Combination of Lactobacillus plantarum and Saccharomyces cerevisiae DV10 as Starter Culture to Produce Mango Slurry: Microbiological, Chemical Parameters and Antioxidant Activity
Abstract
:1. Introduction
2. Results and Discussion
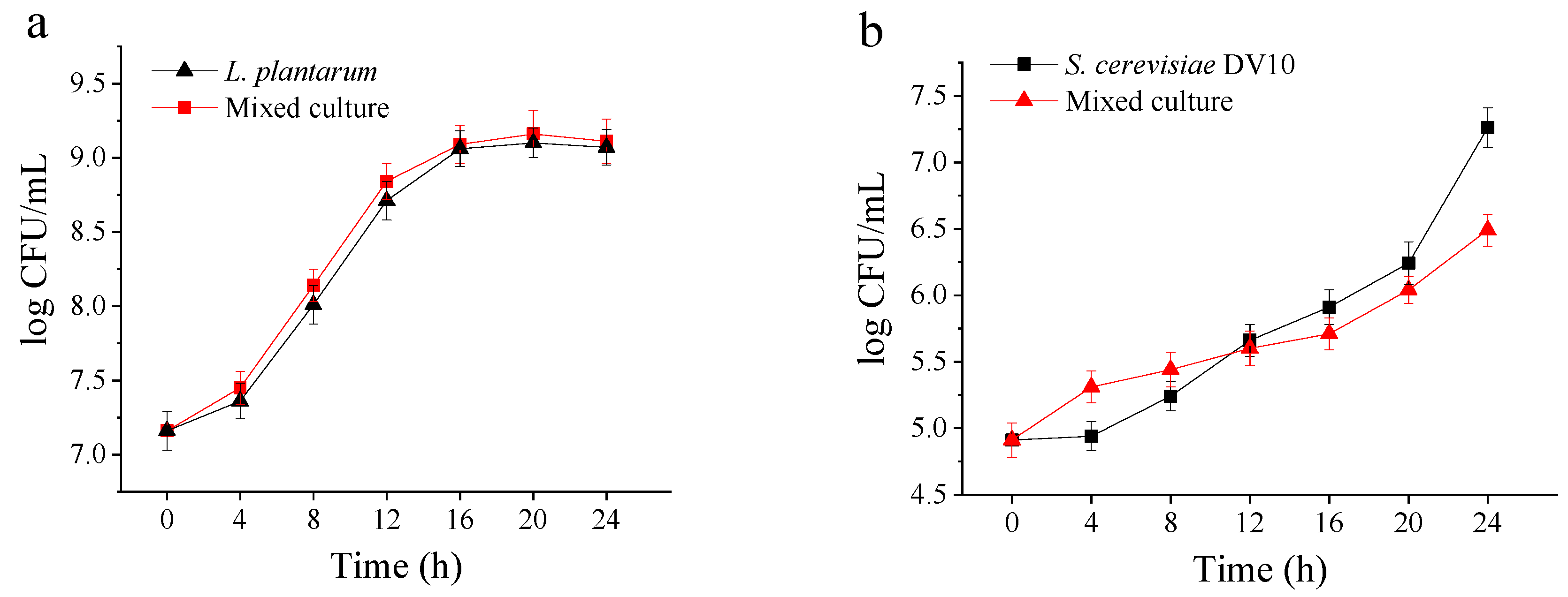
2.1. Microbial Growth Performance During Mango Slurry Fermentation
2.2. Quality Parameters
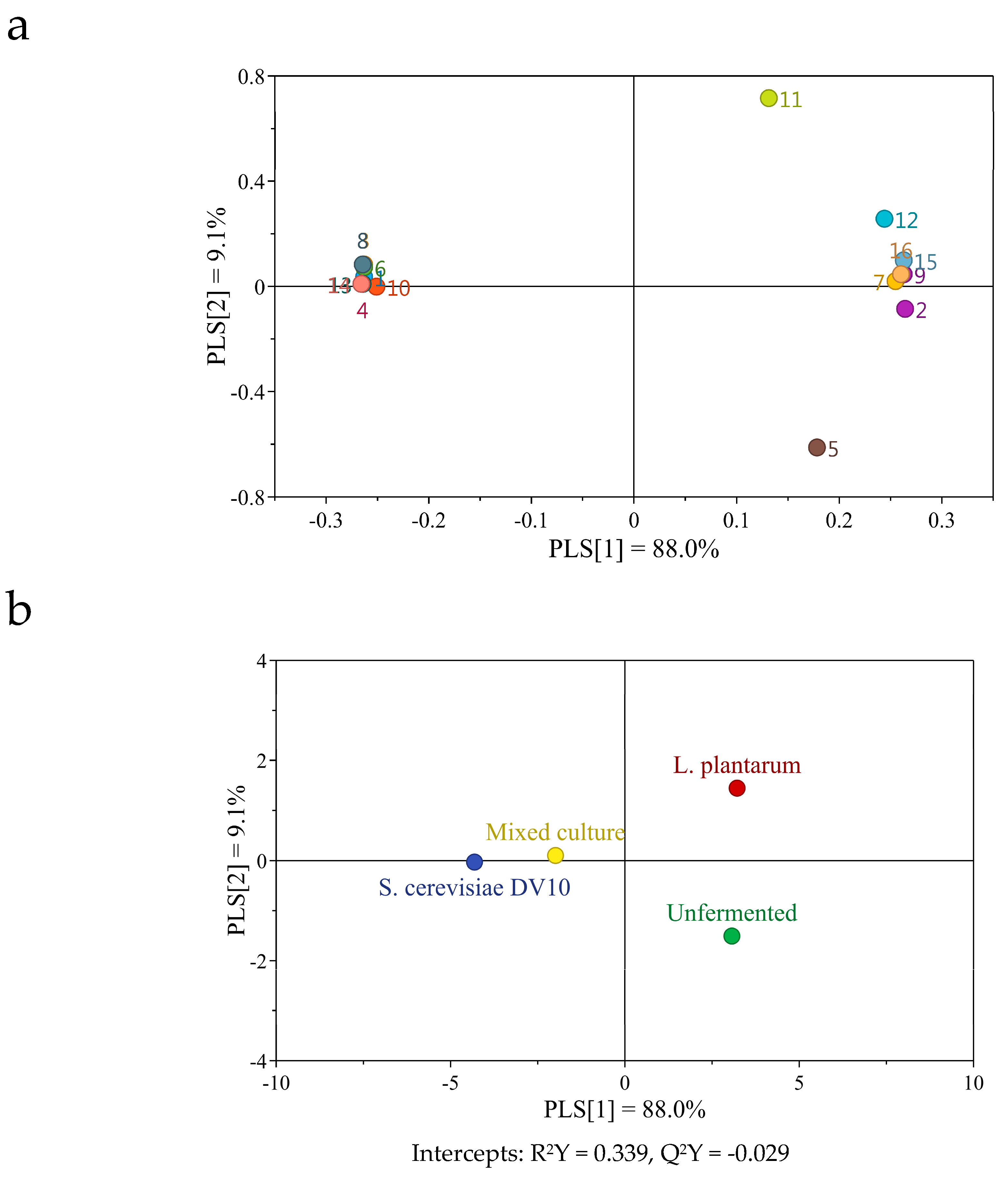
2.3. Changes in TPC, ABTS and FRAP
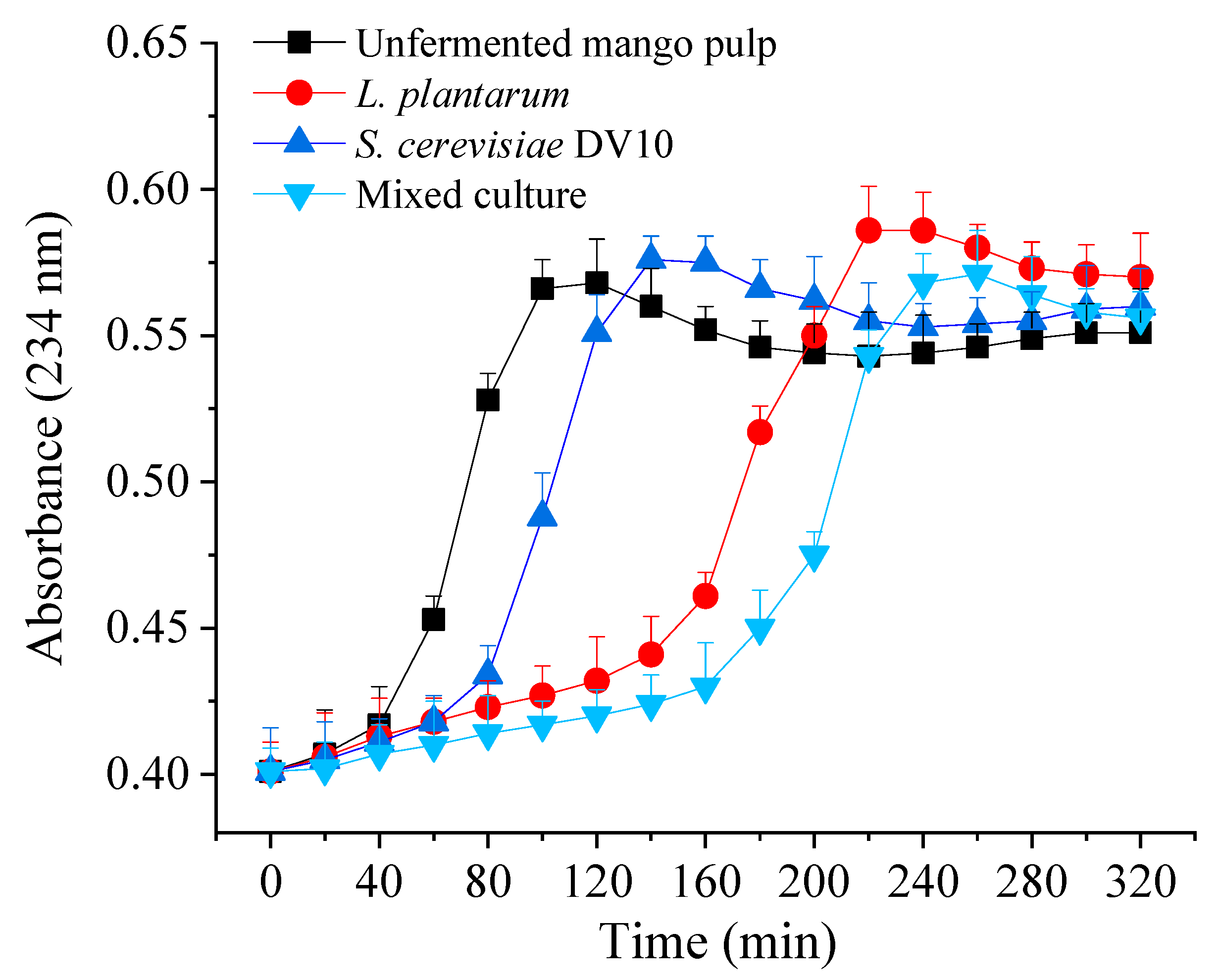
2.4. Inhibition of LDL Oxidation
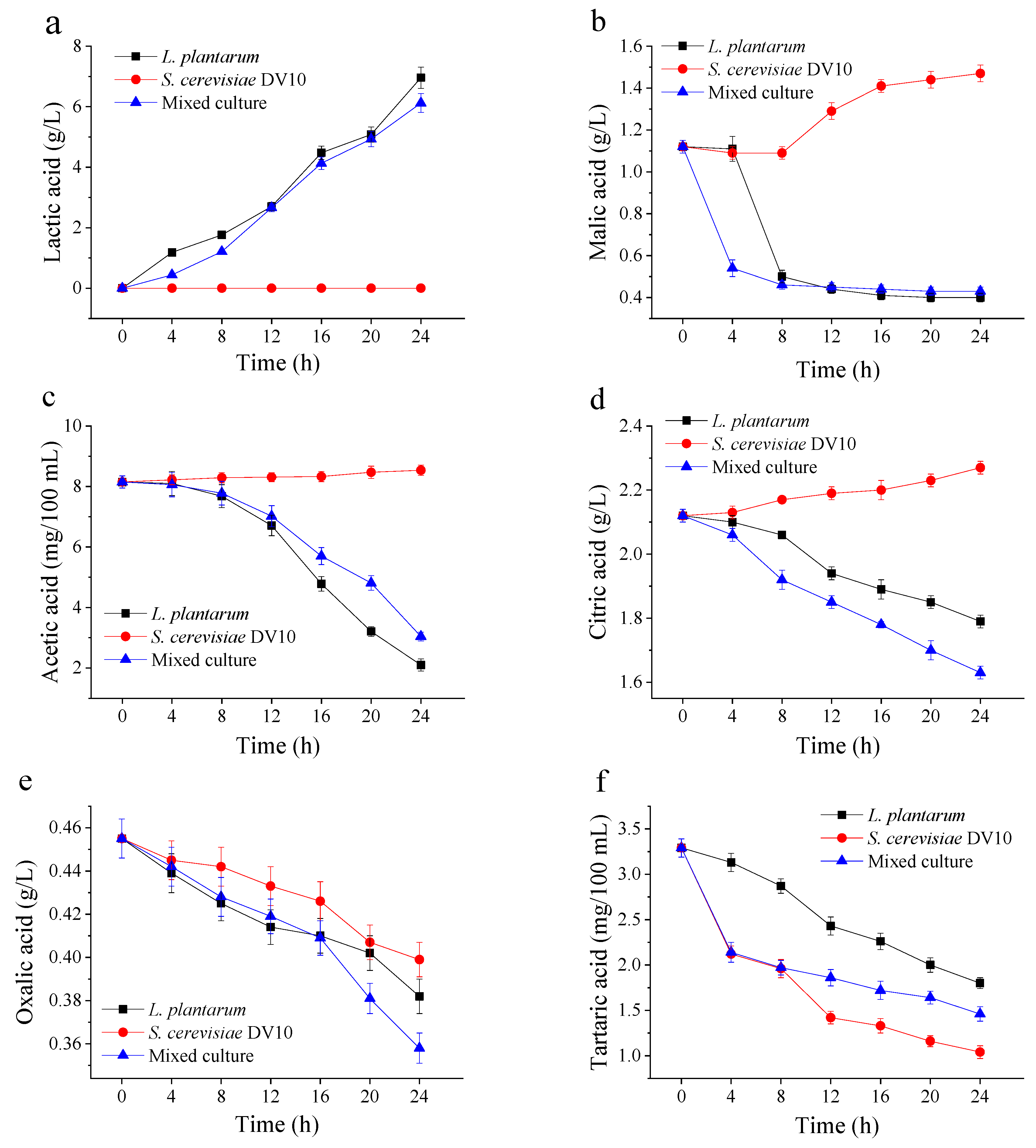
2.5. Changes in Organic Acids
2.6. Changes in Volatile Compounds
3. Materials and Methods
3.1. Materials
3.2. Fermented Mango Slurry
3.3. Enumeration of Microorganisms
3.4. The pH, Total Soluble Solids and Reducing Sugar Content
3.5. TPC
3.6. Scavenging Effect on ABTS Radical
3.7. Determination of FRAP
3.8. Copper-Induced LDL Oxidation
3.9. Organic Acid Content
3.10. Volatile Compound Content
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Kandylis, P.; Pissaridi, K.; Bekatorou, A.; Kanellaki, M.; Koutinas, A.A. Dairy and non-dairy probiotic beverages. Curr. Opin. Food Sci. 2016, 7, 58–63. [Google Scholar] [CrossRef]
- Panda, S.K.; Behera, S.K.; Qaku, X.W.; Sekar, S.; Ndinteh, D.T.; Nanjundaswamy, H.M.; Ray, R.C.; Kayitesi, E. Quality enhancement of prickly pears (Opuntia sp.) juice through probiotic fermentation using Lactobacillus fermentum-ATCC 9338. LWT-Food Sci. Technol. 2017, 75, 453–459. [Google Scholar] [CrossRef]
