amgoune, A.; Thomas, C.M.; Roisnel, T.; Carpentier, J.-F. Ring-Opening Polymerization of Lactide with Group 3 Metal Complexes Supported by Dianionic Alkoxy-Amino-Bisphenolate Ligands: Combining High Activity, Productivity, and Selectivity. Chem. Eur. J. 2006, 12, 169–179. [Google Scholar] [CrossRef]
- Abderramane, A.; Thomas, C.M.; Carpentier, J.-F. Controlled ring-opening polymerization of lactide by group 3 metal complexes. Pure Appl. Chem. 2007, 79, 2013. [Google Scholar]
- Kricheldorf, H.R.; Bornhorst, K.; Hachmann-Thiessen, H. Bismuth(III) n-Hexanoate and Tin(II) 2-Ethylhexanoate Initiated Copolymerizations of ε-Caprolactone and l-Lactide. Macromolecules 2005, 38, 5017–5024. [Google Scholar] [CrossRef]
- Castro-Osma, J.A.; Alonso-Moreno, C.; Márquez-Segovia, I.; Otero, A.; Lara-Sánchez, A.; Fernández-Baeza, J.; Rodríguez, A.M.; Sánchez-Barba, L.F.; García-Martínez, J.C. Synthesis, structural characterization and catalytic evaluation of the ring-opening polymerization of discrete five-coordinate alkyl aluminium complexes. Dalton Trans. 2013, 42, 9325–9337. [Google Scholar] [CrossRef] [PubMed]
- Bero, M.; Kasperczyk, J.; Adamus, G. Coordination polymerization of lactides, 3. Copolymerization of L,L-lactide and ε-caprolactone in the presence of initiators containing Zn and Al. Makromol. Chem. 1993, 194, 907–912. [Google Scholar] [CrossRef]
- Kim, Y.; Jnaneshwara, G.K.; Verkade, J.G. Titanium Alkoxides as Initiators for the Controlled Polymerization of Lactide. Inorg. Chem. 2003, 42, 1437–1447. [Google Scholar] [CrossRef]
- Stassin, F.; Jérôme, R. Polymerization of (L,L)-lactide and copolymerization with ϵ-caprolactone initiated by dibutyltin dimethoxide in supercritical carbon dioxide. J. Polym. Sci. Part Polym. Chem. 2005, 43, 2777–2789. [Google Scholar] [CrossRef]
