Anti-Cancer Activity of Porphyran and Carrageenan from Red Seaweeds
Abstract
:1. Introduction
2. Anti-Cancer Activity from Red Seaweeds
3. Porphyran
4. Carrageenan
5. Combination with Conventional Anti-Cancer Drugs
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Global Cancer Observatory. Available online: http://gco.iarc.fr/ (accessed on 18 November 2019).
- Senthilkumar, K.; Manivasagan, P.; Venkatesan, J.; Kim, S.-K. Brown seaweed fucoidan: Biological activity and apoptosis, growth signaling mechanism in cancer. Int. J. Biol. Macromol. 2013, 60, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Atashrazm, F.; Lowenthal, R.; Woods, G.; Holloway, A.; Dickinson, J. Fucoidan and Cancer: A Multifunctional Molecule with Anti-Tumor Potential. Mar. Drugs 2015, 13, 2327–2346. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Rodríguez, A.G.; Juárez-Portilla, C.; Olivares-Bañuelos, T.; Zepeda, R.C. Anticancer activity of seaweeds. Drug Discov. Today 2018, 23, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Dotan, E.; Aggarwal, C.; Smith, M.R. Impact of Rituximab (Rituxan) on the Treatment of B-Cell Non-Hodgkin’s Lymphoma. Pharm. Ther. 2010, 35, 148–157. [Google Scholar]
- Sithranga Boopathy, N.; Kathiresan, K. Anticancer Drugs from Marine Flora: An Overview. J. Oncol. 2010, 2010, 214186. [Google Scholar] [CrossRef] [PubMed]
- Appeltans, W.; Ahyong, S.T.; Anderson, G.; Angel, M.V.; Artois, T.; Bailly, N.; Bamber, R.; Barber, A.; Bartsch, I.; Berta, A.; et al. The Magnitude of Global Marine Species Diversity. Curr. Biol. 2012, 22, 2189–2202. [Google Scholar] [CrossRef] [PubMed]
- Jimeno, J.; Faircloth, G.; Sousa-Faro, J.F.; Scheuer, P.; Rinehart, K. New Marine Derived Anticancer Therapeutics ─ A Journey from the Sea to Clinical Trials. Mar. Drugs 2004, 2, 14–29. [Google Scholar] [CrossRef]
- Cho, M.; Park, G.-M.; Kim, S.-N.; Amna, T.; Lee, S.; Shin, W.-S. Glioblastoma-Specific Anticancer Activity of Pheophorbide a from the Edible Red Seaweed Grateloupia elliptica. J. Microbiol. Biotechnol 2014, 24, 346. [Google Scholar] [CrossRef]
- Yang, Y.; Chai, Z.; Wang, Q.; Chen, W.; He, Z.; Jiang, S. Cultivation of seaweed Gracilaria in Chinese coastal waters and its contribution to environmental improvements. Algal Res. 2015, 9, 236–244. [Google Scholar] [CrossRef]
- Lange, K.W.; Hauser, J.; Nakamura, Y.; Kanaya, S. Dietary seaweeds and obesity. Food Sci. Hum. Wellness 2015, 4, 87–96. [Google Scholar] [CrossRef]
- Miyashita, K.; Mikami, N.; Hosokawa, M. Chemical and nutritional characteristics of brown seaweed lipids: A review. J. Funct. Foods 2013, 5, 1507–1517. [Google Scholar] [CrossRef]
- Olivares-Bañuelos, T.; Gutiérrez-Rodríguez, A.G.; Méndez-Bellido, R.; Tovar-Miranda, R.; Arroyo-Helguera, O.; Juárez-Portilla, C.; Meza-Menchaca, T.; Aguilar-Rosas, L.E.; Hernández-Kelly, L.C.R.; Ortega, A.; et al. Brown Seaweed Egregia menziesii’s Cytotoxic Activity against Brain Cancer Cell Lines. Molecules 2019, 24, 260. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Xiao, L.; Liu, C.; Qi, H.; Zhang, Z. In vivo antihyperlipidemic and antioxidant activity of porphyran in hyperlipidemic mice. Carbohydr. Polym. 2017, 174, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Pádua, D.; Rocha, E.; Gargiulo, D.; Ramos, A.A. Bioactive compounds from brown seaweeds: Phloroglucinol, fucoxanthin and fucoidan as promising therapeutic agents against breast cancer. Phytochem. Lett. 2015, 14, 91–98. [Google Scholar] [CrossRef]
