Abstract
A halogen-bonded complex formed between methane and chlorine monofluoride has been isolated in the gas phase before the reaction between the components and has been characterised through its rotational spectrum, which is of the symmetric-top type but only exhibits K = 0 type transitions at the low effective temperature of the pulsed-jet experiment. Spectroscopic constants for two low-lying states that result from internal rotation of the CH4 subunit were detected for each of the two isotopic varieties H4C···35ClF and H4C···37ClF and were analysed to show that ClF lies on the symmetry axis with Cl located closer than F to the C atom, at the distance r0(C···Cl) Å and with an intermolecular stretching force constant kσ 4 N m−1. Ab initio calculations at the explicitly correlated level CCSD(T)(F12c)/cc-pVTZ-F12 show that in the equilibrium geometry, the ClF molecule lies along a C3 axis of CH4 and Cl is involved in a halogen bond. The Cl atom points at the nucleophilic region identified on the C3 axis, opposite the unique C–H bond and somewhere near the C atom and the tetrahedron face centre, with re(C···Cl) = 3.191 Å. Atoms-in-molecules (AIM) theory shows a bond critical point between Cl and C, confirming the presence of a halogen bond. The energy that is required to dissociate the complex from the equilibrium conformation into its CH4 and ClF components is only De 5 kJ mol−1. A likely path for the internal rotation of the CH4 subunit is identified by calculations at the same level of theory, which also provide the variation of the energy of the system as a function of the motion along that path. The barrier to the motion along the path is only 20 cm−1.
1. Introduction
It was established, by a comparison of several series of complexes of the type B···HCl and B···ClF, where B is a Lewis base, that a close parallelism exists among the angular geometries of the hydrogen-bonded and halogen-bonded species for a given Lewis base B [1,2]. This parallelism can be readily rationalised by considering the following set of rules for predicting angular geometries:
The equilibrium angular geometry of a hydrogen-bonded complex B···HX or a halogen-bonded complex B···XY can be predicted by assuming that the internuclear axis of HX or XY lies either:
- (1)
- Along the axis of a non-bonding electron (n) pair carried by the acceptor atom Z of B with the order of the atoms Z···δ+H–Xδ- or Z···δ+X–Yδ-, as appropriate, or
- (2)
- Alongthe local symmetry axis of a π- or pseudo-π pair if B carries no n pairs, with the order of the atoms as ⁎···δ+H–Xδ- or ⁎···δ+X–Yδ-, where ⁎ lies on the π-pair axis or
- (3)
- Alongthe axis of a n-pair when B carries both n- and π- electron pairs.
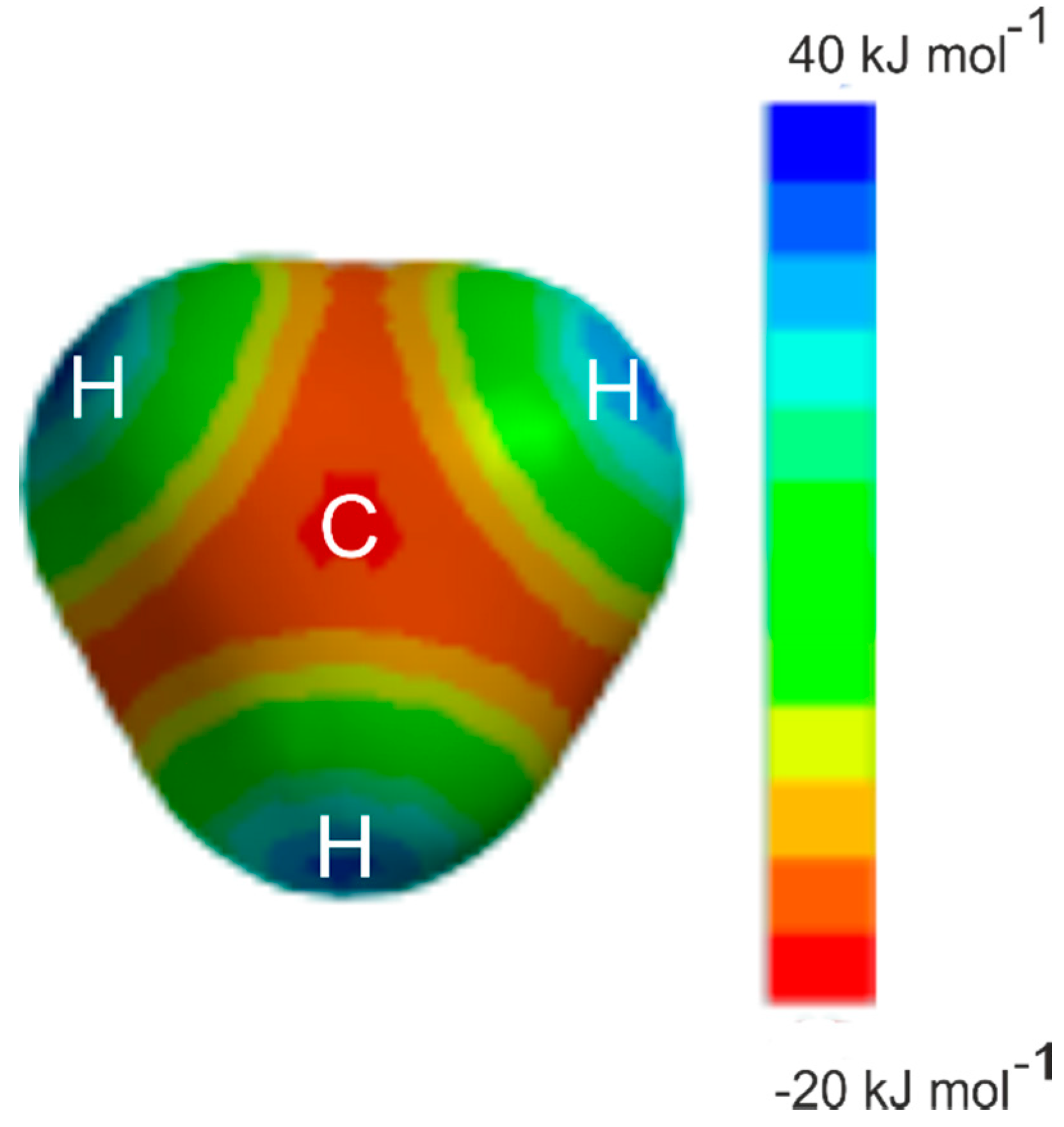
Examples for which the angular geometries are predicted by Rule 2 include several prototype hydrocarbons, for example, ethyne [3,4], ethene [5,6], allene [7,8], cyclopropane [9,10], and benzene [11,12]. The prototype alkane methane has no occupied π-pair orbitals or n-pair orbitals and hence, appears not to fall within the scope of the rules. Nevertheless, it was shown to form very weak complexes of the symmetric-top type with proton donors HX when X is either CN [13,14] or Cl [15,16] or Br [17], that is with the δ+H atom of HX forming a hydrogen bond to a face centre of the methane molecule with a very low potential energy barrier to the internal rotation of the CH4 subunit. Hydrogen fluoride was initially thought to form a complex with methane having a different type of geometry [15], but the rotational spectrum that led to this conclusion arose from H4C···H2O [18] generated from water in the tank in which the gases were mixed. H4C···HF has since been identified [19] through its rotational spectrum and shown to be isomorphous with the other H4C···HX. Evidently, the face centre of methane is a nucleophilic region, as may be seen by the molecular electrostatic surface potential (MESP) of methane shown in Figure 1. The MESP was calculated at the 0.001 e/bohr3 isosurface at the MP2/6-311++G** level of theory with the Spartan program [20]. The MESP calculated at the MP2(Full)/aug-cc-pVTZ level is given in the Supplementary Material and the results are very similar. It was shown some years ago [21] that HCN forms a hydrogen bond to the centre of the face of ethane to give a complex of C3v symmetry. The region near to C, along a C3 axis and opposite a C–H bond in methane or a C–C bond in ethane, is an example of a local maximum in electrostatic potential which is the site for potential electrophilic attack, which could accept a hydrogen bond [22]. On the other hand, the region just outside each H and along a C–H bond direction in methane is clearly electrophilic (positive, blue) and is an example of what is called a σ hole [23]. Raghavendra and Arunan reported atoms-in-molecules (AIM) theory [24] results on H4C···HX (X = F/Cl/OH/SH) complexes and showed that bond critical points were observed for both geometries, showing that CH4 can be both a hydrogen bond donor and acceptor [25].