- Kasperczyk, J.; Jelonek, K.; Dobrzyñski, P.; Jarz, B. The influence of copolymer chain microstructure on cyclosporine a (CyA) and Sirolimus prolonged and sustained release from PLA/TMC and PLA/PCL matrices. J. Control. Release 2006, 116, e5–e6. [Google Scholar] [CrossRef] [PubMed]
- Kricheldorf, H.R.; Damrau, D.-O. Polylactones, 43. Polymerization of L-lactide catalyzed by zinc amino acid salts. Macromol. Chem. Phys. 1998, 199, 1747–1752. [Google Scholar] [CrossRef]
- González, D.M.; Cisterna, J.; Brito, I.; Roisnel, T.; Hamon, J.-R.; Manzur, C. Binuclear Schiff-base zinc(II) complexes: Synthesis, crystal structures and reactivity toward ring opening polymerization of rac-lactide. Polyhedron 2019, 162, 91–99. [Google Scholar] [CrossRef]
- McKeown, P.; McCormick, S.N.; Mahon, M.F.; Jones, M.D. Highly active Mg(ii) and Zn(ii) complexes for the ring opening polymerisation of lactide. Polym. Chem. 2018, 9, 5339–5347. [Google Scholar] [CrossRef]
- Munzeiwa, W.A.; Nyamori, V.O.; Omondi, B. N,O-Amino-phenolate Mg(II) and Zn(II) Schiff base complexes: Synthesis and application in ring-opening polymerization of ε-caprolactone and lactides. Inorg. Chim. Acta 2019, 487, 264–274. [Google Scholar] [CrossRef]
- Wang, B.; Wei, Y.; Li, Z.-J.; Pan, L.; Li, Y.-S. From Zn(C6F5)2 to ZnEt2-based Lewis Pairs: Significantly Improved Catalytic Activity and Monomer Adaptability for the Ring-opening Polymerization of Lactones. ChemCatChem 2018, 10, 5287–5296. [Google Scholar] [CrossRef]
- Hu, Q.; Jie, S.; Braunstein, P.; Li, B.-G. Highly active tridentate amino-phenol zinc complexes for the catalytic ring-opening polymerization of ε-caprolactone. J. Organomet. Chem. 2019, 882, 1–9. [Google Scholar] [CrossRef]