- Shori, B.A. Influence of food matrix on the viability of probiotic bacteria: A review based on dairy and non-dairy beverages. Food Biosci. 2016, 13, 1–8. [Google Scholar] [CrossRef]
- Asif, A.; Farooq, U.; Akram, K.; Hayat, Z.; Shafi, A.; Sarfraz, F.; Sidhu, M.A.I.; Rehman, H.; Aftab, S. Therapeutic potentials of bioactive compounds from mango fruit wastes. Trends Food Sci. Technol. 2016, 53, 102–112. [Google Scholar] [CrossRef]
- Ntsoanea, M.L.; Zude-Sassea, M.; Mahajana, P.; Sivakumar, D. Quality assesment and postharvest technology of mango: A review of its current status and future perspectives. Sci. Hortic-Amst. 2019, 249, 77–85. [Google Scholar] [CrossRef]
- Sivakumar, D.; Jiang, Y.; Yahia, E.M. Maintaining mango (Mangifera indica L.) fruit quality during the export chain. Food Res. Int. 2011, 44, 1254–1263. [Google Scholar] [CrossRef]
- Jahurul, M.; Zaidul, I.; Ghafoor, K.; Al-Juhaimi, F.Y.; Nyam, K.; Norulaini, N.; Sahena, F.; Omar, A.M. Mango (Mangifera indica L.) by-products and their valuable components: A review. Food Chem. 2015, 183, 173–180. [Google Scholar] [CrossRef]
- Santos, C.C.; Libeck, B.S.; Schwan, R.F. Co-culture fermentation of peanut-soy milk for the development of a novel functional beverage. Int. J. Food Microbiol. 2014, 186, 32–41. [Google Scholar] [CrossRef]