- Sun, X.; Zhong, Y.; Luo, H.; Yang, Y. Selenium-Containing Polysaccharide-Protein Complex in Se-Enriched Ulva fasciata Induces Mitochondria-Mediated Apoptosis in A549 Human Lung Cancer Cells. Mar. Drugs 2017, 15, 215. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Wang, J.; Zhang, Z.; Yue, Y.; Zhang, Q. Structure and Bioactivities of Porphyrans and Oligoporphyrans. Curr. Pharm. Des. 2019. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Jayawardena, T.U.; Kim, S.-Y.; Kim, H.-S.; Ahn, G.; Kim, J.; Jeon, Y.-J. Fucoidan isolated from invasive Sargassum horneri inhibit LPS-induced inflammation via blocking NF-κB and MAPK pathways. Algal Res. 2019, 41, 101561. [Google Scholar] [CrossRef]
- Murphy, C.; Hotchkiss, S.; Worthington, J.; McKeown, S.R. The potential of seaweed as a source of drugs for use in cancer chemotherapy. J. Appl. Phycol. 2014, 26, 2211–2264. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- de Souza, L.A.R.; Dore, C.M.P.; Castro, A.J.; de Azevedo, T.C.; de Oliveira, M.T.B.; Maria de Fátima, V.M.; Benevides, N.M.; Leite, E.L. Galactans from the red seaweed Amansia multifida and their effects on inflammation, angiogenesis, coagulation and cell viability. Biomed. Prev. Nutr. 2012, 2, 154–162. [Google Scholar] [CrossRef]
- Peng, J.; Yuan, J.-P.; Wu, C.-F.; Wang, J.-H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef] [PubMed]
- Lins, K.O.A.L.; Bezerra, D.P.; Alves, A.P.N.N.; Alencar, N.M.N.; Lima, M.W.; Torres, V.M.; Farias, W.R.L.; Pessoa, C.; de Moraes, M.O.; Costa-Lotufo, L.V. Antitumor properties of a sulfated polysaccharide from the red seaweed Champia feldmannii (Diaz-Pifferer). J. Appl. Toxicol. 2009, 29, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, W.; Song, W.; Chen, H.; Teng, A.; Liu, A. Partial characterization and anti-tumor activity of an acidic polysaccharide from Gracilaria lemaneiformis. Carbohydr. Polym. 2012, 88, 1313–1318. [Google Scholar] [CrossRef]
- Han, J.G.; Syed, A.Q.; Kwon, M.; Ha, J.H.; Lee, H.Y. Antioxident, immunomodulatory and anticancer activity of fucoidan isolated from Fucus vesiculosus. J. Biotechnol. 2008, 136, S571. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Lin, T.-Y.; Lu, M.-K.; Leng, P.-J.; Tsao, S.-M.; Wu, Y.-C. Fucoidan induces Toll-like receptor 4-regulated reactive oxygen species and promotes endoplasmic reticulum stress-mediated apoptosis in lung cancer. Sci. Rep. 2017, 7, 44990. [Google Scholar] [CrossRef]
- Yamasaki-Miyamoto, Y.; Yamasaki, M.; Tachibana, H.; Yamada, K. Fucoidan Induces Apoptosis through Activation of Caspase-8 on Human Breast Cancer MCF-7 Cells. J. Agric. Food Chem. 2009, 57, 8677–8682. [Google Scholar] [CrossRef]
- Yan, M.-D.; Lin, H.-Y.; Hwang, P.-A. The anti-tumor activity of brown seaweed oligo-fucoidan via lncRNA expression modulation in HepG2 cells. Cytotechnology 2019, 71, 363–374. [Google Scholar] [CrossRef]