- Posada, F.A.; Macías, A.M.; Movilla, S.; Miscione, P.G.; Pérez, D.L.; Hurtado, J.J. Polymers of ε-Caprolactone Using New Copper(II) and Zinc(II) Complexes as Initiators: Synthesis, Characterization and X-Ray Crystal Structures. Polymers 2018, 10, 1239. [Google Scholar] [CrossRef] [PubMed]
- Honrado, M.; Otero, A.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; Garcés, A.; Lara-Sánchez, A.; Rodríguez, A.M. Copolymerization of Cyclic Esters Controlled by Chiral NNO-Scorpionate Zinc Initiators. Organometallics 2016, 35, 189–197. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Karroonnirun, O. Ring-Opening Polymerization of l-Lactide and ε-Caprolactone Utilizing Biocompatible Zinc Catalysts. Random Copolymerization of l-Lactide and ε-Caprolactone. Macromolecules 2010, 43, 8880–8886. [Google Scholar] [CrossRef]
- D’Auria, I.; Lamberti, M.; Mazzeo, M.; Milione, S.; Roviello, G.; Pellecchia, C. Coordination Chemistry and Reactivity of Zinc Complexes Supported by a Phosphido Pincer Ligand. Chem. Eur. J. 2012, 18, 2349–2360. [Google Scholar] [CrossRef]
- Żółtowska, K.; Sobczak, M.; Olędzka, E. Novel Zinc-Catalytic Systems for Ring-Opening Polymerization of ε-Caprolactone. Molecules 2015, 20, 2816–2827. [Google Scholar] [CrossRef]
- Żółtowska, K.; Piotrowska, U.; Oledzka, E.; Sobczak, M. Efficient Diethylzinc/Gallic Acid and Diethylzinc/Gallic Acid Ester Catalytic Systems for the Ring-Opening Polymerization of rac-Lactide. Molecules 2015, 20, 21909–21923. [Google Scholar] [CrossRef]
- Żółtowska, K.; Oledzka, E.; Kuras, M.; Skrzypczak, A.; Nałęcz-Jawecki, G.; Sobczak, M. Cyto- and genotoxicity evaluation of the biomedical polyesters obtained in the presence of new zinc catalytic systems. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 768–772. [Google Scholar] [CrossRef]
