Graphene Oxide: A Smart (Starting) Material for Natural Methylxanthines Adsorption and Detection
Abstract
:1. Introduction
2. Graphene Oxide (GO) and Reduced Graphene Oxide (RGO) as Adsorbents for Extraction and Determination of Natural Methylxanthines
3. Graphene Oxide (GO) in Electrochemical Sensors for Natural Methylxanthines
4. Reduced Graphene Oxide (RGO) and Electrochemically Reduced Graphene Oxide (ERGO) in Electrochemical Sensors for Natural Methylxanthines
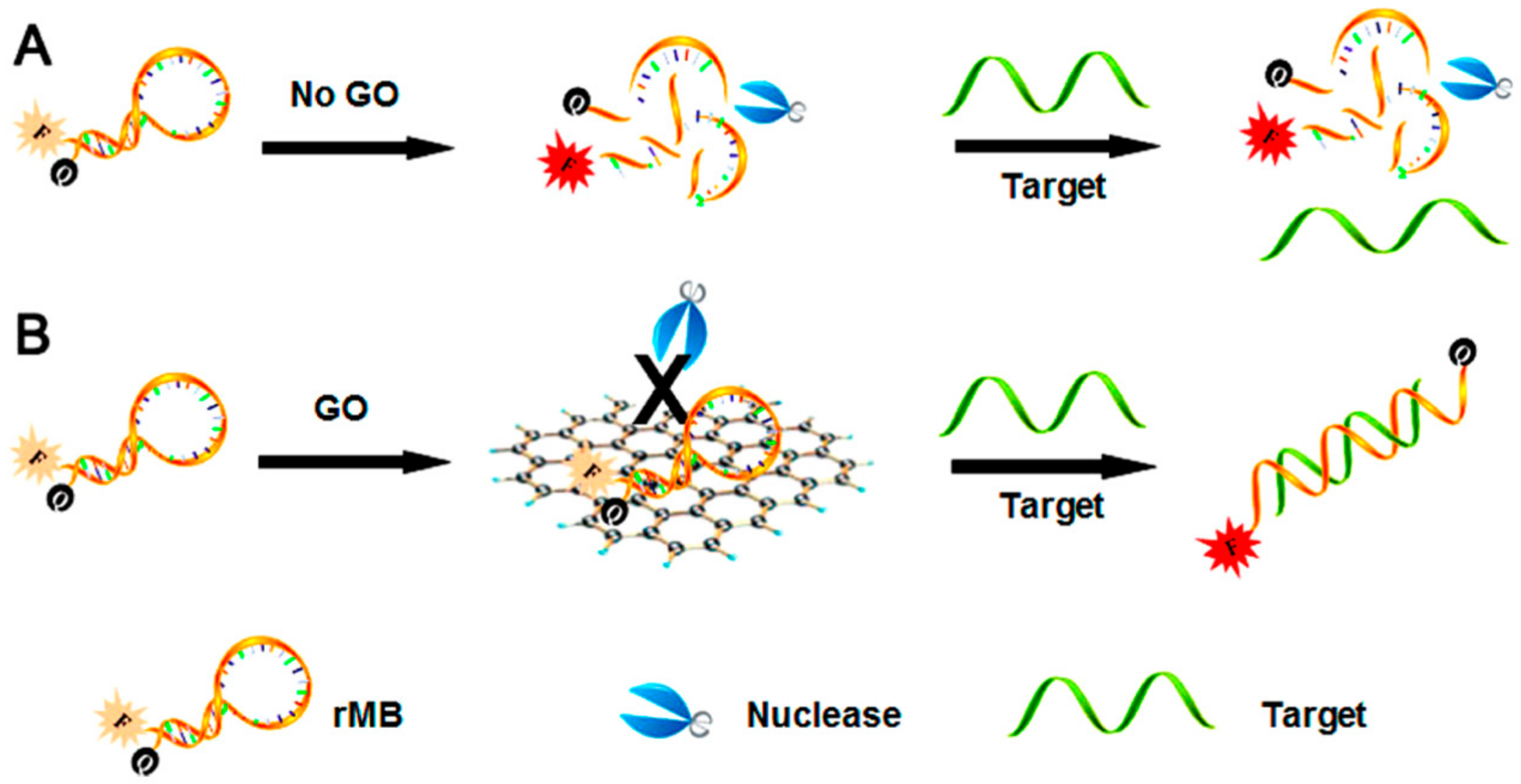
5. GO in Fluorescence Sensors for Natural Methylxanthines
6. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jiang, H. Chemical Preparation of Graphene-Based Nanomaterials and Their Applications in Chemical and Biological Sensors. Small 2011, 7, 2413–2427. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene Oxide: Preparation, Functionalization, and Electrochemical Applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, L.; Chen, J.; Jiang, T.; Ye, H.; Ji, H.; Tsang, S.; Zhao, Z.; Yi, T.; Chen, H. Recent progress in nanomaterial-based assay for the detection of phytotoxins in foods. Food Chem. 2019, 277, 162–178. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos S., V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.-B.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Azman, N.H.N.; Mamat, M.D.S.; Nazir, M.; Ngee, L.H.; Sulaiman, Y. Graphene-based ternary composites for supercapacitors. Int. J. Energy. Res. 2018, 42, 2104–2116. [Google Scholar] [CrossRef]
- Hada, M.; Miyata, K.; Ohmura, S.; Arashida, Y.; Ichiyanagi, K.; Katayama, I.; Suzuki, T.; Chen, W.; Mizote, S.; Sawa, T.; et al. Selective Reduction Mechanism of Graphene Oxide Driven by the Photon Mode versus the Thermal Mode. ACS Nano 2019. [Google Scholar] [CrossRef] [PubMed]
- Marrani, A.G.; Coico, A.C.; Giacco, D.; Zanoni, R.; Scaramuzzo, F.A.; Schrebler, R.; Dini, D.; Bonomo, M.; Dalchiele, E.A. Integration of graphene onto silicon through electrochemical reduction of graphene oxide layers in non-aqueous medium. Appl. Surf. Sci. 2018, 445, 404–414. [Google Scholar] [CrossRef]
- Fredholm, B.B. Methylxanthines; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Monteiro, J.; Alves, M.G.; Oliveira, P.F.; Silva, B.M. Pharmacological potential of methylxanthines: Retrospective analysis and futureexpectations. Crit. Rev. Food Sci. Nutrit. 2019, 59, 2597–2625. [Google Scholar] [CrossRef]
- Petrucci, R.; Zollo, G.; Curulli, A.; Marrosu, G. A new insight into the oxidative mechanism of caffeine and related methylxanthines in aprotic medium: May caffeine be really considered as an antioxidant? Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1781–1789. [Google Scholar] [CrossRef]