- Fuller, R.W.; Cardellina, J.H.; Kato, Y.; Brinen, L.S.; Clardy, J.; Snader, K.M.; Boyd, M.R. A pentahalogenated monoterpene from the red alga Portieria hornemannii produces a novel cytotoxicity profile against a diverse panel of human tumor cell lines. J. Med. Chem. 1992, 35, 3007–3011. [Google Scholar] [CrossRef]
- Rocha, D.; Seca, A.; Pinto, D. Seaweed Secondary Metabaolites In Vitro and In Vivo Anticancer Activity. Mar. Drugs 2018, 16, 410. [Google Scholar] [CrossRef]
- Antunes, E.M.; Afolayan, A.F.; Chiwakata, M.T.; Fakee, J.; Knott, M.G.; Whibley, C.E.; Hendricks, D.T.; Bolton, J.J.; Beukes, D.R. Identification and in vitro anti-esophageal cancer activity of a series of halogenated monoterpenes isolated from the South African seaweeds Plocamium suhrii and Plocamium cornutum. Phytochemistry 2011, 72, 769–772. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-X.; Wijesekara, I.; Li, Y.; Kim, S.-K. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Fucaceae: A Source of Bioactive Phlorotannins. Int. J. Mol. Sci. 2017, 18, 1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namvar, F.; Mohamed, S.; Fard, S.G.; Behravan, J.; Mustapha, N.M.; Alitheen, N.B.M.; Othman, F. Polyphenol-rich seaweed (Eucheuma cottonii) extract suppresses breast tumour via hormone modulation and apoptosis induction. Food Chem. 2012, 130, 376–382. [Google Scholar] [CrossRef]
- ElKassas, H.Y.; ElSheekh, M.M. Cytotoxic activity of biosynthesized gold nanoparticles with an extract of the red seaweed Corallina officinalis on the MCF-7 human breast cancer cell line. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 4311. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Choi, E.J.; Zheng, Z.; Zhu, L.; Cho, S.B.; Kim, K.-Y.; Kim, J.; Cha, I.-H. Apoptotic effect of pheophorbide a-mediated photodynamic therapy on DMBA/TPA-induced mouse papillomas. Lasers Med. Sci. 2015, 30, 51–57. [Google Scholar] [CrossRef]
- Yu, X.; Zhou, C.; Yang, H.; Huang, X.; Ma, H.; Qin, X.; Hu, J. Effect of ultrasonic treatment on the degradation and inhibition cancer cell lines of polysaccharides from Porphyra yezoensis. Carbohydr. Polym. 2015, 117, 650–656. [Google Scholar] [CrossRef]
- Wang, J.; Hou, Y.; Duan, D.; Zhang, Q. The Structure and Nephroprotective Activity of Oligo-Porphyran on Glycerol-Induced Acute Renal Failure in Rats. Mar. Drugs 2017, 15, 135. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Hama, Y.; Yamaguchi, K.; Oda, T. Inhibitory effect of sulphated polysaccharide porphyran on nitric oxide production in lipopolysaccharide-stimulated RAW264.7 macrophages. J. Biochem. (Tokyo) 2012, 151, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Inoue, N.; Yamano, N.; Sakata, K.; Nagao, K.; Hama, Y.; Yanagita, T. The Sulfated Polysaccharide Porphyran Reduces Apolipoprotein B100 Secretion and Lipid Synthesis in HepG2 Cells. Biosci. Biotechnol. Biochem. 2009, 73, 447–449. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Wang, S.C.; Xu, L.W.; He, J.B.; Xu, X.M. Extraction of Porphyran from Porphyra yezoensis for Gel Formulation Preparation. Key Eng. Mater. 2014, 636, 133–137. [Google Scholar] [CrossRef]