- Gálico, D.A.; Nova, C.V.; Guerra, R.B.; Bannach, G. Thermal and spectroscopic studies of the antioxidant food additive propyl gallate. Food Chem. 2015, 182, 89–94. [Google Scholar] [CrossRef]
- Pappalardo, D.; Annunziata, L.; Pellecchia, C. Living Ring-Opening Homo- and Copolymerization of ε-Caprolactone and l- and d,l-Lactides by Dimethyl(salicylaldiminato)aluminum Compounds. Macromolecules 2009, 42, 6056–6062. [Google Scholar] [CrossRef]
- Kasperczyk, J.; Bero, M. Coordination polymerization of lactides, 4. The role of transesterification in the copolymerization of L,L-lactide and ε-caprolactone. Makromol. Chem. 1993, 194, 913–925. [Google Scholar] [CrossRef]
- Bero, M.; Kasperczyk, J. Coordination polymerization of lactides, 5. Influence of lactide structure on the transesterification processes in the copolymerization with ε-caprolactone. Macromol. Chem. Phys. 1996, 197, 3251–3258. [Google Scholar] [CrossRef]
- Vanhoorne, P.; Dubois, P.; Jerome, R.; Teyssie, P. Macromolecular engineering of polylactones and polylactides. 7. Structural analysis of copolyesters of ε-caprolactone and L- or D,L-lactide initiated by triisopropoxyaluminum. Macromolecules 1992, 25, 37–44. [Google Scholar] [CrossRef]
- Zambelli, A.; Caprio, M.; Grassi, A.; Bowen, D.E. Syndiotactic styrene-butadiene block copolymers synthesized with CpTiX3/MAO (Cp = C5H5, X = Cl, F; Cp = C5Me5, X = Me) and TiXn/MAO (n = 3, X = acac; n = 4, X = O-tert-Bu). Macromol. Chem. Phys. 2000, 201, 393–400. [Google Scholar] [CrossRef]
- Cuomo, C.; Serra, M.C.; Maupoey, M.G.; Grassi, A. Copolymerization of Styrene with Butadiene and Isoprene Catalyzed by the Monocyclopentadienyl Titanium Complex Ti(η5-C5H5)(η2-MBMP)Cl. Macromolecules 2007, 40, 7089–7097. [Google Scholar] [CrossRef]