- Angelov, A.; Gotcheva, V.; Hristozova, T.; Gargova, S. Application of pure and mixed probiotic lactic acid bacteria and yeast cultures for oat fermentation. J. Sci. Food Agric. 2005, 85, 2134–2141. [Google Scholar] [CrossRef]
- Rathore, S.; Salmerón, I.; Pandiella, S.S. Production of potentially probiotic beverages using single and mixed cereal substrates fermented with lactic acid bacteria cultures. Food Microbiol. 2012, 30, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Sahu, U.C.; Behera, S.K.; Ray, R.C. Bio-processing of bael [Aegle marmelos L.] fruits into wine with antioxidants. Food Biosci. 2014, 5, 34–41. [Google Scholar] [CrossRef]
- Pérez-Gregorio, M.R.; Regueiro, J.; Alonso-González, E.; Pastrana-Castro, L.M.; Simal-Gándara, J. Influence of alcoholic fermentation process on antioxidant activity and phenolic levels from mulberries (Morus nigra L.). LWT-Food Sci. Technol. 2011, 44, 1793–1801. [Google Scholar] [CrossRef]
- Ankolekar, C.; Pinto, M.; Greene, D.; Shetty, K. In vitro bioassay based screening of antihyperglycemia and antihypertensive activities of Lactobacillus acidophilus fermented pear juice. Innov. Food Sci. Emerg. 2012, 13, 221–230. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, L.X.; Rui, X.; Li, W.; Chen, X.H.; Jiang, M.; Dong, M.S. Enhancement of the antioxidant capacity of soy whey by fermentation with Lactobacillus plantarum B1-6. J. Funct. Foods 2015, 12, 33–44. [Google Scholar] [CrossRef]