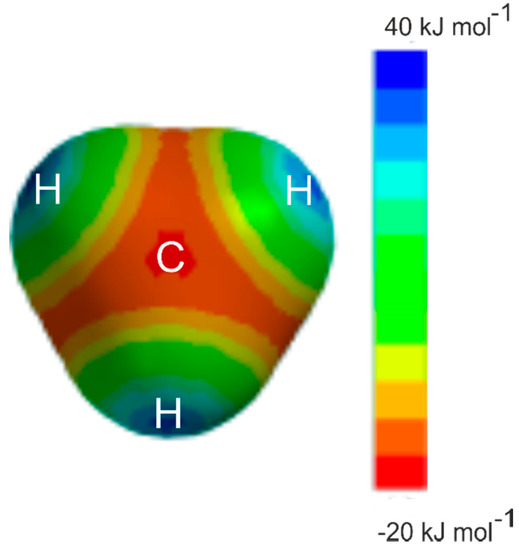
Figure 1.
The molecular electrostatic surface potential of CH4 at the 0.001 e/bohr3 isodensity surface, as calculated at the MP2/6-311++G** level of theory. The view is along a C3 axis of the molecule. The deepest red region along this axis is at the face centre (almost exactly beneath the C symbol), indicating a nucleophilic (negative, −20 kJ mol−1) region of electrostatic potential energy. There are clearly paths to the other three equivalent regions that are only slightly less nucleophilic (orange, −15 kJ mol−1) than the face centre region. The blue areas indicate electrophilic regions known as sigma holes (positive +40 kJ mol−1).
Given the recent growth of interest in the halogen bond and the parallelism of B···HCl and B···ClF angular geometries already referred to, it is of interest to investigate whether methane forms a halogen-bonded complex with ClF that is isomorphous with H4C···HCl. In this article, we report such an investigation conducted by rotational spectroscopy and ab initio calculations. Computational results on the isomer CH4···FCl geometry, having a C-H···F hydrogen bond, have been included for comparison.
2. Experimental and Theoretical Methods
The rotational spectrum of a complex of methane with chlorine monofluoride was observed with a pulsed-jet, Fourier-transform microwave spectrometer of the Balle-Flygare type [26,27], but was modified to include a fast-mixing nozzle [28] to generate the complexes, thereby avoiding a reaction between methane and ClF. A mixture composed of approximately 1% to 2% ClF in argon and held at a stagnation pressure of 3 bar was pulsed via a Series 9 solenoid valve (Parker-Hannifin Corp.Cleveland, ohio, USA.) down the outer of the two concentric, nearly coterminal tubes that form the mixing nozzle. The gas pulse emerging from this nozzle into the evacuated Fabry-Perot cavity of the spectrometer encountered methane that flowed continuously through the inner glass tube of the mixing nozzle into the cavity and complexes H4C···ClF were formed at the cylindrical interface of the two gas flows. The methane flow rate was adjusted to give a nominal background pressure of approximately 4 × 10−4 mbar at room temperature. When the complexes were between the Fabry-Perot mirrors, they were rotationally polarized by suitably timed pulses of microwave radiation of 1.1 μs duration. The subsequent free-induction decay at rotational transition frequencies was collected, Fourier-transformed, and processed in the usual way. Individual nuclear quadrupole hyperfine components (full-width at a half-height of approximately 15–20 kHz) in the rotational spectrum of H4C···ClF arising from the presence of Cl nuclei were well resolved and could be measured with an estimated accuracy of 2 kHz. Rotational transitions of both isotopologues H4C···35ClF and H4C···37ClF were readily observed in natural abundance. Unfortunately, flowing the methane through the outer component of the nozzle continuously consumed too much sample to allow expensive isotopically substituted methane samples to be used. The ClF was prepared and purified by the method of Shack and Wilson [29] while natural gas was used, without purification, as the source of methane.
Ab initio geometry optimisations for H4C···ClF, H4C···HCl and CH4···FCl were carried out at the CCSD(T)(F12c) explicitly correlated level of theory [30] with appropriately optimised cc-pVTZ-F12 basis functions [31] by using the MOLPRO program [32]. The potential energy as a function of relative angular orientations of the two subunits (see later) was calculated by optimising all other geometrical parameters at a fixed orientation and repeating this procedure in suitable increments of the angle. The AIM analysis was performed using AIMALL software [33]. The wavefunction that was appropriate to the optimised geometry at the MP2/aug-cc-pVTZ level using the GAUSSIAN electronic structure package [34] was employed in the AIM analysis. Molecular electrostatic surface potentials (MESPs) were calculated at the 0.001 bohr/Å3 iso-surface by employing the MP2/6-311++G** level of theory in the SPARTAN electronic structure program [20] and at the MP2/aug-cc-pVTZ level using the GAUSSIAN electronic structure package [34] (see Supplementary Information).
3. Results
3.1. Determination of Spectroscopic Constants
The observed rotational spectra of the two isotopologues H4C···35ClF and H4C···37ClF had the form expected for a symmetric-top molecule in its vibrational ground state, with the quantum number K = 0 and a single Cl nucleus on the symmetric-top axis. Two J +1 ← J, K = 0 ← 0 rotational transitions, with J = 1 and 2, were identified for each isotopologue within the frequency range (6–20 GHz) of the spectrometer. Each transition exhibited the characteristic quadrupole hyperfine structure arising from a Cl nucleus (spin quantum number I = 3/2) located on the top axis. In fact, for each J +1 ← J, K = 0 ← 0 transition, two almost identical hyperfine patterns were detected close together in frequency compared with the spacing between the two consecutive J transitions that were investigated. The two groups of hyperfine transitions of a given J were assigned to states with the symmetry labels A and F, where the nomenclature in use is that introduced by Ohshima and Endo [16] in their analysis of the similar spectrum of H4C···HCl. These authors treated the coupling of the angular momentum arising from the internal rotation of the CH4 subunit with that of the rotation of the whole molecular framework. They thereby produced an energy level diagram which revealed the correlation between the free-rotor states j = 0, 1, and 2 of CH4 and various states of the complex as a function of the barrier hindering the free rotation. For H4C···HCl, the population at the end of the supersonic expansion appears to be limited to the three lowest internal-rotation states. These states were predicted to belong to symmetry species, A, E, and F, and were shown to have relative intensities of 9:2:5, respectively, when nuclear spin statistical effects were included. The A and F state transitions correspond to the K = 0 components while the E state corresponds to K = 1 for H4C···HCl. The E state transitions were readily distinguished by their characteristic K = 1 type of Cl-nuclear quadrupole hyperfine structure.
In the case of H4C···ClF, a similar behaviour might be expected. The two sets of transitions that were observed for each J for H4C···ClF are of the K = 0 type, as indicated by the nuclear quadrupole hyperfine structure of the J = 2 ← 1 and J = 3 ← 2 transitions, hence the labels A and F that are assigned. Presumably, the different nozzle type used here, with different conditions compared with those used for H4C···HCl in references 15 and 16, led to a lower effective temperature of the expansion and this coupled with the smaller statistical weight of the E state and a somewhat weaker spectrum precluded the observation of the E (K = 1) state group of transitions. The observed frequencies of the hyperfine components in the J = 2 ← 1 and 3 ← 2 transitions for the 35Cl and 37Cl isotopologues are available in Table S1 as Supplementary Material. The residuals from the final cycles of the fits described below are also given in Table S1.