- Sarasua, J.R.; López-Rodríguez, N.; Zuza, E.; Petisco, S.; Castro, B.; del Olmo, M.; Palomares, T.; Alonso-Varona, A. Crystallinity assessment and in vitro cytotoxicity of polylactide scaffolds for biomedical applications. J. Mater. Sci. Mater. Med. 2011, 22, 2513–2523. [Google Scholar] [CrossRef]
- Lin, L.; Xu, Y.; Wang, S.; Xiao, M.; Meng, Y. Ring-opening polymerization of l-lactide and ε-caprolactone catalyzed by versatile tri-zinc complex: Synthesis of biodegradable polyester with gradient sequence structure. Eur. Polym. J. 2016, 74, 109–119. [Google Scholar] [CrossRef]
- International Organization for Standardization. Available online: https://www.iso.org/standard/73342.html (accessed on 6 April 2019).
- Nałęcz-Jawecki, G. Spirotox—Spirostomum ambiguum acute toxicity test—10 years of experience. Environ. Toxicol. 2004, 19, 359–364. [Google Scholar] [CrossRef]
- Oda, Y.; Nakamura, S.; Oki, I.; Kato, T.; Shinagawa, H. Evaluation of the new system (umu-test) for the detection of environmental mutagens and carcinogens. Mutat. Res. Mutagen. Relat. Subj. 1985, 147, 219–229. [Google Scholar] [CrossRef]
Sample Availability: Samples of the all compounds are available from the authors. |
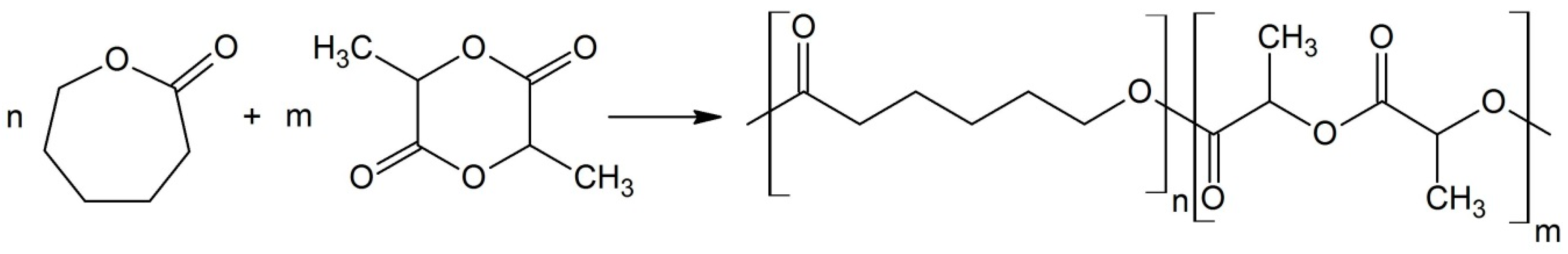
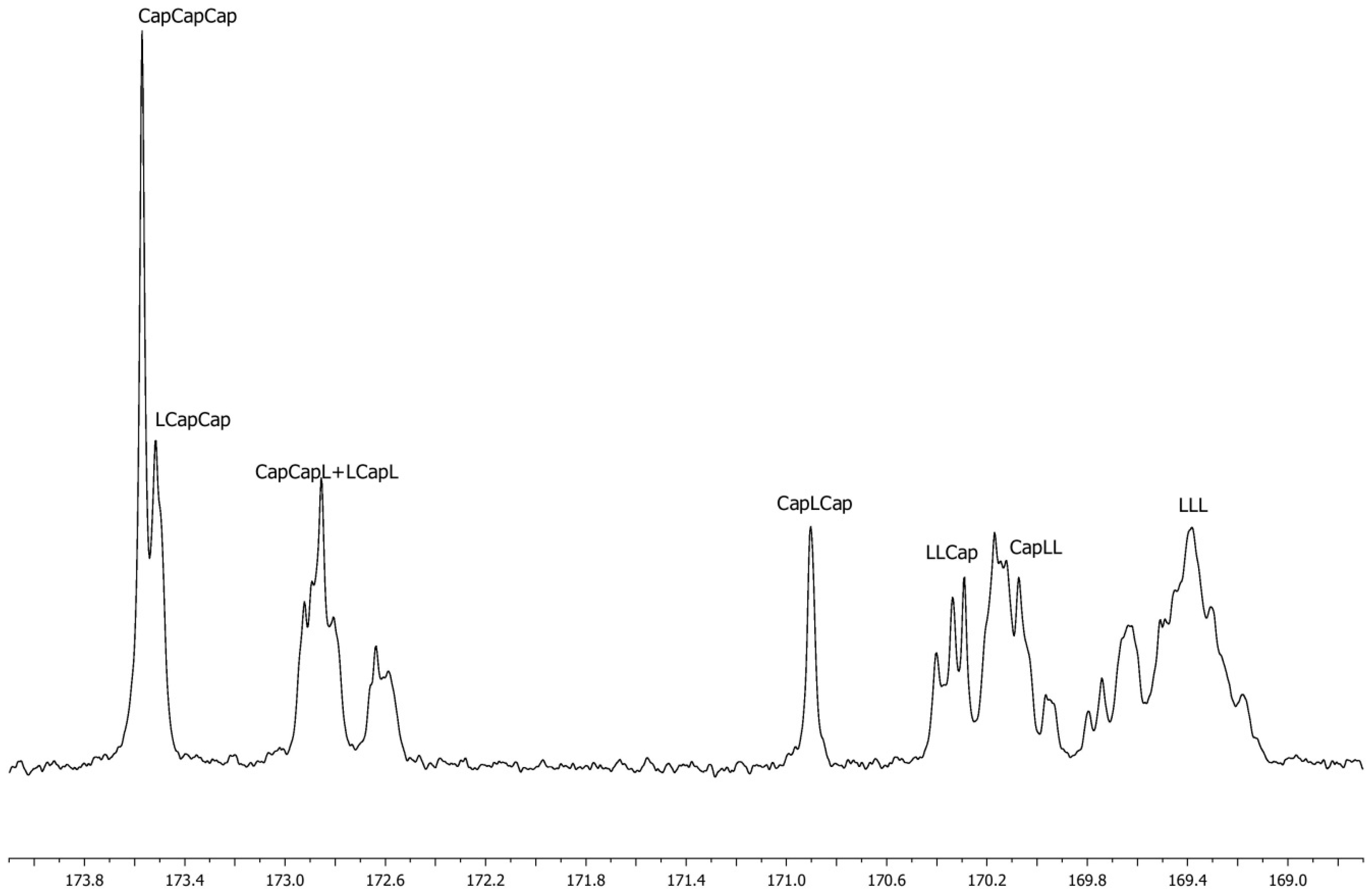
| Entry | Molar Ratio CL/rac-LA | Molar Ratio Zn/Monomers | Reaction Time [h] | Temp. [°C] | Conv.LA | Conv.CL | [L] a | Yield b [%] | lCap c | lLL d | R e | Mnf | Đf | TII [%] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1/1 | 1/100 | 16 | 80 | 0.88 | 0.92 | 0.77 | 64 | 2.32 | 4.83 | 0.45 | 9000 | 1.42 | 31 |
| 2 | 1/1 | 1/100 | 24 | 80 | 0.85 | 0.96 | 0.60 | 83 | 2.24 | 2.65 | 0.47 | 9700 | 1.54 | 47 |
| 3 | 1/1 | 1/100 | 48 | 80 | 0.82 | 0.97 | 0.58 | 87 | 1.85 | 1.63 | 0.74 | 9000 | 1.61 | 74 |