- Kwon, M.-J.; Nam, T.-J. Porphyran induces apoptosis related signal pathway in AGS gastric cancer cell lines. Life Sci. 2006, 79, 1956–1962. [Google Scholar] [CrossRef]
- Kwon, M.-J.; Nam, T.-J. Chromatographically Purified Porphyran from Porphyra yezoensis Effectively Inhibits Proliferation of Human Cancer Cells. Food Sci. Biotechnol. 2007, 16, 873–878. [Google Scholar]
- Noda, H.; Amano, H.; Arashima, K.; Hashimoto, S.; Nisizawa, K. Antitumour Activity of Polysaccharides and Lipids from Marine Algae. Nippon Suisan Gakkaishi 1989, 55, 1265–1271. [Google Scholar] [CrossRef] [Green Version]
- Noda, H.; Amano, H.; Arashima, K.; Nisizawa, K. Antitumor activity of marine algae. Hydrobiologia 1990, 204–205, 577–584. [Google Scholar] [CrossRef]
- Min, H.-K.; Kim, H.-J.; Chang, H.-C. Growth-inhibitory Effect of the Extract of Porphyran-Chungkookjang on Cancer Cell. J. Korean Soc. Food Sci. Nutr. 2008, 37, 826–833. [Google Scholar] [CrossRef]
- Zhang, L.-X.; Cai, C.-E.; Guo, T.-T.; Gu, J.-W.; Xu, H.-L.; Zhou, Y.; Wang, Y.; Liu, C.-C.; He, P.-M. Anti-cancer effects of polysaccharide and phycocyanin from Porphyra yezoensis. J. Mar. Sci. Technol. 2011, 19, 6. [Google Scholar]
- He, D.; Wu, S.; Yan, L.; Zuo, J.; Cheng, Y.; Wang, H.; Liu, J.; Zhang, X.; Wu, M.; Choi, J.; et al. Antitumor bioactivity of porphyran extracted from Pyropia yezoensis Chonsoo2 on human cancer cell lines. J. Sci. Food Agric. 2019, 99, 6722–6730. [Google Scholar] [CrossRef]
- Ma, C.; Feng, M.; Zhai, X.; Hu, M.; You, L.; Luo, W.; Zhao, M. Optimization for the extraction of polysaccharides from Ganoderma lucidum and their antioxidant and antiproliferative activities. J. Taiwan Inst. Chem. Eng. 2013, 44, 886–894. [Google Scholar] [CrossRef]
- Chen, T.; Zhao, X.; Wu, J.; Yu, D.; Wu, Y. Supercritical fluid CO2 extraction, simultaneous determination of components in ultra-fine powder of Ganoderma sinense by HPLC–ESI-MS method. J. Taiwan Inst. Chem. Eng. 2011, 42, 428–434. [Google Scholar] [CrossRef]
- Liu, W.; Wang, H.; Pang, X.; Yao, W.; Gao, X. Characterization and antioxidant activity of two low-molecular-weight polysaccharides purified from the fruiting bodies of Ganoderma lucidum. Int. J. Biol. Macromol. 2010, 46, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Zhang, Q.; Qi, H.; Zhang, H.; Niu, X.; Xu, Z.; Li, Z. Degradation of porphyran from Porphyra haitanensis and the antioxidant activities of the degraded porphyrans with different molecular weight. Int. J. Biol. Macromol. 2006, 38, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Isaka, S.; Cho, K.; Nakazono, S.; Abu, R.; Ueno, M.; Kim, D.; Oda, T. Antioxidant and anti-inflammatory activities of porphyran isolated from discolored nori (Porphyra yezoensis). Int. J. Biol. Macromol. 2015, 74, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Geng, L.; Zhang, J.; Wang, J.; Zhang, Q.; Duan, D.; Zhang, Q. Oligo-Porphyran Ameliorates Neurobehavioral Deficits in Parkinsonian Mice by Regulating the PI3K/Akt/Bcl-2 Pathway. Mar. Drugs 2018, 16, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasedya, E.S.; Miyake, M.; Kobayashi, D.; Hazama, A. Carrageenan delays cell cycle progression in human cancer cells in vitro demonstrated by FUCCI imaging. BMC Complement. Altern. Med. 2016, 16, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiner, M.L. Food additive carrageenan: Part II: A critical review of carrageenan in vivo safety studies. Crit. Rev. Toxicol. 2014, 44, 244–269. [Google Scholar] [CrossRef]