- Panusa, A.; Petrucci, R.; Lavecchia, R.; Zuorro, A. UHPLC-PDA-ESI-TOF/MS metabolic profiling and antioxidant capacity of arabica and robusta coffee silverskin: Antioxidants vs phytotoxins. Food Res. Int. 2017, 99, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.P.; Alves, M.G.; Oliveira, P.F.; Silva, B.M. Structure-Bioactivity Relationships of Methylxanthines: Trying to Make Sense of All the Promises and the Drawbacks. Molecules 2016, 21, 974. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Oñatibia-Astibia, A.; Martínez-Pinilla, E. Health Benefits of Methylxanthines in Cacao and Chocolate. Nutrients 2013, 5, 4159–4173. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, F.; Mattiello, L.; Zane, D.; Feroci, M. Electrochemical behaviour of 9-methylcaffeinium iodide and in situ electrochemical synthesis of hymeniacidin. Electrochim. Acta 2018, 280, 71–76. [Google Scholar] [CrossRef]
- Pandolfi, F.; Chiarotto, I.; Mattiello, L.; Rocco, D.; Feroci, M. Cathodic reduction of caffeine: Amino-functionalized imidazole from a bio-based reagent. Synlett 2019, 30, 1215–1218. [Google Scholar] [CrossRef]
- Chiarotto, I.; Mattiello, L.; Pandolfi, F.; Rocco, D.; Feroci, M.; Petrucci, R. Electrochemical oxidation of theophylline in organic solvents: HPLC-PDA-ESI-MS/MS analysis of the oxidation products. ChemElectroChem 2019, 6, 4511–4521. [Google Scholar] [CrossRef]
- Andreeva, E.Y.; Dmitrienko, S.G.; Zolotov, Y.A. Methylxanthines: Properties and determination in various objects. Russ. Chem. Rev. 2012, 81, 397–414. [Google Scholar] [CrossRef]
- Trani, A.; Petrucci, R.; Marrosu, G.; Zane, D.; Curulli, A. Selective electrochemical determination of caffeine at a gold-chitosan nanocomposite sensor: May little change on nanocomposites synthesis affect selectivity? J. Electroanal. Chem. 2017, 788, 99–106. [Google Scholar] [CrossRef]
- Trani, A.; Petrucci, R.; Marrosu, G.; Curulli, A. Determination of caffeine @ gold nanoparticles modified gold (Au) electrode: A preliminary study. Lect. Notes Electric. Eng. 2015, 319, 147–151. [Google Scholar] [CrossRef]
- Zhang, Z.; Schniepp, H.C.; Adamson, D.H. Characterization of graphene oxide: Variations in reported approaches. Carbon 2019, 154, 510. [Google Scholar] [CrossRef]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-Based Materials: Synthesis, Characterization, Properties, and Applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Cubadda, F.; Dini, L.; Terranova, M.L.; Aureli, F.; Sorbo, A.; Passeri, D. Scientific basis of nanotechnology, implications for the food sector and future trends. Trends Food Sci. Technol. 2014, 40, 127–148. [Google Scholar] [CrossRef]