| 4 | 2/1 | 1/100 | 16 | 80 | 0.76 | 0.96 | 0.50 | 65 | 3.13 | 1.82 | 0.55 | 9200 | 1.35 | 39 |
| 5 | 2/1 | 1/100 | 24 | 80 | 0.74 | 0.97 | 0.39 | 93 | 2.58 | 1.09 | 0.76 | 8000 | 1.46 | 67 |
| 6 | 2/1 | 1/100 | 48 | 80 | 0.75 | 0.97 | 0.43 | 85 | 3.03 | 1.34 | 0.65 | 9200 | 1.53 | 60 |
| 7 | 1/2 | 1/100 | 16 | 80 | 0.89 | 0.92 | 0.77 | 68 | 1.76 | 4.45 | 0.49 | 8600 | 1.60 | 38 |
| 8 | 1/2 | 1/100 | 24 | 80 | 0.85 | 0.93 | 0.68 | 90 | 1.68 | 3.34 | 0.47 | 7900 | 1.51 | 40 |
| 9 | 1/2 | 1/100 | 48 | 80 | 0.86 | 0.94 | 0.73 | 85 | 1.78 | 3.82 | 0.49 | 8600 | 1.70 | 23 |
| 10 | 1/1 | 2/100 | 16 | 80 | 0.80 | 0.92 | 0.65 | 64 | 2.00 | 2.16 | 0.67 | 5400 | 1.32 | 60 |
| 11 | 1/1 | 2/100 | 24 | 80 | 0.80 | 0.95 | 0.58 | 58 | 2.32 | 1.87 | 0.64 | 5400 | 1.24 | 40 |
| 12 | 1/1 | 2/100 | 48 | 80 | 0.88 | 0.92 | 0.58 | 63 | 2.41 | 1.82 | 0.66 | 5300 | 1.38 | 45 |
| 13 | 2/1 | 2/100 | 16 | 80 | 0.67 | 0.96 | 0.42 | 67 | 2.21 | 1.09 | 0.80 | 6000 | 1.39 | 89 |
| 14 | 2/1 | 2/100 | 24 | 80 | 0.73 | 0.96 | 0.46 | 69 | 2.61 | 1.18 | 0.79 | 6700 | 1.35 | 69 |
| 15 | 2/1 | 2/100 | 48 | 80 | 0.76 | 0.98 | 0.44 | 62 | 2.84 | 1.24 | 0.72 | 6700 | 1.45 | 60 |
| 16 | 1/2 | 2/100 | 16 | 80 | 0.88 | 0.92 | 0.78 | 67 | 1.70 | 3.96 | 0.58 | 7100 | 1.52 | 46 |
| 17 | 1/2 | 2/100 | 24 | 80 | 0.82 | 0.95 | 0.79 | 64 | 1.85 | 4.14 | 0.56 | 7100 | 1.42 | 39 |
| 18 | 1/2 | 2/100 | 48 | 80 | 0.84 | 0.87 | 0.77 | 66 | 1.27 | 2.67 | 0.83 | 7700 | 1.56 | 100 |
| 19 | 1/1 | 6/100 | 24 | 60 | 0.61 | 0.75 | 0.51 | 20 | 11.68 | 3.10 | 0.33 | 7700 | 1.22 | 7.4 |
| 20 | 1/1 | 6/100 | 48 | 60 | 0.90 | 0.83 | 0.64 | 46 | 5.19 | 7.51 | 0.18 | 8400 | 1.29 | 11 |
| 21 | 1/1 | 6/100 | 16 | 80 | 0.50 | 0.55 | 0.70 | 81 | 7.15 | 7.43 | 0.23 | 8400 | 1.38 | 6.5 |