- Chandrasekaran, R.; Bian, W.; Okuyama, K. Three-dimensional structure of guaran. Carbohydr. Res. 1998, 312, 219–224. [Google Scholar] [CrossRef]
- Fedorov, S.; Ermakova, S.; Zvyagintseva, T.; Stonik, V. Anticancer and Cancer Preventive Properties of Marine Polysaccharides: Some Results and Prospects. Mar. Drugs 2013, 11, 4876–4901. [Google Scholar] [CrossRef] [Green Version]
- Mendoza, W.G.; Ganzon-Fortes, E.T.; Villanueva, R.D.; Romero, J.B.; Montano, M.N.E. Tissue age as a factor affecting carrageenan quantity and quality in farmed Kappaphycus striatum (Schmitz) Doty ex Silva. Bot. Mar. 2006, 49, 57–64. [Google Scholar] [CrossRef]
- Knutsen, S.H.; Moe, S.T.; Larsen, B.; Grasdalen, H. Molecular cut-off values of dialysis membranes for alginate and kappa-carrageenan oligosaccharides. Hydrobiologia 1993, 260–261, 667–672. [Google Scholar] [CrossRef]
- Cheong, K.-L.; Qiu, H.-M.; Du, H.; Liu, Y.; Khan, B.M. Oligosaccharides Derived from Red Seaweed: Production, Properties, and Potential Health and Cosmetic Applications. Molecules 2018, 23, 2451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, Q.; Sun, W.; Shan, X.; Jiang, H.; Yu, G. Carrageenan-induced colitis is associated with decreased population of anti-inflammatory bacterium, Akkermansia muciniphila, in the gut microbiota of C57BL/6J mice. Toxicol. Lett. 2017, 279, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Necas, J.; Bartosikova, L. Carrageenan: A review. Veterinární Medicína 2013, 58, 187–205. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-Y.; Huang, S.-S.; Lee, M.-M.; Deng, J.-S.; Huang, G.-J. Anti-inflammatory activities of cardamonin from Alpinia katsumadai through heme oxygenase-1 induction and inhibition of NF-κB and MAPK signaling pathway in the carrageenan-induced paw edema. Int. Immunopharmacol. 2015, 25, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, M.; Bani, D.; Cinci, L.; Nistri, S.; Uliva, C.; Ragazzo, E.; Vannacci, A.; Manoni, M.; Gori, A.M.; Abbate, R.; et al. Anti-inflammatory effects of low molecular weight heparin derivative in a rat model of carrageenan-induced pleurisy. J. Cell. Mol. Med. 2009, 13, 2704–2712. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanzadeh, B.; Mansouri, M.; Hemmati, A.; Naghizadeh, B.; Mard, S.; Rezaie, A. A study of the mechanisms underlying the anti-inflammatory effect of ellagic acid in carrageenan-induced paw edema in rats. Indian J. Pharmacol. 2015, 47, 292. [Google Scholar] [CrossRef] [Green Version]
- Shree, N.; Venkategowda, S.; Venkatranganna, M.V.; Bhonde, R.R. Treatment with adipose derived mesenchymal stem cells and their conditioned media reverse carrageenan induced paw oedema in db/db mice. Biomed. Pharmacother. 2017, 90, 350–353. [Google Scholar] [CrossRef]