- Wu, J.-F.; Gao, X.; Ge, L.; Zhao, G.-C.; Wang, G.-F. A fluorescence sensing platform of theophylline based on the interaction of RNA aptamer with graphene oxide. RSC Adv. 2019, 9, 19813–19818. [Google Scholar] [CrossRef]
- Ghann, W.E.; Kang, H.; Uddin, J.; Chowdhury, F.A.; Khondaker, S.I.; Moniruzzaman, M.; Kabir, H.; Rahman, M.M. Synthesis and Characterization of Reduced Graphene Oxide and Their Application in Dye-Sensitized Solar Cells. ChemEngineering 2019, 3, 7. [Google Scholar] [CrossRef]
- Matassa, R.; Orlanducci, S.; Tamburri, E.; Guglielmotti, V.; Sordi, D.; Terranova, M.L.; Passeri, D.; Rossi, M. Characterization of carbon structures produced by graphene self-assembly. J. Appl. Cryst. 2014, 47, 222–227. [Google Scholar] [CrossRef]
- Park, S.; Ruoff, R.S. Synthesis and characterization of chemically modified graphenes. Curr. Opin. Colloid Interface Sci. 2015, 20, 322–328. [Google Scholar] [CrossRef]
- Teklu, A.; Barry, C.; Palumbo, M.; Weiwadel, C.; Kuthirummal, N.; Flagg, J. Mechanical Characterization of Reduced Graphene Oxide Using AFM. Adv. Condens. Matter Phys. 2019, 8713965. [Google Scholar] [CrossRef]
- Passeri, D.; Bettucci, A.; Rossi, M. Acoustics and atomic force microscopy for the mechanical characterization of thin films. Anal. Bioanal. Chem. 2010, 396, 2769–2783. [Google Scholar] [CrossRef]
- Dinelli, F.; Pingue, P.; Kay, N.D.; Kolosov, O.V. Subsurface imaging of two-dimensional materials at the nanoscale. Nanotechnology 2017, 28, 085706. [Google Scholar] [CrossRef]
- Pashaee, F.; Sharifi, F.; Fanchini, G.; Lagugne´-Labarthet, F. Tip-enhanced Raman spectroscopy of graphene-like and graphitic platelets on ultraflat gold nanoplates. Phys. Chem. Chem. Phys. 2015, 17, 21315. [Google Scholar] [CrossRef]
- Bhattarai, A.; Krayev, A.; Temiryazev, A.; Evplov, D.; Crampton, K.T.; Hess, W.P.; El-Khoury, P.Z. Tip-Enhanced Raman Scattering from Nanopatterned Graphene and Graphene Oxide. Nano Lett. 2018, 18, 4029–4033. [Google Scholar] [CrossRef] [PubMed]
- Seabra, A.B.; Paula, A.J.; de Lima, R.; Alves, O.L.; Durán, N. Nanotoxicity of Graphene and Graphene Oxide. Chem. Res. Toxicol. 2014, 27, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.; Song, B.; Liang, H.; Liu, J.; Feng, X.; Deng, B.; Sun, T.; Shao, L. Toxicity of graphene-family nanoparticles: A general review of the origins and mechanisms. Particl. Fibr. Toxicol. 2016, 13, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelin, M.; Fusco, L.; Martín, C.; Sosa, S.; Frontiñán-Rubio, J.; González-Domínguez, J.M.; Durán-Prado, M.; Vázquez, E.; Prato, M.; Tubaro, A. Graphene and graphene oxide induce ROS production in human HaCaT skin keratinocytes: the role of xanthine oxidase and NADH dehydrogenase. Nanoscale 2018, 10, 11820–11830. [Google Scholar] [CrossRef] [Green Version]