Observed frequencies of nuclear quadrupole components of the J = 2 ← 1 and 3 ← 2 transitions were fitted in an iterative, least-squares analysis using the Pickett’s SPFIT program [35]. The Hamiltonian that was chosen had the form
in which HR is the familiar Hamiltonian for a semi-rigid, symmetric-rotor molecule in its vibrational ground state. The second term is the energy operator for the interaction of the Cl nuclear electric quadrupole moment Q(Cl) with the electric field gradient ∇E(Cl) at the Cl nucleus. The third term of Equation (1) accounts for the coupling of the F nuclear spin angular momentum IF to J by means of the magnetic spin-rotation mechanism, where M(F) is the fluorine spin-rotation coupling tensor. The contributions of Cl spin-rotation coupling to transition frequencies can be ignored because the magnetic moment of Cl is 3.2 times smaller than that of F and even the F spin-rotation effect is only just detectable. The four protons of CH4 have magnetic moments of a similar magnitude to that of F and lead to a very complicated, largely unresolved hyperfine structure which manifests itself as line broadening and shape distortion on some of the Cl nuclear quadrupole components.
H = HR − 1/6 Q(Cl):∇E(Cl) + IF M(F)J
The matrix of H was constructed in the ICl + J = F1; F1 + IF = F coupled basis and diagonalized in blocks of F. For a vibrational ground-state, symmetric-top molecule in a K = 0 state, only the components χaa(Cl) = eQ(Cl) ∂2V(Cl)/∂a2 and Mbb(F) = Mcc(F) of the two coupling tensors are determinable. Only the quartic centrifugal distortion constant DJ was necessary to produce a fit with an R.M.S. error σ similar to the estimated error (2 kHz) of the frequency measurement. The observable spectroscopic constants that were determined for the two investigated isotopologues of H4C···ClF are given in Table 1, along with their standard errors, which were obtained using Kisiel’s PIFORM program [36]. Residuals of the fits are in Table S1.

Table 1.
Determined spectroscopic constants of H4C···35ClF and H4C···37ClF.
3.2. The Equilibrium Geometry of H4C···ClF and Variation of the Potential Energy Function with Internal Rotation of the CH4 Subunit
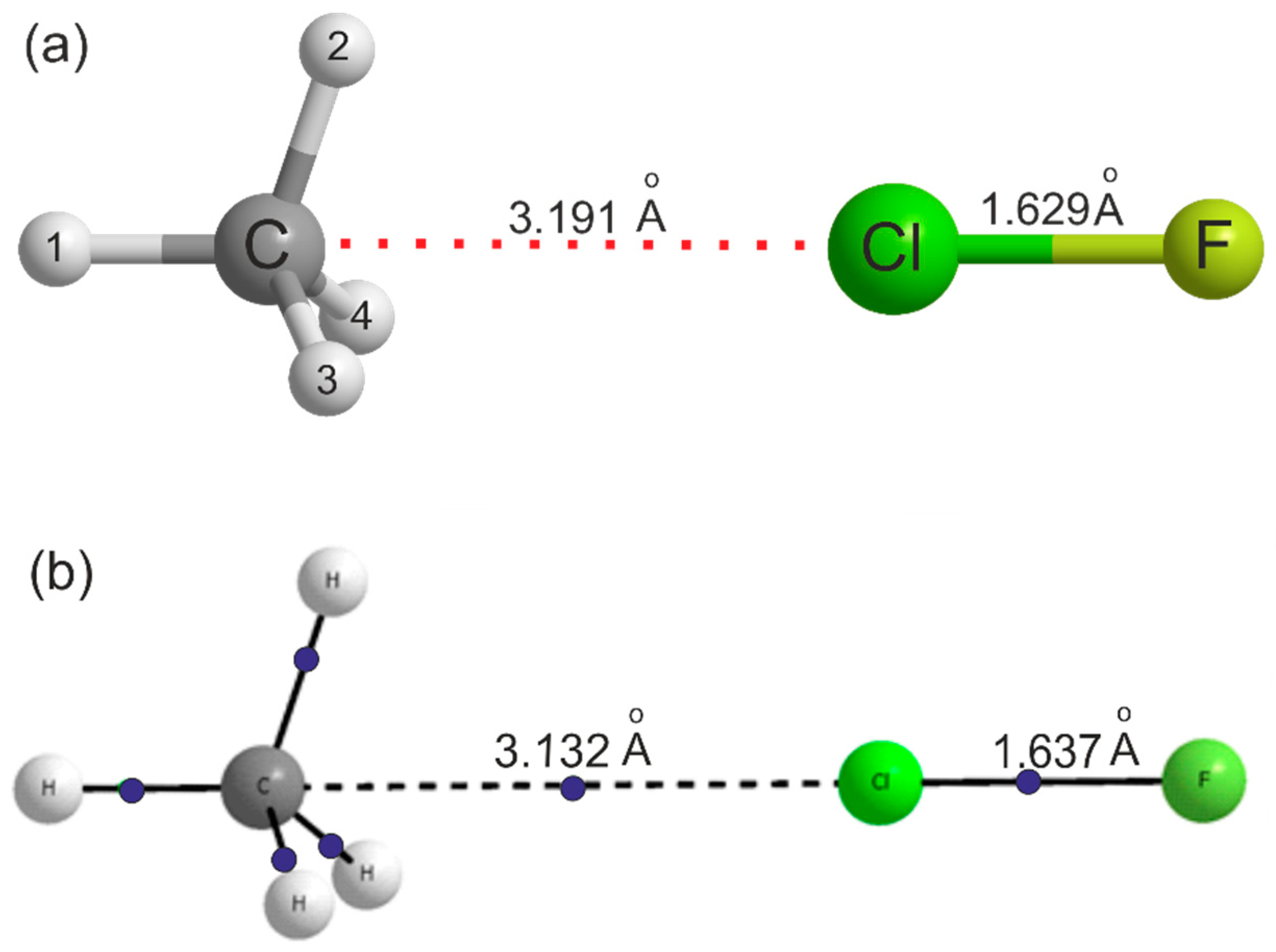
For the reason already given, no isotopic substitution in the methane subunit was possible in this study. The main question of interest in H4C···ClF is whether the ClF molecule forms a halogen bond to the centre of a face of the methane molecule. 13C substitution is unhelpful in that respect and although a single D substitution in the methane subunit in the case of H4C···HCl [15] gave a hint of a low barrier to internal rotation of CH4, no firm conclusion was possible. Ab initio calculations at the CCSD(T)(F12c)/cc-pVTZ-F12 level of theory reported here reveal that the equilibrium geometry of H4C···ClF is as shown in Figure 2a, that is with Cl forming a halogen bond to the centre of a face of the methane molecule.
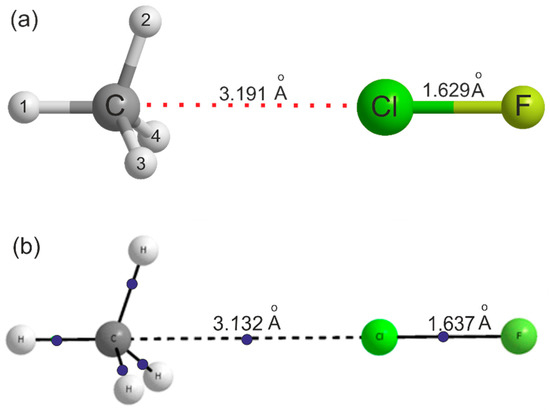
Figure 2.
(a) The symmetry and geometry of the H4C···ClF complex as optimised ab initio at the CCSD(T)(F12c)/cc-pVTZ-F12 level of theory. Further bond lengths and angles are in Table 2. (b) The result of an atoms-in-molecules (AIM) calculation conducted on the geometry optimised at the MP2/Aug-cc-pVTZ level using the GAUSSIAN electronic structure package. Bond critical points are indicated by blue dots. The important distances in the complexes calculated at the two levels are shown in the structure and indicate that the results are similar. Note that there is a bond critical point between the CH4 face centre and the Cl atom.