- Li, X.C.; Xing, Y.; Cao, L.; Xu, Q.L.; Li, S.H.; Wang, R.R.; Jiang, Z.J.; Che, Z.M.; Lin, H.B. Effects of six commercial, Saccharomyces cerevisiae, strains on phenolic attributes, antioxidant activity, and aroma of kiwifruit (Actinidia deliciosa cv.) wine. Biomed Res. Int. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Kim, J.; Choi, J.N.; Kang, D.; Son, G.H.; Kim, Y.; Choi, H.; Kwon, D.Y.; Lee, C.H. Correlation between antioxidative activities and metabolite changes during Cheonggukjang fermentation. Biosci. Biotechnol. Biochem. 2011, 75, 732–739. [Google Scholar] [CrossRef]
- Vadivel, V.; Stuetz, W.; Scherbaum, V.; Biesalski, H.K. Total free phenolic content and health relevant functionality of Indian wild legume grains: Effect of indigenous processing methods. J. Food Compos. Anal. 2011, 24, 935–943. [Google Scholar] [CrossRef]
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [CrossRef]
- Camargo, A.C.D.; Regitano-d’Arce, M.A.B.; Biasoto, A.C.T.; Shahidi, F. Low molecular weight phenolics of grape juice and winemaking byproducts: Antioxidant activities and inhibition of oxidation of human low-density lipoprotein cholesterol and DNA strand breakage. J. Agric. Food Chem. 2014, 62, 12159–12171. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Jang, S.; Baek, E.H.; Kim, M.J.; Lee, K.S.; Shin, H.S.; Chung, M.J.; Kim, J.E.; Lee, K.O.; Ha, N.J. Lactic acid bacteria affect serum cholesterol levels, harmful fecal enzyme activity, and fecal water content. Lipids Health Dis. 2009, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.; Li, Y.; Li, P.; Wang, H. Polyphenolic compounds and antioxidant properties of selected China wines. Food Chem. 2009, 112, 454–460. [Google Scholar] [CrossRef]
- Duarte, W.F.; Dias, D.R.; Pereira, G.V.M.; Gervásio, I.M.; Schwan, R.F. Indigenous and inoculated yeast fermentation of gabiroba (Campomanesia pubescens) pulp for fruit wine production. J. Ind. Microbiol. Biotechnol. 2009, 36, 557–569. [Google Scholar] [CrossRef]
- Menezes, A.G.T.; Ramos, C.L.; Dias, D.R.; Schwan, R.F. Combination of probiotic yeast and lactic acid bacteria as starter culture to produce maize-based beverages. Food Res. Int. 2018, 111, 187–197. [Google Scholar] [CrossRef]
- Álvarez-Martín, P.; Flórez, A.B.; Hernández-Barranco, A.; Mayo, B. Interaction between dairy yeasts and lactic acid bacteria strains during milk fermentation. Food Control 2008, 19, 62–70. [Google Scholar] [CrossRef]
- Liu, S.N.; Han, Y.; Zhou, Z.J. Lactic acid bacteria in traditional fermented Chinese foods. Food Res. Int. 2011, 44, 643–651. [Google Scholar] [CrossRef]
- Pino, J.A.; Mesa, J.; Muñoz, Y.; Marti, M.P.; Marbot, R. Volatile components from mango (Mangifera indica L.) cultivars. J. Agric. Food Chem. 2005, 53, 2213–2223. [Google Scholar] [CrossRef]