- Amrollahi-Sharifabadi, M.; Kazem Koohi, M.; Zayerzadeh, E.; Hablolvarid, M.H.; Hassan, J.; Seifalian, A.M. In vivo toxicological evaluation of graphene oxide nanoplatelets for clinical application. Int. J. Nanomed. 2018, 13, 4757–4769. [Google Scholar] [CrossRef] [Green Version]
- Vu, T.H.T.; Tran, T.T.T.; Le, H.N.T.; Nguyen, P.H.T.; Bui, N.Q.; Essayem, N. A new green approach for the reduction of graphene oxide nanosheets using caffeine. Bull. Mater. Sci. 2015, 38, 667–671. [Google Scholar]
- Ma, G.; Zhang, M.; Zhu, L.; Chen, H.; Liu, X.; Lu, C. Facile synthesis of amine-functional reduced graphene oxides asmodified quick, easy, cheap, effective, rugged and safe adsorbent for multi-pesticide residues analysis of tea. J. Chromatogr. A 2018, 1531, 22–31. [Google Scholar] [CrossRef]
- Akpotu, S.O.; Moodley, B. MCM-48 encapsulated with reduced graphene oxide/graphene oxide and as-synthesised MCM-48 application in remediation of pharmaceuticals from aqueous system. J. Mol. Liquids 2018, 261, 540–549. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, G.; Zhang, L.; Chen, H.; Zhu, L.; Wang, C.; Liu, X. Chitosan-reduced graphene oxides composites with 3D structures as an effective reverse dispersed solid phase extraction adsorbent for pesticides analysis. Analyst 2019, 144, 5164–5171. [Google Scholar] [CrossRef]
- Delhiraja, K.; Vellingiri, K.; Boukhvalov, D.W.; Philip, L. Development of Highly Water Stable Graphene Oxide-Based Composites for the Removal of Pharmaceuticals and Personal Care Products. Ind. Eng. Chem. Res. 2019, 58, 2899–2913. [Google Scholar] [CrossRef]
- Sereshti, H.; Khosraviani, M.; Samadi, S.; Amini-Fazl, M.S. Simultaneous determination of theophylline, theobromine and caffeine in different tea beverages by graphene-oxide based ultrasonicassisted dispersive micro solid-phase extraction combined with HPLC-UV. RSC Adv. 2014, 4, 47114–47120. [Google Scholar] [CrossRef]
- Rahimi, A.; Zanjanchi, M.A.; Bakhtiari, S.; Dehsaraei, M. Selective determination of caffeine in foods with 3D-graphene based ultrasound-assisted magnetic solid phase extraction. Food Chem. 2018, 262, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, H.İ.; Yılmaz, E.; Soylak, M. Magnetic solid phase extraction of trace paracetamol and caffeine in synthetic urine and wastewater samples by a using core shell hybrid material consisting of graphene oxide/multiwalled carbon nanotube/Fe3O4/SiO2. Microchem. J. 2019, 145, 843–851. [Google Scholar] [CrossRef]
- Panusa, A.; Zuorro, A.; Lavecchia, R.; Marrosu, G.; Petrucci, R. Recovery of Natural Antioxidants from Spent Coffee Grounds. J. Agric. Food Chem. 2013, 61, 4162–4168. [Google Scholar] [CrossRef]