- Karama, Z.B.; Samar, M.; Amina, T.; Lobna, J.; Mohamed, T.; Slim, T. Effects of Juniperus phoenicea Hydroalcoholic Extract on Inflammatory Mediators and Oxidative Stress Markers in Carrageenan-Induced Paw Oedema in Mice. BioMed Res. Int. 2018, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, S.; Mapp, P.I.; Walsh, D.A. Angiogenesis and the persistence of inflammation in a rat model of proliferative synovitis. Arthritis Rheum. 2010, 62, 1890–1898. [Google Scholar] [CrossRef]
- Arslan, R.; Bektas, N.; Bor, Z.; Sener, E. Evaluation of the antithrombotic effects of Crataegus monogyna and Crataegus davisii in the carrageenan-induced tail thrombosis model. Pharm. Biol. 2015, 53, 275–279. [Google Scholar] [CrossRef] [Green Version]
- Ma, N.; Liu, X.W.; Yang, Y.J.; Li, J.Y.; Mohamed, I.; Liu, G.R.; Zhang, J.Y. Preventive Effect of Aspirin Eugenol Ester on Thrombosis in κ-Carrageenan-Induced Rat Tail Thrombosis Model. PLoS ONE 2015, 10, e0133125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-L.; Xi, M.-Z.; Choi, Y.-B.; Lee, B.-H. Antithrombotic Effect of Fermented Ophiopogon japonicus in Thrombosis-Induced Rat Models. J. Med. Food 2017, 20, 637–645. [Google Scholar] [CrossRef]
- Ehrke, M.J. Immunomodulation in cancer therapeutics. Int. Immunopharmacol. 2003, 3, 1105–1119. [Google Scholar] [CrossRef]
- Luo, M.; Shao, B.; Nie, W.; Wei, X.-W.; Li, Y.-L.; Wang, B.-L.; He, Z.-Y.; Liang, X.; Ye, T.-H.; Wei, Y.-Q. Antitumor and Adjuvant Activity of λ-carrageenan by Stimulating Immune Response in Cancer Immunotherapy. Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Song, J.; Li, X.; Li, N.; Dai, J. Immunomodulation and antitumor activity of κ-carrageenan oligosaccharides. Cancer Lett. 2006, 243, 228–234. [Google Scholar] [CrossRef]
- Ling, N. Growth Inhibition and Cell Cycle Arrest of Kappa-Selenocarrageenan and Paclitaxel on HepG2 Cells. Adv. Mater. Res. 2011, 343–344, 530–534. [Google Scholar] [CrossRef]
- Jin, Z.; Han, Y.-X.; Han, X.-R. Degraded Iota-Carrageenan Can Induce Apoptosis in Human Osteosarcoma Cells Via the Wnt/β-Catenin Signaling Pathway. Nutr. Cancer 2013, 65, 126–131. [Google Scholar] [CrossRef]
- Yao, Z.; Wu, H.; Zhang, S.; Du, Y. Enzymatic preparation of κ-carrageenan oligosaccharides and their anti-angiogenic activity. Carbohydr. Polym. 2014, 101, 359–367. [Google Scholar] [CrossRef]
- Paper, D.H.; Vogl, H.; Franz, G.; Hoffman, R. Defined carrageenan derivatives as angiogenesis inhibitors. Macromol. Symp. 1995, 99, 219–225. [Google Scholar] [CrossRef]
- Poupard, N.; Badarou, P.; Fasani, F.; Groult, H.; Bridiau, N.; Sannier, F.; Bordenave-Juchereau, S.; Kieda, C.; Piot, J.-M.; Grillon, C.; et al. Assessment of Heparanase-Mediated Angiogenesis Using Microvascular Endothelial Cells: Identification of λ-Carrageenan Derivative as a Potent Anti Angiogenic Agent. Mar. Drugs 2017, 15, 134. [Google Scholar] [CrossRef]
- Chen, H.; Yan, X.; Lin, J.; Wang, F.; Xu, W. Depolymerized Products of λ-Carrageenan as a Potent Angiogenesis Inhibitor. J. Agric. Food Chem. 2007, 55, 6910–6917. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Song, J.; Li, X.; Li, N.; Liu, S. Enhanced immunostimulatory and antitumor activity of different derivatives of κ-carrageenan oligosaccharides from Kappaphycus striatum. J. Appl. Phycol. 2011, 23, 59–65. [Google Scholar] [CrossRef]