- Li, X.; Yu, B.; Curran, P.; Liu, S.Q. Chemical and volatile composition of mango wines fermented with different Saccharomyces cerevisiae yeast strains. S. Afr. J. Enol. Vitic. 2011, 32, 117–128. [Google Scholar] [CrossRef]
- Longo, M.A.; Sanromán, M.A. Production of food aroma compounds: Microbial and enzymatic methodologies. Food Technol. Biotechnol. 2006, 44, 335–353. [Google Scholar] [CrossRef]
- Freire, A.L.; Ramos, C.L.; Schwan, R.F. Microbiological and chemical parameters during cassava based-substrate fermentation using potential starter cultures of lactic acid bacteria and yeast. Food Res. Int. 2015, 76, 787–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saqib, A.A.N.; Whitney, P.J. Differential behaviour of the dinitrosalicylic acid (DNS) reagent towards mono- and di-saccharide sugars. Biomass Bioenergy 2011, 35, 4748–4750. [Google Scholar] [CrossRef]
- De Sá, L.Z.C.M.; Castro, P.F.S.; Lino, F.M.A.; Bernardes, M.J.C.; Viegas, J.C.J.; Dinis, T.C.P.; Santana, M.J.; Romao, W.; Vaz, B.G.; Lião, L.M.; et al. Antioxidant potential and vasodilatory activity of fermented beverages of jabuticaba berry (Myrciaria jaboticaba). J. Funct. Foods 2014, 8, 169–179. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Rufián-Henares, J.A.; Morales, F.J. Assessing the antioxidant activity of melanoidins from coffee brews by different antioxidant methods. J. Agric. Food Chem. 2005, 53, 