- Petrucci, R.; Marrosu, G.; Astolfi, P.; Lupidi, G.; Greci, L. Cyclic voltammetry, spectroelectrochemistry and electron spin resonance as combined tools to study thymoquinone in aprotic medium. Electrochim. Acta 2012, 60, 230–238. [Google Scholar] [CrossRef]
- Stetter, J.R.; Penrose, W.R.; Yao, S. Sensors, Chemical Sensors, Electrochemical Sensors, and ECS. J. Electrochem. Soc. 2003, 150, S11–S16. [Google Scholar] [CrossRef]
- Pandikumar, A.; Rameshkumar, P. Graphene-Based Electrochemical Sensors for Biomolecules; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, F.; Zhao, W.; Zhou, J.; Liu, Y.; Zou, L.; Ye, B. Voltammetric sensor for caffeine based on a glassy carbon electrode modified with Nafion and graphene oxide. Microchim Acta 2011, 174, 383–390. [Google Scholar] [CrossRef]
- Wong, A.; Santos, A.M.; Almeida Silva, T.; Fatibello-Filho, O. Simultaneous determination of isoproterenol, acetaminophen, folic acid, propranolol and caffeine using a sensor platform based on carbon black, graphene oxide, copper nanoparticles and PEDOT:PSS. Talanta 2018, 183, 329–338. [Google Scholar] [CrossRef]
- Murugan, E.; Kumar, K. Fabrication of SnS/TiO2@GO Composite Coated Glassy Carbon Electrode for Concomitant Determination of Paracetamol, Tryptophan, and Caffeine in Pharmaceutical Formulations. Anal. Chem. 2019, 91, 5667–5676. [Google Scholar] [CrossRef]
- Shetti, N.P.; Malode, S.J.; Nayak, D.S.; Bagihalli, G.B.; Reddy, K.R.; Ravindranadh, K.; Reddy, C.V. A novel biosensor based on graphene oxide-nanoclay hybrid electrode for the detection of Theophylline for healthcare applications. Microchem. J. 2019, 149, 103985. [Google Scholar] [CrossRef]
- Shehata, M.; Azab, S.M.; Fekry, A.M. May glutathione and graphene oxide enhance the electrochemical detection of caffeine on carbon paste sensor in aqueous and surfactant media for beverages analysis? Synt. Met. 2019, 256, 116122. [Google Scholar] [CrossRef]
- Cui, F.; Zhang, X. A method based on electrodeposition of reduced graphene oxide on glassy carbon electrode for sensitive detection of theophylline. J. Solid State Electrochem. 2013, 17, 167–173. [Google Scholar] [CrossRef]
- Khoo, W.Y.H.; Pumera, M.; Bonanni, A. Graphene platforms for the detection of caffeine in real samples. Anal. Chim. Acta 2013, 804, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Raj, M.A.; John, S.A. Simultaneous determination of uric acid, xanthine, hypoxanthine and caffeine in human blood serum and urine samples using electrochemically reduced graphene oxide modified electrode. Anal. Chim. Acta 2013, 771, 14–20. [Google Scholar] [CrossRef]