Full details of the geometries of H4C···ClF, CH4 and ClF optimised at this level of theory are given in Table 2. Note that for H4C···ClF, there are two optimised geometries listed in Table 2. In column A, the angle ∠H1–C···Cl was fixed at 180.00°, but in column B, the angle was released and optimised at 180.39° (see Supplementary Material for the MOLPRO output files). The deviation from 180° in column B results from use of an incomplete basis set. The following discussion refers to the results in column A. The energy difference between the two geometries is 5.0 kJ mol−1, which, as expected, is very small. The changes in the methane geometry on complex formations are extremely small, with increases of only 0.001 Å in the length of the three equivalent C–H bonds nearest to the Cl atom and 0.2° in the three equivalent HCH angles. The Cl–F bond lengthens by 0.0036 Å. The equilibrium dissociation energy calculated at the CCSD(T)(F12c)/cc-pVTZ-F12 level is De = 5.5 kJ mol-1 and at the MP2/aug-cc-pVTZ level is De = 5.4 kJ mol−1, after BSSE correction, thereby confirming that the complex is very weakly bound. The latter value reduces to D0 = 4.4 kJ mol−1 after zero-point energy corrections. For comparison, the corresponding De value and ClF bond lengthening for the van der Waals molecule Ar···ClF are 3.0 kJ mol−1 and 0.001 Å, respectively, when calculated at the CCSD(T)(F12c)/cc-pVTZ-F12 level. Figure 2b shows the result of the AIM calculation at the MP2(Full)/Aug-cc-pVTZ level, with bond critical points (bcp) indicated by blue dots. Although the AIM calculations were done at different levels, comparison of the dissociation energy and geometry at these two levels indicate that the major conclusions presented here would not change with the level of calculations. There is a bcp between the C atom in methane through the face centre and the Cl atom in ClF. The electron density ρ and the Laplacian of the electron density at this bcp are 0.0085 au and +0.0411 au, respectively, thereby confirming the presence of the halogen bond. These may be compared to the values 0.0107 au and +0.0407 au, respectively, for H4C···HCl [24]. All the AIM parameters for H4C…ClF and H4C…HCl complexes are given in Table S2 in the Supplementary Material. For comparison, results for the CH4…FCl geometry having a C-H…F hydrogen bond are included as well. The electron density at the bcp for this geometry is slightly smaller than that for the halogen-bonded geometry, 0.0071 au, indicating that the hydrogen bonded complex is weaker than the halogen-bonded geometry that was observed in the experiments. The BSSE corrected interaction energy for this structure is −0.9 kJ mol−1 and this structure is not stable when zero-point vibrational energy corrections are taken into account (BSSE+ZP corrected energy is +1.0 kJ mol−1). All AIM parameters indicate that the interactions in all these complexes are closed shell in nature and are comparable to other halogen/hydrogen bonded complexes [24].

Table 2.
Ab initio equilibrium geometries of H4C···ClF, CH4, and ClF optimised at the CCSD(T)(F12c)/cc-pVTZ-F12 level of theory.
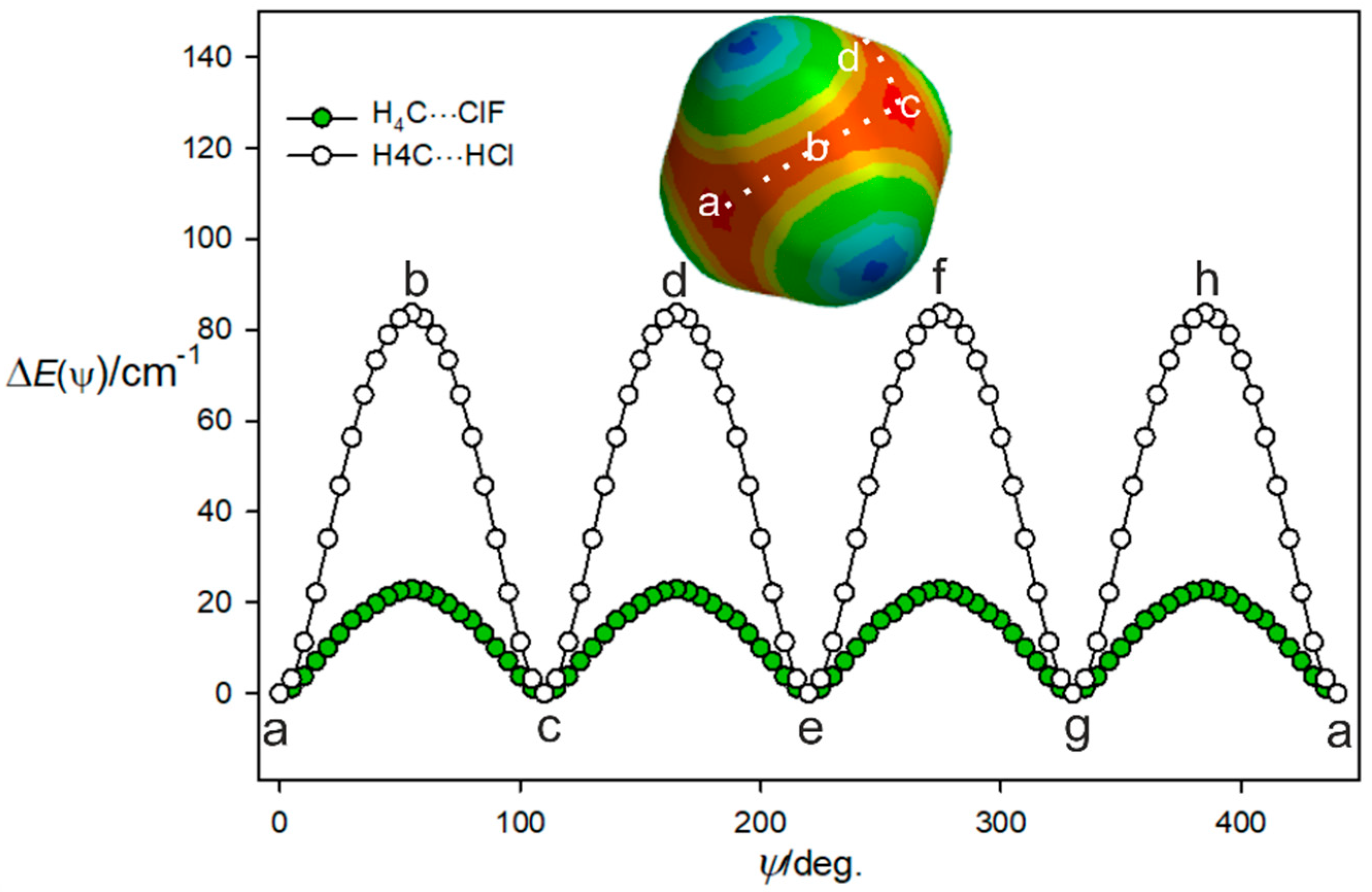
Given the weakness of the interaction of the ClF and CH4 molecules and the fact that methane has four equivalent face centres, it is likely that there is a low barrier to internal rotation of the CH4 subunit between the four equivalent equilibrium positions. This can be illustrated by the potential energy as a function of the path shown as a dotted white line on the MESP diagram of methane in Figure 3 and described by an angle ψ. The angle ψ is 0° when the ClF molecules lies along the C3 axis at position (a) in Figure 3 and the Cl atom is involved in a halogen bond to that face centre. The angle ψ was then increased in 5° steps by rotating the CH4 subunit, with the C, Cl, and F nuclei constrained to be collinear at each step and to lie in the plane that contains the initial C3 axis at position (a), a C2 axis at position (b), and the next C3 axis at position (c). The geometry and energy E(ψ) were calculated at each step by optimisation of all parameters other than ψ. This path is, thus, along the centre of the red nucleophilic region in the MESP of methane, which is included in Figure 3. The angle ψ = 54.74° corresponds to ClF lying along a C2 axis of the methane subunit at position (b), where Cl is looking at the tetrahedron edge centre and lies on the C2 axis. When the ClF molecule is in position (c) and therefore, lies along the next C3 axis, the angle ψ is 109.47°. The CH4 subunit and path then turn, as indicated, to follow the next (identical) nucleophilic routes (c), (d), (e), and so on until all four most nucleophilic centres are sampled. There is clearly a very low potential energy barrier at the centre of each tetrahedron edge along the path. In the complex, the internal motion of the CH4 subunit is likely to be constrained mainly to describe the path (a), (b), (c), (d), (e), etc., illustrated in Figure 3 because it is the most nucleophilic route.