| 22 | 1/1 | 6/100 | 24 | 80 | 0.55 | 0.65 | 0.72 | 79 | 10.07 | 2.54 | 0.69 | 8500 | 1.43 | 12 |
| 23 | 1/1 | 6/100 | 48 | 80 | 0.89 | 0.86 | 0.62 | 52 | 4.40 | 3.29 | 0.40 | 9600 | 1.51 | 28 |
| 24 | 2/1 | 8/100 | 24 | 60 | 0.83 | 0.29 | 0.79 | 25 | 8.12 | 12.94 | 0.19 | 7900 | 1.19 | 1.7 |
| 25 | 2/1 | 8/100 | 48 | 60 | 0.86 | 0.96 | 0.48 | 52 | 9.89 | 3.54 | 0.27 | 8400 | 1.26 | 9.6 |
| 26 | 2/1 | 8/100 | 16 | 80 | 0.78 | 0.93 | 0.49 | 83 | 7.76 | 4.05 | 0.24 | 8600 | 1.38 | 6.1 |
| 27 | 2/1 | 8/100 | 24 | 80 | 0.85 | 0.96 | 0.49 | 59 | 7.23 | 3.21 | 0.31 | 9200 | 1.36 | 14 |
| 28 | 2/1 | 8/100 | 48 | 80 | 0.84 | 0.96 | 0.49 | 32 | 8.82 | 2.95 | 0.33 | 9800 | 1.40 | 5.3 |
| 29 | 1/2 | 8/100 | 24 | 60 | 0.85 | 0.60 | 0.82 | 44 | 5.96 | 22.04 | 0.13 | 7300 | 1.32 | 0 |
| 30 | 1/2 | 8/100 | 48 | 60 | 0.90 | 0.87 | 0.80 | 46 | 6.01 | 15.96 | 0.16 | 8000 | 1.40 | 15 |
| 31 | 1/2 | 8/100 | 16 | 80 | 0.77 | 0.90 | 0.81 | 73 | 6.39 | 15.97 | 0.17 | 8000 | 1.54 | 3.0 |
| 32 | 1/2 | 8/100 | 24 | 80 | 0.88 | 0.90 | 0.80 | 42 | 3.97 | 13.31 | 0.19 | 8600 | 1.59 | 39 |
| 33 | 1/2 | 8/100 | 48 | 80 | 0.87 | 0.90 | 0.75 | 34 | 4.67 | 7.31 | 0.28 | 9300 | 1.71 | 12 |
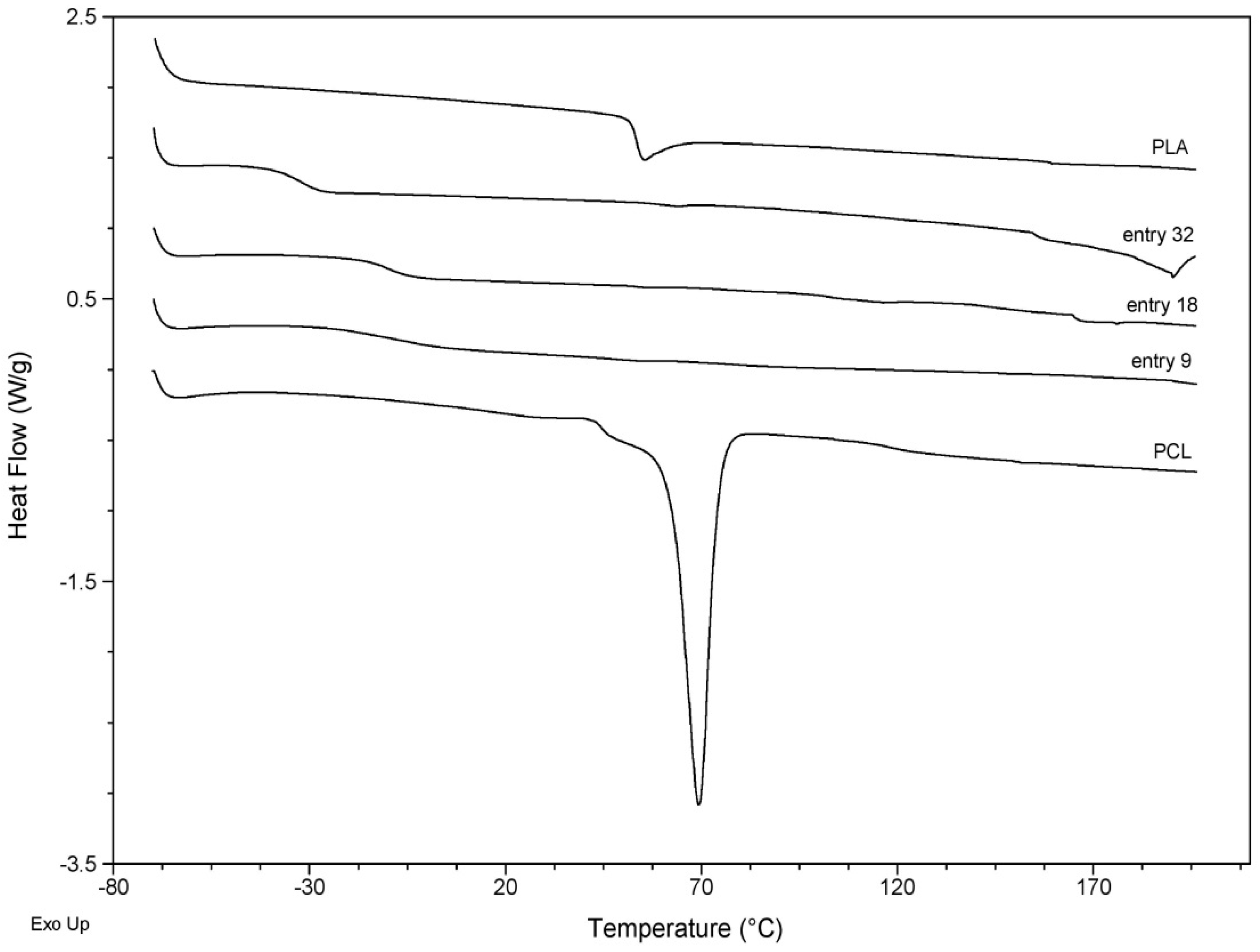
| Entry | Tm1 [°C] a | Tm2 [°C] | Tg [°C] b |
|---|---|---|---|
| PCL | 69.4 | - | −60.0 |
| PLA | - | - | 53.4 |
| 9 | 51.6 | - | −3.8 |
| 18 | 52.5 | 164.9 | −10.0 |
| 32 | 56.4 | 181.8 | −32.5 |
| Entry | Spirotox 24 h-PE 1 | Microtox 15 min-PE 1 |
|---|---|---|
| 1 | 0 | 13 ± 12 |
| 2 | 0 | 25 ± 6 |
| 3 | 0 | 10 ± 6 |
| 4 | 0 | 16 ± 3 |
| 5 | 0 | 34 ± 3 |
| 6 | 0 | 18 ± 4 |
| 7 | 0 | 10 ± 6 |
| 8 | 0 | 21 ± 3 |
| 9 | 0 | 22 ± 2 |
| Entry | −S9 a | +S9 b | ||
|---|---|---|---|---|
| G c ± SD | IR d ± SD | G c ± SD | IR d ± SD | |
| 1 | 1.02 ± 0.02 | 0.86 ± 0.11 | 0.91 ± 0.02 | 1.00 ± 0.02 |