- Raj, M.A.; John, S.A. Graphene layer modified glassy carbon electrode for the determination of norepinephrine and theophylline in pharmaceutical formulations. Anal. Methods 2014, 6, 2181–2188. [Google Scholar] [CrossRef]
- Vasilescu, I.; Eremia, S.A.V.; Penu, R.; Albu, C.; Radoi, A.; Litescu, S.C.; Radu, G.-L. Disposable dual sensor array for simultaneous determination of chlorogenic acid and caffeine from coffee. RSC Adv. 2015, 5, 261–268. [Google Scholar] [CrossRef]
- Kesavan, S.; Raj, M.A.; John, S.A. Formation of electrochemically reduced graphene oxide on melamine electrografted layers and its application toward the determination of methylxanthines. Anal. Biochem. 2016, 496, 14–24. [Google Scholar] [CrossRef]
- Velmurugan, M.; Karikalan, N.; Chen, S.-M.; Karuppiah, C. Core-shell like Cu2O nanocubes enfolded with Co(OH)2 on reduced graphene oxide for the amperometric detection of caffeine. Microchim. Acta 2016, 183, 2713–2721. [Google Scholar] [CrossRef]
- Rocha, D.P.; Dornellas, R.M.; Nossol, E.; Richter, E.M.; Silva, S.G.; Santana, M.H.P.; Munoz, R.A.A. Electrochemically Reduced Graphene Oxide for Forensic Electrochemistry: Detection of Cocaine and its Adulterants Paracetamol, Caffeine and Levamisole. Electroanalysis 2017, 29, 2418–2422. [Google Scholar] [CrossRef]
- Marques Petroni, J.; Lucca, B.G.; da Silva Júnior, L.C.; Barbosa Alves, D.C.; Ferreira, V.S. Paper-based Electrochemical Devices Coupled to External Graphene-Cu Nanoparticles Modified Solid Electrode through Meniscus Configuration and their Use in Biological Analysis. Electroanalysis 2017, 29, 2628–2637. [Google Scholar] [CrossRef]
- Phong, N.H.; Toan, T.T.T.; Tinh, M.X.; Tuyen, T.N.; Mau, T.X.; Khieu, D.Q. Simultaneous Voltammetric Determination of Ascorbic Acid, Paracetamol, and Caffeine Using Electrochemically Reduced Graphene-Oxide-Modified Electrode. J. Nanomat. 2018, 5348016. [Google Scholar] [CrossRef]
- Gao, L.; Yue, R.; Xu, J.; Liu, Z. One-pot Synthesis of Fe2O3/PEDOT/rGO Nanocomposite for Sensitive Determination of Caffeine. Int. J. Electrochem. Sci. 2018, 13, 6791–6802. [Google Scholar] [CrossRef]
- Raj, M.; Goyal, R.N. Silver nanoparticles and electrochemically reduced graphene oxide nanocomposite based biosensor for determining the effect of caffeine on Estradiol release in women of child-bearing age. Sens. Actuators B Chem. 2019, 284, 759–767. [Google Scholar] [CrossRef]
- Li, R.; Yao, L.; Wang, Z.; Lv, W.; Wang, W.; Kong, F.; Wang, W. Facile Synthesis Gold-Polyindole-Reduced Graphene Oxide Ternary Nanocomposites with Enhanced Electrocatalytic Activity for the Electrochemical Sensing of Caffeine. J. Electrochem. Soc. 2019, 166, B212–B218. [Google Scholar] [CrossRef]