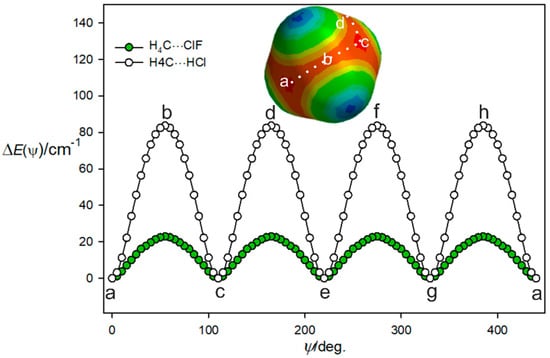
Figure 3.
Variation of the energy of H4C···ClF with the angular orientation ψ of the two subunits that follows the lowest energy path a, b, c, etc. between the four equivalent minima. See the text for a definition of the angle ψ.
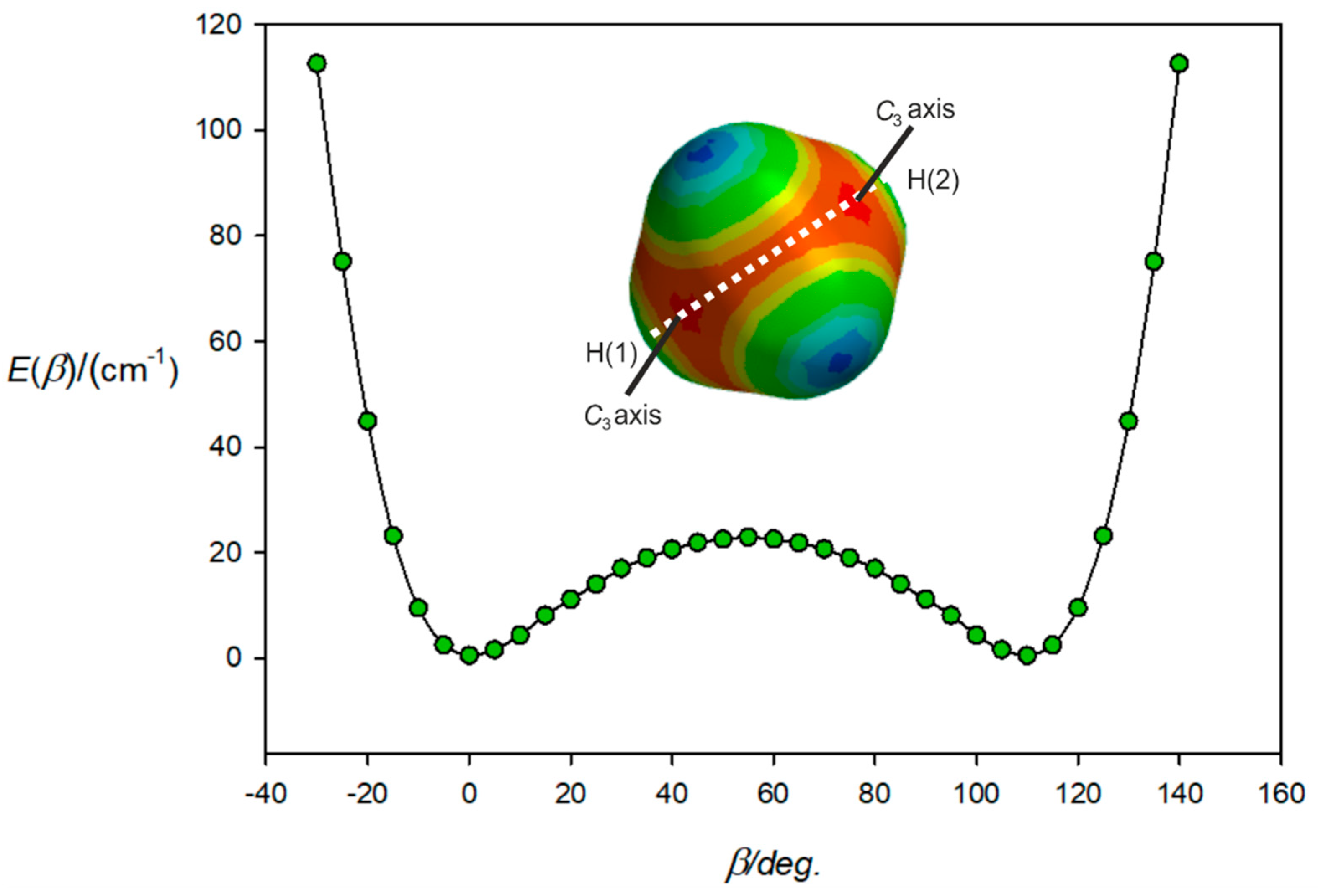
Figure 4 shows the result of the calculations (with the same constraints of collinearity, but this time defining the angle as β) when the path is confined to the H(1)-(a)-(c)-H(2) plane and starts near to one (electrophilic, blue) hydrogen atom H(1), passes through one face centre, through a tetrahedron edge, through another face centre, and finishes near H(2). Clearly it is the high electrophilicity (and therefore, potential energy) near the H atoms that acts to confine the path indicated in Figure 3.
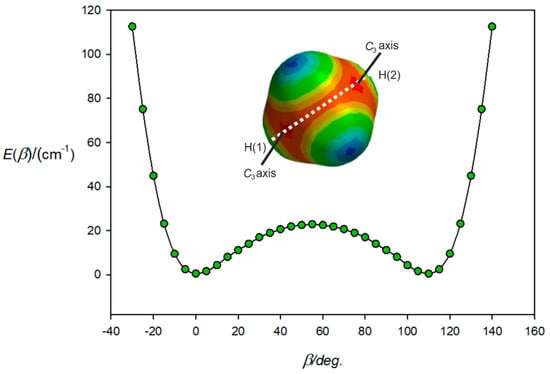
Figure 4.
Variation of the energy of H4C···ClF with the angle β made by the ClF subunit with the C atom of CH4 and when ClF lies in the H(1)-C-H(2) plane. At β = 0°, ClF lies along a C3 axis and at β = 54.7°, lies along the C2 axis associated with the edge between atoms H(3) and H(4). At β = 109.47°, ClF lies along the other C3 axis indicated. As β further increases, the energy rises until the H(2) atom is encountered.
The same procedure as used to produce the potential function for H4C···ClF in Figure 3 was followed for H4C···HCl, with the calculations again conducted at the CCSD(T)(F12c)/cc-pVTZ-F12 level. The result is included in Figure 3, which shows that the barrier to the motion described is about four times larger than that established for H4C···ClF. Presumably, the smaller size of the H atom in HCl means that it can more closely approach each nucleophilic region that is near to the face centre of methane and opposite a C–H bond. Moreover, at point b, the negative region around the Cl atom could have attractive interaction with the positive potential around the H atoms, stabilizing this orientation, also leading to a reduction in the barrier. This is confirmed by NBO calculations, which show the overlap between the lone pairs of electrons in the Cl atom with the C-H anti-bonding orbitals in CH4, leading to more stabilization compared to that of the HCl complex. These results are presented in the Supplementary Material.
Figure 4 in the paper by Ohshima and Endo [16] shows the correlation between the energy of the free rotor states j = 0, 1, and 2 of CH4 and the energies of various states of the complex H4C···HCl as a function of the barrier hindering the free rotation of the CH4 subunit. Examination of this figure suggests that the lower barrier that exists for H4C···ClF is likely to lead to higher energies for the K = 1, E, and F states that correlate with the j = 1 and 2 free-rotor states of methane, respectively, than exist for H4C···HCl. This is probably another reason why a careful search failed to reveal rotational transitions in K = 1 states for H4C···ClF.