| 2 | 1.00 ± 0.01 | 0.77 ± 0.11 | 0.90 ± 0.04 | 1.00 ± 0.31 |
| 3 | 1.05 ± 0.03 | 0.73 ± 0.08 | 0.91 ± 0.13 | 1.01 ± 0.15 |
| 4 | 1.08 ± 0.02 | 0.77 ± 0.08 | 1.03 ± 0.19 | 0.79 ± 0.07 |
| 5 | 1.00 ± 0.06 | 0.75 ± 0.09 | 0.87 ± 0.05 | 1.06 ± 0.02 |
| 6 | 0.97 ± 0.07 | 0.90 ± 0.14 | 1.05 ± 0.15 | 0.81 ± 0.09 |
| 7 | 1.13 ± 0.05 | 0.85 ± 0.06 | 1.04 ± 0.06 | 1.02 ± 0.08 |
| 8 | 1.02 ± 0.13 | 0.88 ± 0.28 | 1.01 ± 0.16 | 0.75 ± 0.09 |
| 9 | 0.99 ± 0.02 | 0.84 ± 0.13 | 1.07 ± 0.10 | 0.77 ± 0.09 |
| Negative Control | 1.01 ± 0.09 | 0.91 ± 0.17 | 1.00 ± 0.10 | 0.99 ± 0.14 |
| Solvent Control | 0.92 ± 0.12 | 0.83 ± 0.12 | 0.93 ± 0.07 | 0.84 ± 0.07 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wyrębiak, R.; Oledzka, E.; Figat, R.; Sobczak, M. Application of Diethylzinc/Propyl Gallate Catalytic System for Ring-Opening Copolymerization of rac-Lactide and ε-Caprolactone. Molecules 2019, 24, 4168. https://doi.org/10.3390/molecules24224168
Wyrębiak R, Oledzka E, Figat R, Sobczak M. Application of Diethylzinc/Propyl Gallate Catalytic System for Ring-Opening Copolymerization of rac-Lactide and ε-Caprolactone. Molecules. 2019; 24(22):4168. https://doi.org/10.3390/molecules24224168
Chicago/Turabian StyleWyrębiak, Rafał, Ewa Oledzka, Ramona Figat, and Marcin Sobczak. 2019. "Application of Diethylzinc/Propyl Gallate Catalytic System for Ring-Opening Copolymerization of rac-Lactide and ε-Caprolactone" Molecules 24, no. 22: 4168. https://doi.org/10.3390/molecules24224168
APA StyleWyrębiak, R., Oledzka, E., Figat, R., & Sobczak, M. (2019). Application of Diethylzinc/Propyl Gallate Catalytic System for Ring-Opening Copolymerization of rac-Lactide and ε-Caprolactone. Molecules, 24(22), 4168. https://doi.org/10.3390/molecules24224168