- Cui, L.; Chen, Z.; Zhu, Z.; Lin, X.; Chen, X.; Yang, C.J. Stabilization of ssRNA on Graphene Oxide Surface: An Effective Way to Design Highly Robust RNA Probes. Anal. Chem. 2013, 85, 2269–2275. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Song, J.; Wang, Y.; Cheng, T. Cyclically amplified fluorescent detection of theophylline andthiamine pyrophosphate by coupling self-cleaving RNA ribozymewith endonuclease. Anal. Chim. Acta 2013, 797, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Lau, P.S.; Liu, M.; Shuang, S.; Dong, C.; Li, Y. A General Strategy to Create RNA Aptamer Sensors Using “Regulated” Graphene Oxide Adsorption. ACS Appl. Mater. Interfaces 2014, 6, 21806–21812. [Google Scholar] [CrossRef]
- Ling, K.; Jiang, H.; Li, Y.; Tao, X.; Qiu, C.; Li, F.-R. A self-assembling RNA aptamer-based graphene oxide sensor for the turn-on detection of theophylline in serum. Biosens. Bioelectronics 2016, 86, 8–13. [Google Scholar] [CrossRef]
- Lou, Y.-F.; Peng, Y.-B.; Luo, X.; Yang, Z.; Wang, R.; Sun, D.; Li, L.; Tan, Y.; Huang, J.; Cui, L. A universal aptasensing platform based on cryonase-assisted signal amplification and graphene oxide induced quenching of the fluorescence of labeled nucleic acid probes: application to the detection of theophylline and ATP. Microchim. Acta 2019, 186, 494. [Google Scholar] [CrossRef]
- Kant, R.; Tabassum, R.; Gupta, B.D. Integrating nanohybrid membranes of reduced graphene oxide: chitosan: silica sol gel with fiber optic SPR for caffeine detection. Nanotechnology 2017, 28, 195502. [Google Scholar] [CrossRef]


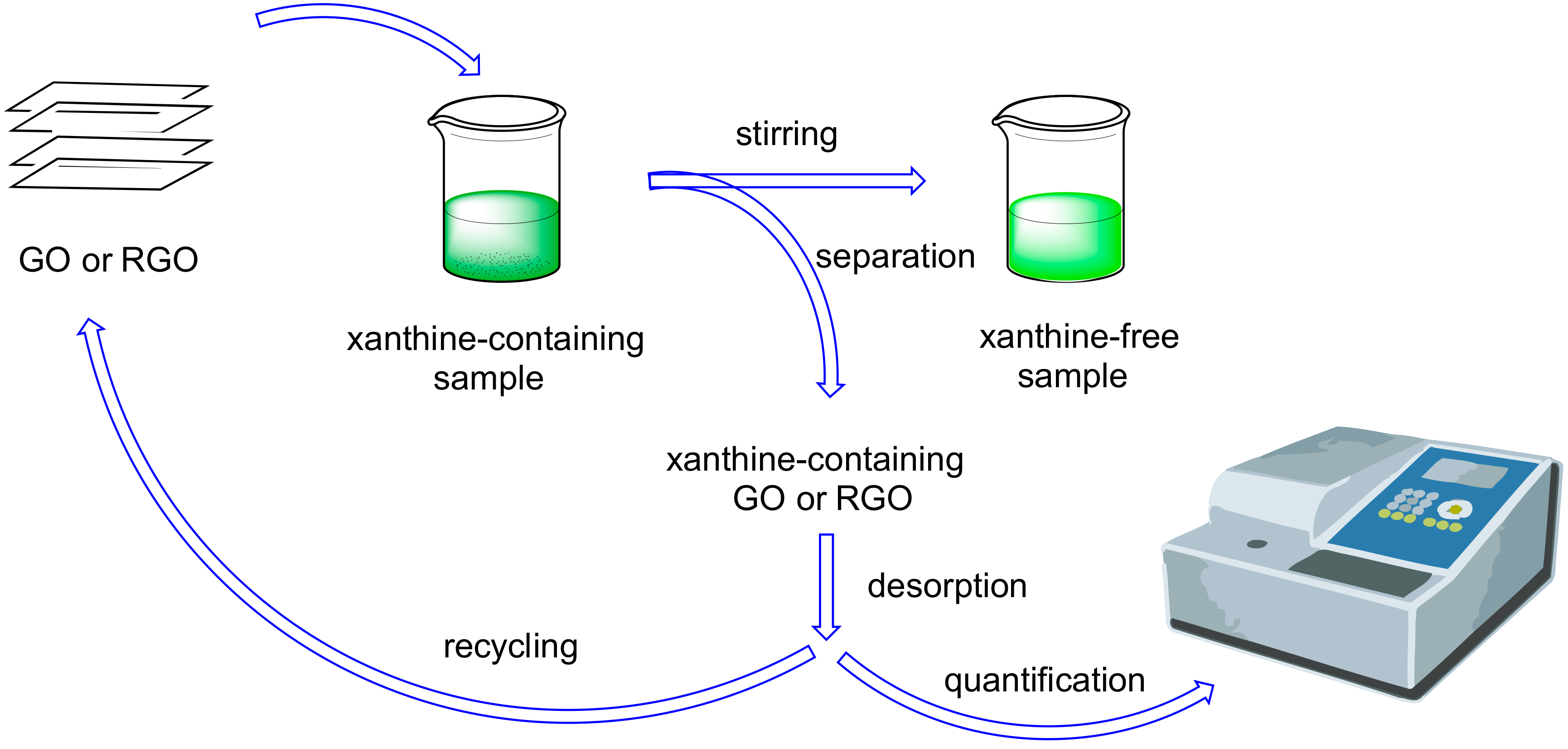
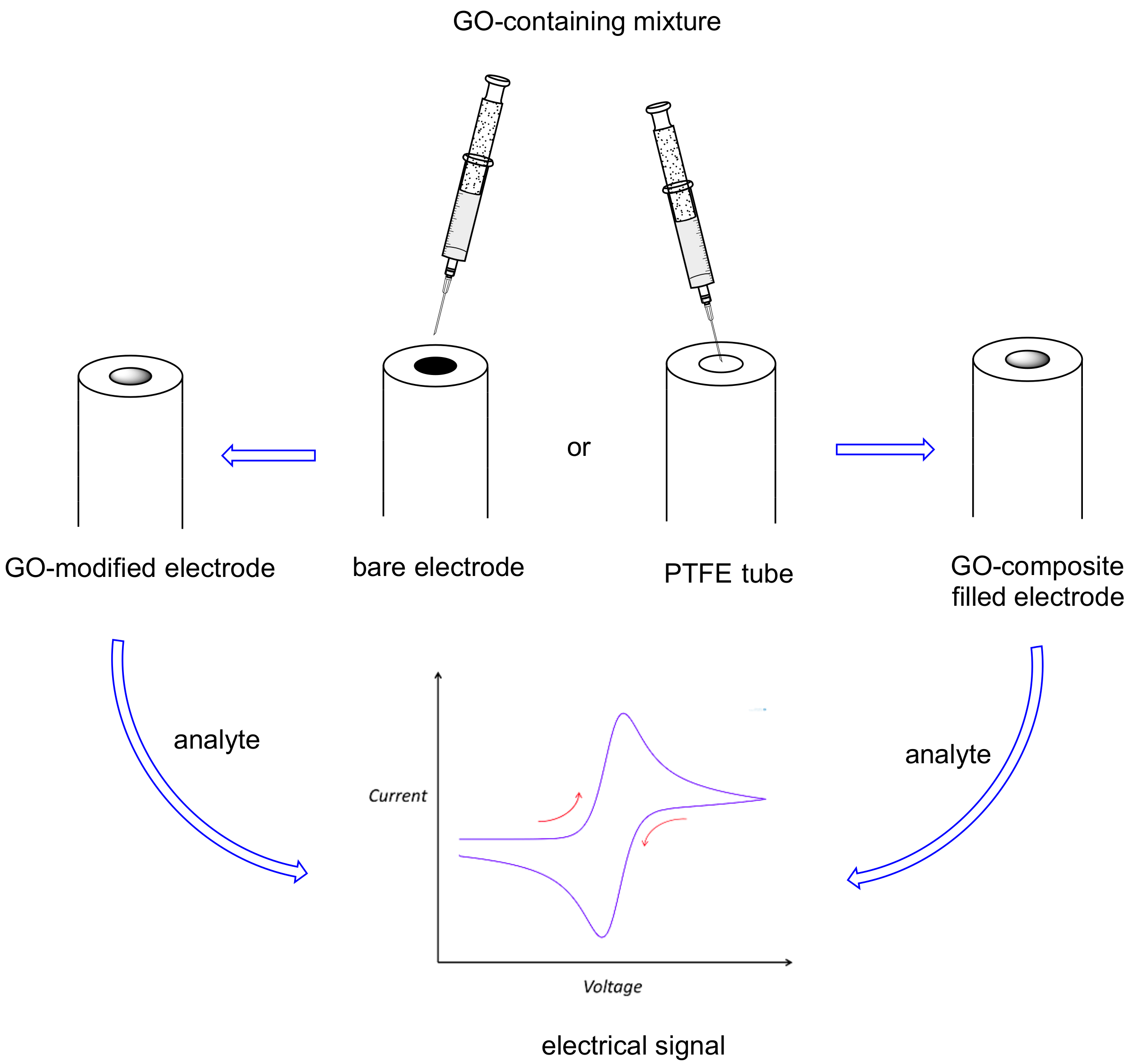
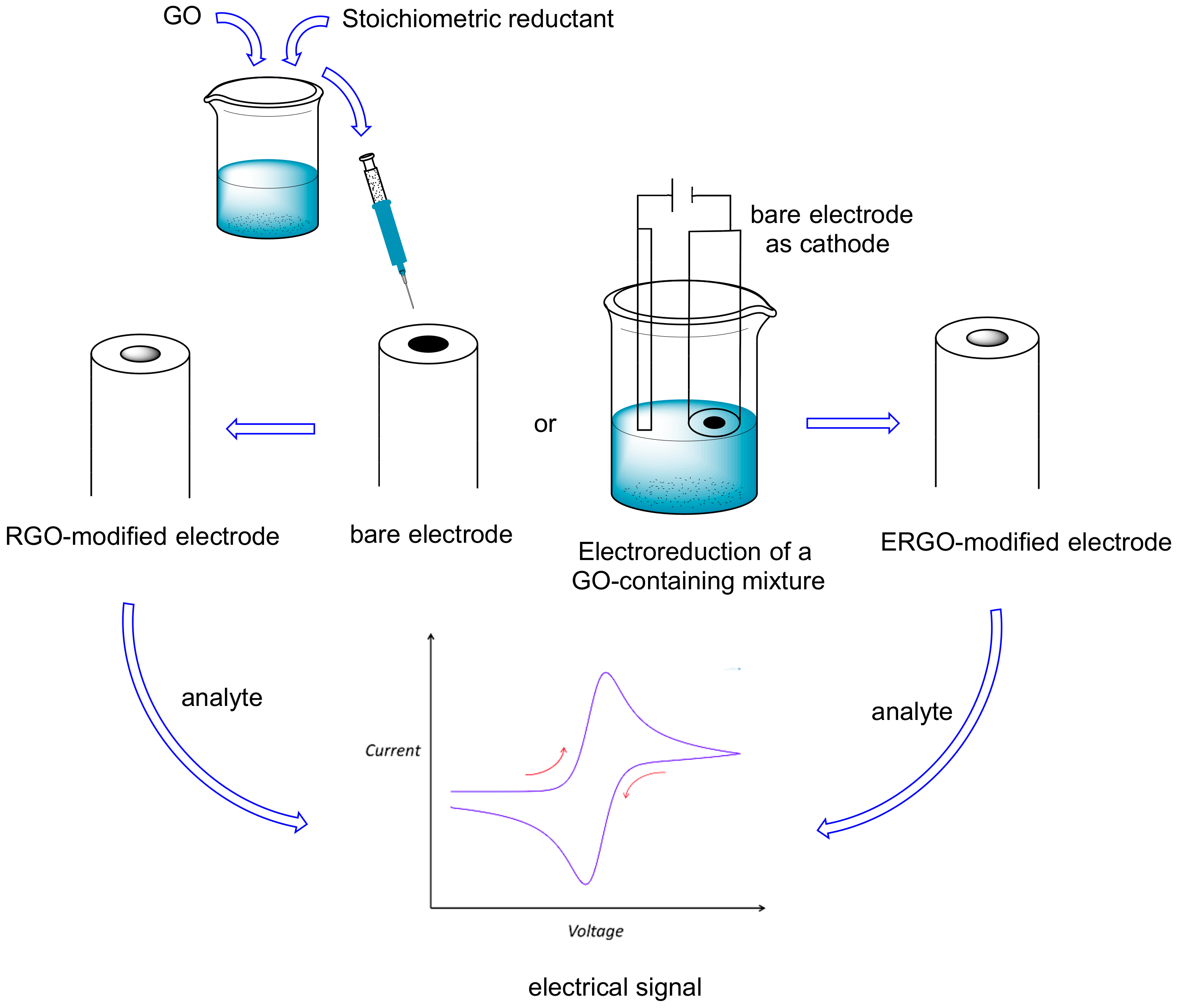
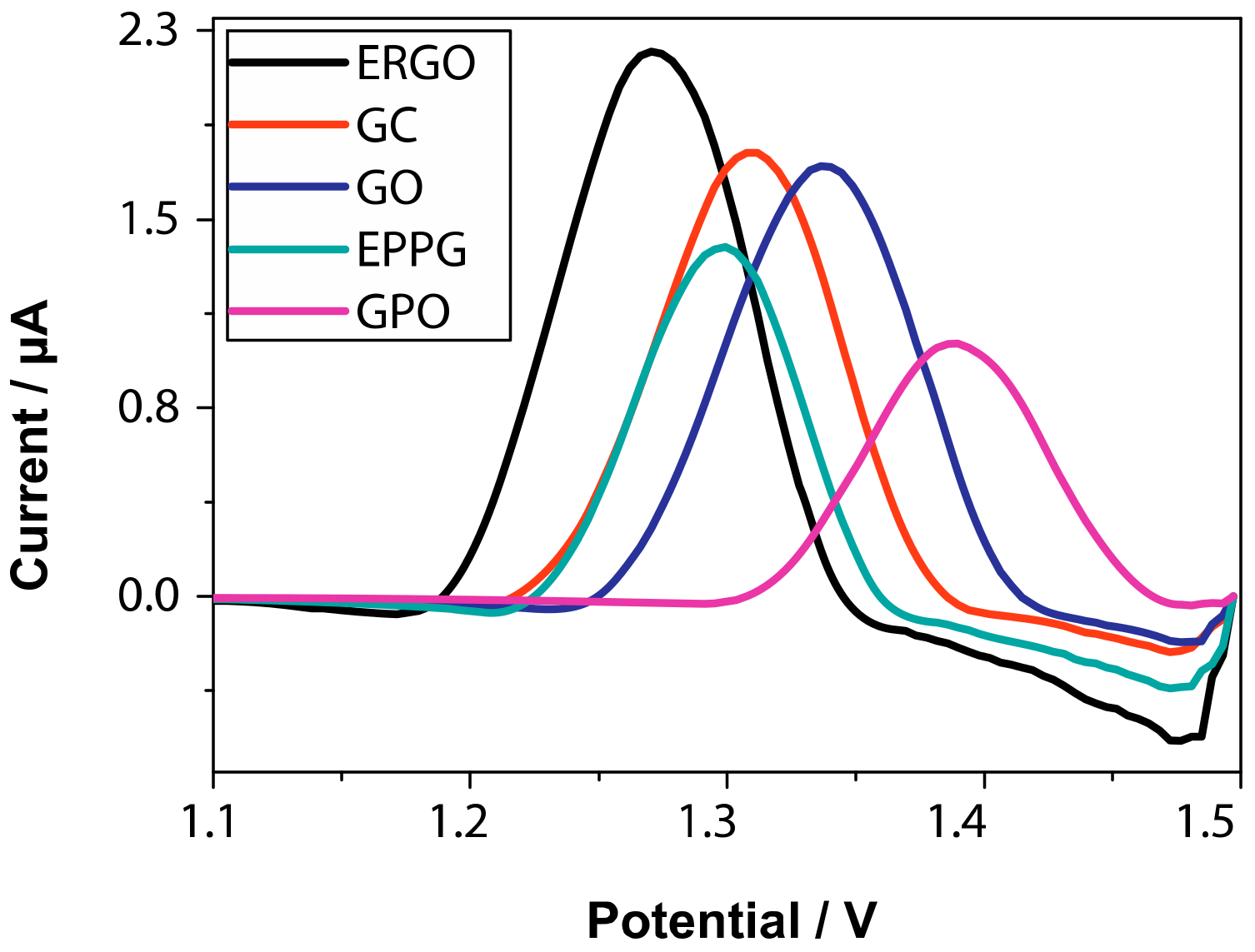
| Entry | Adsorbent | Adsorbed Analyte 1 | Adsorption Capacity | Sample | Reference |
|---|---|---|---|---|---|
| 1 | Bu3N-RGO | Cf | 203.7 mg g−1 | beverages | [38] |
| 2 | M48-RGO 2 | Cf | 153.8 mg g−1 | water | [39] |
| 3 | GO-AC-CS 3 | Cf | 14.8 mg g−1 | wastewater | [40] |
| 4 | CS-RGO 3 | Cf | 179.3 mg g−1 | beverages | [41] |
| Entry | Graphene Derivative in Adsorbent | Detected Analytes 1 | Detection Limit | Quantification Limit | Sample | Reference |
|---|---|---|---|---|---|---|
| 1 | GO | Cf, Tp, Tb | 0.11–0.90 ng mL−1 | 0.37–3.00 ng mL−1 | beverages | [42] |
| 2 | RGO | Cf | 0.1 ng mL−1 | 0.5–500 ng mL−1 | beverages | [43] |
| 3 | GO | Cf | 1.48 ng mL−1 | 5–800 ng mL−1 | wastewater | [44] |
| Entry | Electrode | Analyte 1 | Detection Limit | Detection Range | Reference |
|---|---|---|---|---|---|
| 1 | GO-Nafion-GC 2 | Cf | 2 × 10−7 mol L−1 | 4·10−7/8 × 10−5 mol L−1 | [49] |
| 2 | GO-CB-CuNPs- PEDOT:PSS-GC 3 | Cf | 3.4 × 10−6 mol L−1 | 1·10−7/6 × 10−7 mol L−1 | [50] |
| 3 | GO-SnS/TiO2-GC | Cf | 4.4 × 10−9 mol L−1 | 2·10−8/3 × 10−4 mol L−1 | [51] |