3.3. Interpretation of the Ground-State Spectroscopic Constant of H4C···ClF
Only zero-point spectroscopic constants were obtained from the rotational spectrum of H4C···ClF (we use the nomenclature “zero-point” and the subscript 0 for both the A and F states for convenience in this section because although the A state has the lower energy, both states must lie close together). These spectroscopic constants can be interpreted to provide a r0-type value of the C to Cl distance for each state and another measure of the binding strength, namely the intermolecular stretching force constant kσ, that is appropriate to the two lowest energy states of this complex. The fact that the difference in B0 of H4C···35ClF and H4C···37ClF is only 1.5 MHz shows that Cl lies close to the centre of mass of the complex and is the atom involved in the (halogen-bond) interaction.
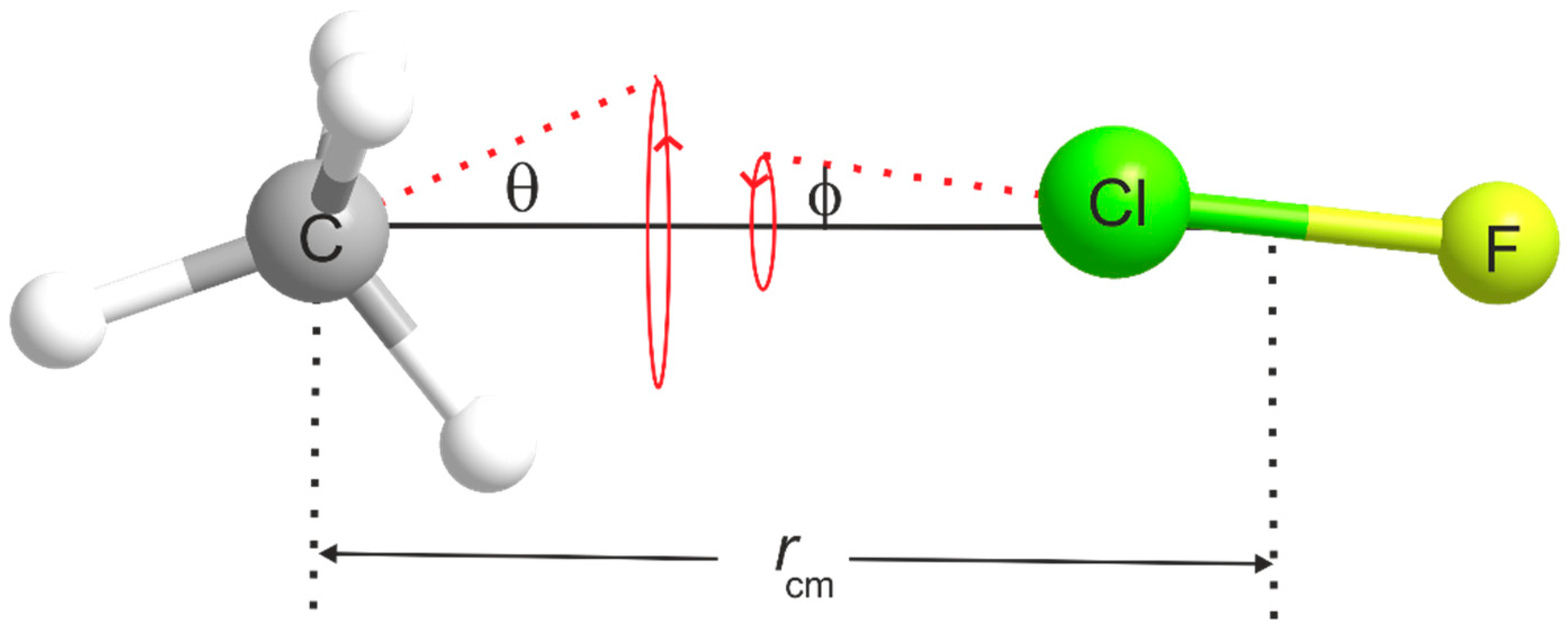
The most significant contributions to the difference between the equilibrium and zero-point moments of inertia of a weakly bound complex such as H4C···ClF originate in the effect of the intermolecular bending and stretching modes. The model [37] of the complex commonly used to allow for the contribution of the intermolecular bending modes is shown in Figure 5. The subunits CH4 and ClF are assumed to undergo the indicated angular oscillations about their mass centres, with amplitudes θ and respectively. Each oscillation is assumed to be two-dimensionally isotropic and the distance rcm between the mass centres is assumed to be fixed. This model, therefore, does not allow for intermolecular stretching. In Figure 5, θ and are the angles made by the C3 axis of CH4 and internuclear axis of ClF, respectively, with the line rcm. The model relates of the complex to the ground-state moments of inertia and of the two components according to
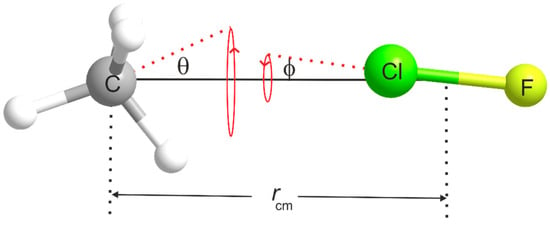
The term is necessarily independent of the angle θ because of the Td symmetry of methane. A value of ⟨⟩ can be estimated from the 35Cl nuclear quadrupole coupling constant because the observed value of this quantity is the projection of the free ClF molecule coupling constant [38] onto the a axis, averaged over the zero-point motion, i.e., . This is strictly correct only if the electric field gradient (efg) at the Cl nucleus is unchanged by the presence of the CH4 electric charge distribution nearby. The values for H4C···35ClF and H4C···37ClF that result are given in Table 3, while the required values of [38] are in Table 4. The values are likely to be good estimates given that the octupole moment is the first non-zero moment of the methane electric charge distribution. Thus, will not be significantly changed by any modification of the efg at Cl due to the presence of methane. When is used in Equation (2) together with the appropriate zero-point moments of inertia calculated from the free molecule ground-state rotational constants of CH4 [39] and ClF [40] given in Table 4, the distance rcm between the centres of mass of CH4 and ClF and the distance r(C···Cl) = rcm − r can be calculated, where r is the distance from Cl to the ClF mass centre. The results of this procedure are collected in Table 3 for the A and F states of both H4C···35ClF and H4C···37ClF. We note that although r(C···Cl) is isotopically invariant within each state, it is considerably longer in the F state than in the A state.

Table 3.
Distances rcm and r(C···Cl), oscillation amplitudes , and intermolecular stretching force constants kσ determined for H4C···ClF a.

Table 4.
Some properties of CH4 and ClF.
The intermolecular quadratic stretching force constant kσ provides a further measure of the strength of the interaction of the subunits CH4 and ClF. It is the restoring force for unit infinitesimal displacement along the weak bond direction (the C3 axis of CH4 in this case). When terms higher than quadratic in the potential energy function are neglected and when the components are assumed rigid and unperturbed by complex formation, Millen [41] showed that kσ is related to the centrifugal distortion constant DJ of an axially symmetric complex such as H4C···ClF by
where is a reduced mass. The rotational constants B, BCH4, and BClF in Equation (3) should be equilibrium values for the complex, CH4 and ClF, respectively, as should the centrifugal distortion constant DJ. In the absence of the equilibrium quantities, the corresponding zero-point values from Table 1 and Table 4 were used. The results from Equation (3) are included in Table 4 and we note that within the experimental error generated from the observed DJ values, the kσ are invariant when 35Cl is substituted by 37Cl within a given state. However, there is clearly a significant change between the A and F states, implying that the F state is more strongly bound than the A state. This observation appears to be in conflict with a distance r(C···Cl) that is longer by 0.02 Å in the F state (see Table 3), but this conflict probably arises from vibration-rotation coupling resulting from the low barrier to internal rotation of the CH4 subunit (see Section 3.2). Nevertheless, the values of kσ are very small, a result that is consistent with the small value of the dissociation De obtained from ab initio calculations.