- de Araújo, C.A.; Noseda, M.D.; Cipriani, T.R.; Gonçalves, A.G.; Duarte, M.E.R.; Ducatti, D.R.B. Selective sulfation of carrageenans and the influence of sulfate regiochemistry on anticoagulant properties. Carbohydr. Polym. 2013, 91, 483–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- dos Santos-Fidencio, G.C.; Gonçalves, A.G.; Noseda, M.D.; Duarte, M.E.R.; Ducatti, D.R.B. Effects of carboxyl group on the anticoagulant activity of oxidized carrageenans. Carbohydr. Polym. 2019, 214, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zhang, W.; Li, X.; Lü, X.; Li, N.; Gao, X.; Song, J. Preparation and in vitro antioxidant activity of κ-carrageenan oligosaccharides and their oversulfated, acetylated, and phosphorylated derivatives. Carbohydr. Res. 2005, 340, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, T.; Yuyama, H.; Vogl, O. Synthesis of poly (ethylene glycol)-bound 3-(5-fluorouracil-1-yl) propanoic acid, its hydrolysis reactivity and antitumor activity. Makromol. Chem. Rapid Commun. 1985, 6, 815–819. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z. The antitumor activity of a red alga polysaccharide complexes carrying 5-fluorouracil. Int. J. Biol. Macromol. 2014, 69, 542–545. [Google Scholar] [CrossRef]
- Raymond, E.; Buquet-Fagot, C.; Djelloul, S.; Mester, J.; Cvitkovic, E.; Allain, P.; Louvet, C.; Gespach, C. Antitumor activity of oxaliplatin in combination with 5-fluorouracil and the thymidylate synthase inhibitor AG337 in human colon, breast and ovarian cancers. Anticancer. Drugs 1997, 8, 876–885. [Google Scholar] [CrossRef]
- Zhou, G.; Xin, H.; Sheng, W.; Sun, Y.; Li, Z.; Xu, Z. In vivo growth-inhibition of S180 tumor by mixture of 5-Fu and low molecular λ-carrageenan from Chondrus ocellatus. Pharmacol. Res. 2005, 51, 153–157. [Google Scholar] [CrossRef]
- Zhou, G.; Sheng, W.; Yao, W.; Wang, C. Effect of low molecular λ-carrageenan from Chondrus ocellatus on antitumor H-22 activity of 5-Fu. Pharmacol. Res. 2006, 53, 129–134. [Google Scholar] [CrossRef]
- Mohammad Ali Faramarzi. Armin Sadighi Insights into biogenic and chemical production of inorganic nanomaterials and nanostructures. Adv. Colloid Interface Sci. 2012, 189–190, 1–20. [Google Scholar]
- Chen, X.; Zhao, X.; Gao, Y.; Yin, J.; Bai, M.; Wang, F. Green Synthesis of Gold Nanoparticles Using Carrageenan Oligosaccharide and Their In Vitro Antitumor Activity. Mar. Drugs 2018, 16, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raman, M.; Devi, V.; Doble, M. Biocompatible ι-carrageenan-γ-maghemite nanocomposite for biomedical applications – synthesis, characterization and in vitro anticancer efficacy. J. Nanobiotechnol. 2015, 13, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]

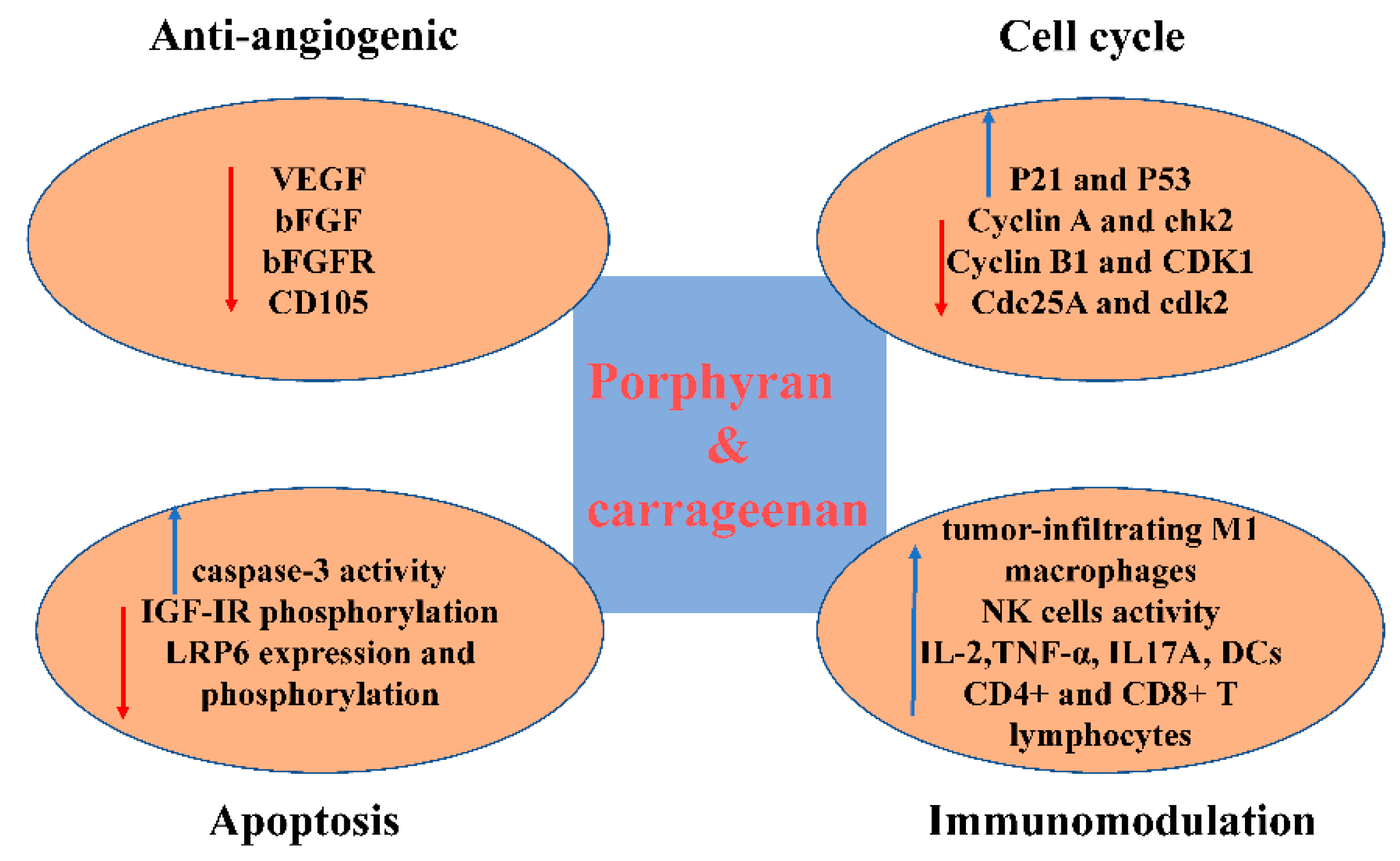

| Source | Target | Type of Activity | Possible Mechanisms | References |
|---|---|---|---|---|
| P. yezoensis | Mice implanted with Ehrlich carcinoma and Meth A fibrosarcoma | Appreciable inhibition of tumor growth | Not referred | [45,46] |
| AGS and HT-29 cancer cells | Antiproliferation | [47] | ||