| 4 | GO-NC-CPE 2 | Tp | 1.8 × 10−9 mol L−1 | 1·10−8/2 × 10−7 mol L−1 | [52] |
| 5 | GO-RG-CPE 2 | Cf | 1.5 × 10−7 mol L−1 | 8·10−6/8 × 10−4 mol L−1 | [53] |
| Entry | Electrode | Analyte 1 | Detection Limit | Detection Range | Reference |
|---|---|---|---|---|---|
| 1 | ERGO-GC 2 | Tp | 1 × 10−7 mol L−1 | 8·10−7/6·10−5 mol L−1 | [54] |
| 2 | ERGO-HDA-GC 2 | Cf | 4.3 × 10−7 mol L−1 | 1·10−5/5·10−4 mol L−1 | [56] |
| 3 | ERGO-HDA-GC 2 | Tp | 2.9 × 10−9 mol L−1 | 5·10−8/4·10−5 mol L−1 | [57] |
| 4 | RGO-Nafion-GC 2 | Cf | 2.2 × 10−7 mol L−1 | 3·10−7/3·10−6 mol L−1 | [58] |
| 5 | RGO-Cu2O- Co(OH)2-GC 2 | Cf | 4.0 × 10−7 mol L−1 | 8·10−7/1·10−3 mol L−1 | [60] |
| 6 | ERGO-GC 2 | Cf | 2.3 × 10−7 mol L−1 | 2·10−7/4·10−6 mol L−1 | [63] |
| 7 | RGO-Fe2O3- PEDOT-GC 2 | Cf | 3.3 × 10−7 mol L−1 | 1·10−6/8·10−4 mol L−1 | [64] |
| 8 | ERGO-AgNPs -PG2,3 | Cf | 5.4 × 10−10 mol L−1 | 1·10−9/2·10−4 mol L−1 | [65] |
| 9 | RGO-Au- PIn-GC 2 | Cf | 2.6 × 10−7 mol L−1 | 8·10−7/4·10−5 mol L−1 | [66] |
| Entry | Aptamer | Analyte 1 | Detection Limit | Detection Range | Reference |
|---|---|---|---|---|---|
| 1 | ssRNA 2 | Tp | 2 × 10−6 mol L−1 | 1·10−6/1·10−4 mol L−1 | [67] |
| 2 | ssDNA 2 | Tp | 1 × 10−7 mol L−1 | 1·10−7/1·10−5 mol L−1 | [68] |
| 3 | RNA/DNA | Tp | 5 × 10−7 mol L−1 | 5·10−7/2·10−3 mol L−1 | [69] |
| 4 | RNA | Tp | 1.6 × 10−7 mol L−1 | 1·10−6/1·10−4 mol L−1 | [70] |
| 5 | ssRNA 2 | Tp | 4 × 10−9 mol L−1 | 1·10−8/3·10−6 mol L−1 | [24] |
| 6 | ssRNA-cryonase 2 | Tp | 4.7 × 10−8 mol L−1 | 5·10−8/5·10−6 mol L−1 | [71] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrucci, R.; Chiarotto, I.; Mattiello, L.; Passeri, D.; Rossi, M.; Zollo, G.; Feroci, M. Graphene Oxide: A Smart (Starting) Material for Natural Methylxanthines Adsorption and Detection. Molecules 2019, 24, 4247. https://doi.org/10.3390/molecules24234247
Petrucci R, Chiarotto I, Mattiello L, Passeri D, Rossi M, Zollo G, Feroci M. Graphene Oxide: A Smart (Starting) Material for Natural Methylxanthines Adsorption and Detection. Molecules. 2019; 24(23):4247. https://doi.org/10.3390/molecules24234247
Chicago/Turabian StylePetrucci, Rita, Isabella Chiarotto, Leonardo Mattiello, Daniele Passeri, Marco Rossi, Giuseppe Zollo, and Marta Feroci. 2019. "Graphene Oxide: A Smart (Starting) Material for Natural Methylxanthines Adsorption and Detection" Molecules 24, no. 23: 4247. https://doi.org/10.3390/molecules24234247
APA StylePetrucci, R., Chiarotto, I., Mattiello, L., Passeri, D., Rossi, M., Zollo, G., & Feroci, M. (2019). Graphene Oxide: A Smart (Starting) Material for Natural Methylxanthines Adsorption and Detection. Molecules, 24(23), 4247. https://doi.org/10.3390/molecules24234247