4. Conclusions
Ab initio calculations at the CCSD(T)(F12c)/cc-pVTZ-F12 level of theory have shown that methane and chlorine monofluoride form a weak complex in which the ClF molecule lies along a C3 axis of methane, with the electrophilic Cl atom interacting with the most nucleophilic region of methane, namely a region opposite a C–H bond, along a C3 axis and somewhere between C and a face centre of the tetrahedron. The four identical energy-minimum positions are linked by a relatively low energy path, as indicated by the molecular electrostatic surface potential calculated at the MP2/6-311++G** level, which is in close agreement with the result of calculations at the MP2/aug-cc-pVTZ level (available in the Supplementary Material). The AIM calculations at the latter level show that there is a bond critical point between C and Cl, confirming the presence of the halogen bond. Calculations of the energy of the complex along the low-energy paths between the minima indicate that the barrier presented to the internal rotation of the CH4 subunit between the four equivalent minima is only about 20 cm−1., which is four times smaller than that similarly calculated for the isomorphous hydrogen-bonded complex H4C···HCl. The equilibrium dissociation energy De of the complex is only 5 kJ mol−1. There is clearly a halogen bond linking the two molecules in this complex, albeit a very weak one.
The rotational spectra of the two isotopologues H4C···35ClF and H4C···37ClF that were observed in a pulsed jet at temperatures of approximately 2 or 3 K by using a fast-mixing nozzle (to arrest any reaction between the two components) confirm that the prototype alkane molecule methane does indeed form complexes with chlorine monofluoride in which the Cl atom is closer to the C atom than is the F atom. Moreover, the fact that the rotational spectrum can be detected in two low-lying energy states (labelled A and F) that almost certainly arise from the internal rotation of CH4 between the four equivalent minima is consistent with the potential energy function calculated ab initio. As expected, the r0-type bond length determined from the rotational constants of the A and F states are longer than the equilibrium quantity calculated ab initio. Small experimental values of the intermolecular stretching force constant are consistent with the small ab initio values of De.
Complexes in which an HX molecule forms a hydrogen bond to a face centre of methane were identified as long ago as the early 1990s for X = HCN [13,14], HCl [15,16], and HBr [17]. The work by Ohshima and Endo [16] concerning the internal rotation of the CH4 subunit in H4C···HCl was important in establishing this conclusion, as was the investigation of C2H6···HCN [21], which demonstrated that HCN forms a hydrogen bond to the centre of the face of a CH3 group in a situation where a facile internal rotation of ethane between face centres was unlikely. Atoms-in-Molecules theory [24] applied to H4C···HX (X = F/Cl/OH/SH) complexes by Raghavendra and Arunan [25] showed that bond critical points exist between H of HX and C of CH4. AIM calculations reported here also reveal a similarly placed bond critical point in the isomorphous complex H4C···ClF. A question of general interest concerns nomenclature in the context of recent definitions of the hydrogen bond [42] and the halogen bond [43]. The nucleophilic region somewhere between C and a face centre of methane can interact with the electrophilic region near H or Cl in forming H4C···HCl or H4C··· ClF, respectively [23]. In reference [25], the H4C···HX (X = F/Cl/OH/SH) were referred to as stabilised by hydrogen bonds to carbon. The geometries of all H4C···HX and H4C···ClF that have been investigated so far show that three of the H atoms of CH4 are closer to H in HX or to the Cl in ClF than to C and therefore, that such structures could possibly be classified as trifurcated hydrogen bonds instead of hydrogen or halogen bonds if one did not look at the electron density. Recently, however, Frontera and co-workers [44] analysed CH3···O contacts in proteins and concluded that these were stabilized by carbon bonds [45] to O and are not trifurcated H bonds.
Supplementary Materials
The following are available online. Figure S1: Electrostatic potential of CH4 calculated at MP2/aug-cc-pVTZ level; Figure S2: Optimized geometry of the CH4…FCl hydrogen bonded geometry, including the bond critical point; Table S1: Rotational transitions for the title complex and their fit; Table S2: AIM parameters for the H4C…ClF, H4C…HCl and CH4…FCl complexes; Table S3: Cartesian coordinates for the optimized geometries of the three complexes; Table S4: Rotational constants for the H4C…ClF and CH4…FCl complexes; Table S5 and S6: NBO results for H4C…ClF and CH4…HCl complexes.
Author Contributions
A.C.L. (Implementation of project, experiments, supervision, ab initio calculations, writing); D.G.L. (Spectroscopic analysis); J.H.H. (Preparation of ClF sample); D.M. (ab initio and AIM calculations); E.A. (Ab initio and AIM calculation, writing, supervision).
Funding
This research was funded by the Engineering and Physical Sciences Research Council of the UK and the Indo-French Centre for Promotion of Advanced Scientific Research.
Acknowledgments
ACL thanks the EPSRC for a Senior Fellowship (1997–2002), during which the experimental work described here was carried out at the University of Exeter. ACL also thanks the University of Bristol for a current Senior Research Fellowship during which the CCSD(T)F12c calculations were executed. EA and DM acknowledge funding from the Indo-French Centre for Promotion of Advanced Scientific Research from 2013–16 for the AIM computational work. The authors thank one reviewer for pointing out an additional interaction that could explain the difference in barrier for CH4 internal rotation between ClF and HCl complexes, which is shown in Figure 3.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Legon, A.C.; Millen, D.J. Determination of properties of hydrogen-bonded dimers by rotational spectroscopy and a classification of dimer geometries. Faraday Discuss. Chem. Soc. 1982, 73, 71–87. [Google Scholar] [CrossRef]
- Legon, A.C. Pre-reactive complexes of dihalogens XY with Lewis bases B in the gas phase: A systematic case for the ‘halogen’ analogue B⋯XY of the hydrogen bond B⋯HX. Angew. Chem. Int. Ed. Engl. 1999, 38, 2686–2714. [Google Scholar] [CrossRef]
- Legon, A.C.; Aldrich, P.D.; Flygare, W.H. The rotational spectrum and molecular structure of the acetylene⋯HCl dimer. J. Chem. Phys. 1981, 75, 625–630. [Google Scholar] [CrossRef]
- Hinds, K.; Holloway, J.H.; Legon, A.C. Evidence for a pre-reactive intermediate in a gaseous mixture of ethyne and chlorine monofluoride. The rotational spectrum of the Mulliken bπ.aσ* complex C2H2⋯ClF. J. Chem. Soc. Faraday Trans. 1996, 92, 1291–1296. [Google Scholar] [CrossRef]
- Aldrich, P.D.; Legon, A.C.; Flygare, W.H. The rotational spectrum, structure and molecular properties of the ethylene⋯HCl dimer. J. Chem. Phys. 1981, 75, 2126–2134. [Google Scholar] [CrossRef]
- Bloemink, H.I.; Holloway, J.H.; Legon, A.C. Geometry and nature of the binding of the pre-reactive complex C2H4⋯ClF from its rotational spectrum. Chem. Phys. Lett. 1996, 250, 567–575. [Google Scholar] [CrossRef]
- Legon, A.C.; Willoughby, L.C. Detection and characterisation of a weakly bound dimer of allene and hydrogen fluoride by rotational spectroscopy. Chem. Phys. Lett. 1988, 143, 214–220. [Google Scholar] [CrossRef]
- Cooke, S.A.; Holloway, J.H.; Legon, A.C. The rotational spectrum and angular geometry of a pre-reactive complex of allene and chlorine monofluoride. Chem. Phys. Lett. 1997, 266, 61–69. [Google Scholar] [CrossRef]
- Legon, A.C.; Aldrich, P.D.; Flygare, W.H. The rotational spectrum, chlorine nuclear quadrupole coupling constants, and molecular geometry of a hydrogen-bonded dimer of cyclopropane and hydrogen chloride. J. Am. Chem. Soc. 1982, 104, 1486–1490. [Google Scholar] [CrossRef]
- Hinds, K.; Holloway, J.H.; Legon, A.C. A pseudo-π analogue of a Mulliken bπ.aσ* type complex: The rotational spectrum of cyclopropane-chlorine monofluoride. J. Chem. Soc. Faraday Trans. 1997, 93, 373–378. [Google Scholar] [CrossRef]
- Read, W.G.; Campbell, E.J.; Henderson, G. The rotational spectrum and molecular structure of the benzene-hydrogen chloride complex. J. Chem. Phys. 1983, 78, 3501–3506. [Google Scholar] [CrossRef]
- Cooke, S.A.; Evans, C.M.; Holloway, J.H.; Legon, A.C. Interactions of benzene and halogens in the gas phase: Rotational spectrum of C6H6⋯ClF. J. Chem. Soc. Faraday Trans. 1998, 94, 2295–2302. [Google Scholar] [CrossRef]
- Legon, A.C.; Wallwork, A.L. The pairwise interaction of methane with hydrogen cyanide: A surprising result from rotational spectroscopy. J. Chem. Soc. Chem. Commun. 1989, 588–589. [Google Scholar] [CrossRef]
- Legon, A.C.; Wallwork, A.L. Methane as a proton acceptor: Rotational spectrum and internal dynamics of a weakly bound dimer of methane and hydrogen cyanide. J. Chem. Soc. Faraday Trans. 1992, 88, 1–9. [Google Scholar] [CrossRef]
- Legon, A.C.; Roberts, B.P.; Wallwork, A.L. Rotational spectra and geometries of the gas-phase dimers (CH4, HF) and (CH4, HCl). Chem. Phys. Lett. 1990, 173, 107–114. [Google Scholar] [CrossRef]
- Ohshima, Y.; Endo, Y. Rotational spectrum and internal rotation of a methane–HCl complex. J. Chem. Phys. 1990, 93, 6256–6265. [Google Scholar] [CrossRef]
- Atkins, M.J.; Legon, A.C.; Wallwork, A.J. The pairwise interaction of methane and hydrogen bromide: Properties of the weakly bound dimer CH4⋯HBr from microwave spectroscopy. Chem. Phys. Lett. 1992, 192, 368–374. [Google Scholar] [CrossRef]
- Suenram, R.D.; Fraser, G.T.; Lovas, F.J.; Kawashima, Y.J. The microwave spectrum of CH4-H2O. Chem. Phys. 1994, 101, 7230–7272. [Google Scholar] [CrossRef]
- Legon, A.C.; Lister, D.G. Rotational spectrum of CH4···HF. in preparation.