| SGC-7901 and 95D cancer cell lines | [38] | |||
| Hep3B cells | Antiproliferation and cell cycle blocked in the G2/M phase | Upregulation of p21 and p53, while negatively regulating cyclin B1and CDK1 | [49] | |
| HO-8910, MCF-7, K562, and SMMC-7721 cells | Antiproliferation and cell cycle arrested at the G0/G1or the G2/M check points | Not referred | [48] | |
| HT-29 colon cancer cells and AGS gastric cancer cells | Antiproliferation and apoptosis induced | Increasing caspase-3 activity | [44] | |
| Commodity provided by Korea Bio Polymer (KBP) company | AGS human gastric cancer cells. | Negatively regulating IGF-IR phosphorylation and inducing caspase-3 activation | [43] |
| Source | Target | Type of Activity | Possible Mechanisms | References |
|---|---|---|---|---|
| λ-carrageenan purchased from Sigma-Aldrich | B16-F10 and 4T1 bearing mice | Inhibition of tumor growth and improving immune system | Increasing the number of tumor-infiltrating M1 macrophages, DCs, and more activated CD4+ CD8+ T lymphocytes and enhancing the secretion of IL17A in spleen and significantly increase the level of TNF-α in tumor | [75] |
| Carrageenan oligosaccharides derived from Kappaphycus striatum | S180-bearing mice | Increase macrophage phagocytosis, the form of antibody secreted by spleen cells, spleen lymphocyte proliferation, NK cells activity, serumal IL-2 and TNF-a level | [76] | |
| κ-carrageenan and λ-carrageenan purchased from Sigma-Aldrich | HeLa cells | Cell cycle delayed in G2/M phase or in both G1 and G2/M phase | Not referred | [56] |
| κ-selenocarrageenan consisted of selenium and κ-carrageenan | HepG2 cells | Cell cycle delayed in S phase | Upregulating Cyclin A and chk2 protein and down-regulating Cdc25A and cdk2 expression. | [77] |
| ι-Carrageenan | Human osteosarcoma cell line | Apoptosis induced and Cell cycle delayed in G1 phase | Downregulation of the Wnt/β-catenin signaling pathway through suppressing LRP6 expression and phosphorylation | [78] |
| κ-carrageenan oligosaccharides prepared from κ-carrageenan with enzyme | MCF-7 xenograft tumor | Antiproliferation and anti-angiogenic | Negative regulation of human VEGF, bFGF, bFGFR, and CD105 | [79] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Gao, T.; Yang, Y.; Meng, F.; Zhan, F.; Jiang, Q.; Sun, X. Anti-Cancer Activity of Porphyran and Carrageenan from Red Seaweeds. Molecules 2019, 24, 4286. https://doi.org/10.3390/molecules24234286
Liu Z, Gao T, Yang Y, Meng F, Zhan F, Jiang Q, Sun X. Anti-Cancer Activity of Porphyran and Carrageenan from Red Seaweeds. Molecules. 2019; 24(23):4286. https://doi.org/10.3390/molecules24234286
Chicago/Turabian StyleLiu, Zhiwei, Tianheng Gao, Ying Yang, Fanxin Meng, Fengping Zhan, Qichen Jiang, and Xian Sun. 2019. "Anti-Cancer Activity of Porphyran and Carrageenan from Red Seaweeds" Molecules 24, no. 23: 4286. https://doi.org/10.3390/molecules24234286
APA StyleLiu, Z., Gao, T., Yang, Y., Meng, F., Zhan, F., Jiang, Q., & Sun, X. (2019). Anti-Cancer Activity of Porphyran and Carrageenan from Red Seaweeds. Molecules, 24(23), 4286. https://doi.org/10.3390/molecules24234286