- Deppmeier, B.J.; Driessen, A.J.; Hehre, T.S.; Hehre, W.J.; Johnson, J.A.; Klunzinger, P.E.; Leonard, J.M.; Pham, I.N.; Pietro, W.J.; Yu, J.; et al. SPARTAN’14 Mechanics Program, Release 1.1.8; Wavefunction Inc.; SPARTAN Inc.: Irvine, CA, USA, 2014. [Google Scholar]
- Legon, A.C.; Wallwork, A.L.; Warner, H.E. Do methyl groups form hydrogen bonds? An answer from the rotational spectrum of ethane-hydrogen cyanide. Chem. Phys. Lett. 1992, 191, 97–101. [Google Scholar] [CrossRef]
- Politzer, P.; Truhlar, D.G. (Eds.) Chemical Applications of Atomic and Molecular Electrostatic Potential; Plenum Press: New York, NY, USA, 1981. [Google Scholar]
- Clark, T.; Hennemann, M.; Murray, J.S.; Politzer, P.A. Halogen bonding: The σ-hole. J. Mol. Model. 2007, 13, 291–296. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Clarendon Press: Oxford, UK, 1990. [Google Scholar]
- Raghavendra, B.; Arunan, E. Hydrogen bonding with a hydrogen bond: The methane-water complex and penta-coordinate carbon. Chem. Phys. Lett. 2008, 467, 37–40. [Google Scholar] [CrossRef]
- Balle, T.J.; Flygare, W.H. Fabry–Perot cavity pulsed Fourier transform microwave spectrometer with a pulsed nozzle particle source. Rev. Sci. Instrum. 1981, 52, 33–45. [Google Scholar] [CrossRef]
- Legon, A.C. Fourier-Transform Microwave Spectroscopy, Chapter 9 in Atomic and Molecular Beam Methods; Scoles, G., Ed.; Oxford University Press: New York, NY, USA, 1992; Volume 2, pp. 289–308. [Google Scholar]
- Legon, A.C. Pre-reactive intermediates in gas-phase chemical reactions: A contribution from rotational spectroscopy. J. Chem. Soc. Chem. Commun. 1996, 109–116. [Google Scholar] [CrossRef]
- Schack, C.J.; Wilson, R.D. The Preparation of Chlorine Monofluoride, Synthesis and Reactivity. Inorg. Met. Org. Chem. 1973, 3, 393–396. [Google Scholar] [CrossRef]
- Knizia, G.; Adler, T.B.; Werner, H.-J. Simplified CCSD(T)-F12 methods: Theory and benchmarks. J. Chem. Phys. 2009, 130, 054104. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.A.; Adler, T.B.; Werner, H.-J. Systematically convergent basis sets for explicitly correlated wavefunctions: The atoms H, He, B–Ne, and Al–Ar. J. Chem. Phys. 2008, 128, 084102. [Google Scholar] [CrossRef]
- Werner, H.-J.; Knowles, P.J.; Knizia, G.; Manby, F.R.; Schütz, M.; Celani, P.; Györffy, W.; Kats, D.; Korona, T.; Lindh, R.; et al. MOLPRO, Version 2009.1, A Package of ab Initio Programs. Available online: http://www.molpro.net (accessed on 8 September 2019).
- Keith, T.A. AIMALL (Version 16.05.19), TK Gristmill Software, Overland Park, KS, USA. 2016. Available online: Aim.tkgristmill.com (accessed on 8 September 2019).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Pickett, H.M. The Fitting and Prediction of Vibration-Rotation Spectra with Spin Interaction. J. Mol. Spectrosc. 1991, 148, 371–377. [Google Scholar] [CrossRef]
- Kisiel, Z. The Program PIFORM. Available online: http://www.ifpan.edu.pl/~kisiel/prospe.htm (accessed on 8 September 2019).
- Fraser, G.T.; Leopold, K.R.; Nelson, D.D., Jr.; Tung, A.; Klemperer, W. The rotational spectrum and structure of NH3-HCN. J. Chem. Phys. 1984, 80, 3073–3077. [Google Scholar] [CrossRef]
- Fabricant, B.; Muenter, J.S. Molecular beam Zeeman effect and dipole moment sign of ClF. J. Chem. Phys. 1977, 66, 5274–5277. [Google Scholar] [CrossRef]
- Abe, M.; Iwakuni, K.; Okubo, S.; Sasada, H. Accurate transition frequency list of the ν3 band of methane from sub-Doppler resolution comb-referenced spectroscopy. J. Opt. Soc. Am. B 2013, 30, 1027–1035. [Google Scholar] [CrossRef]
- McGurk, J.C.; Norris, C.L.; Tigelaar, H.L.; Flygare, W.H. Molecular magnetic properties of FCl. J. Chem. Phys. 1973, 58, 3118–3126. [Google Scholar] [CrossRef]
- Millen, D.J. Determination of stretching force constants of weakly bound dimers from centrifugal distortion constants. Can. J. Chem. 1985, 63, 1477–1479. [Google Scholar] [CrossRef]
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; et al. Definition of the hydrogen bond (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 83, 1637–1641. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Ho, P.S.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.A.; Resnati, G.; Rissanen, K. Definition of the halogen bond (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Bazua, A.; Frontera, A. RCH3···O Interactions in Biological Systems: Are They Trifurcated H-Bonds or Noncovalent Carbon Bonds? Crystals 2016, 6, 26. [Google Scholar] [CrossRef]
- Mani, D.; Arunan, E. The X-C···Y (X = O/F, Y = O/S/F/Cl/Br/N/P) ‘carbon bond’ and hydrophobic interactions. Phys. Chem. Chem. Phys. 2013, 15, 14377–14383. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).