7832–7836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Li, H.; Xi, W.; An, W.; Niu, L.; Cao, Y.; Wang, C.F.; Wang, Y.J.; Yin, Y. Changes in sugars and organic acids in wolfberry (Lycium barbarum L.) fruit during development and maturation. Food Chem. 2015, 173, 718–724. [Google Scholar] [CrossRef]
- Lee, P.R.; Chong, S.M.; Yu, B.; Curran, P.; Liu, S.Q. Effects of sequentially inoculated Williopsis saturnus and Saccharomyces cerevisiae on volatile profiles of papaya wine. Food Res. Int. 2012, 45, 177–183. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
| Samples | pH | TSS (° Brix) | Reducing sugars (g/L) | TPC (mg GAE/100 mL) | ABTS (% Inh) | FRAP (mM FeSO4) |
|---|---|---|---|---|---|---|
| Unfermented | 4.12 ± 0.15b | 21.6 ± 0.5b | 2.24 ± 0.07b | 75.87 ± 1.43a | 10.43 ± 0.25a | 1.11 ± 0.03a |
| L. plantarum | 3.54 ± 0.09a | 21.2 ± 0.4b | 2.13 ± 0.06b | 86.59 ± 1.29c | 15.29 ± 0.31c | 1.47 ± 0.04b |
| S. cerevisiae DV10 | 3.98 ± 0.12b | 20.2 ± 0.5a | 1.97 ± 0.04a | 79.41 ± 1.65b | 12.72 ± 0.29b | 1.16 ± 0.02a |
| Co-culture | 3.55 ± 0.07a | 20.3 ± 0.4a | 2.00 ± 0.05a | 89.25 ± 1.06d | 16.11 ± 0.34d | 1.49 ± 0.05b |
| Volatile Compounds | RT | RI | Unfermented Mango Slurry | L. plantarum | S. cerevisiae DV10 | Co-Culture |
|---|---|---|---|---|---|---|
| Terpenes | ||||||
| (+)-4-Carene | 10.228 | 919 | 517.02 ± 25.85a | 535.5 ± 26.78a | 263.9 ± 18.2b | 275.62 ± 18.78b |
| 3-Carene | 7.312 | 948 | 33.94 ± 1.7b | 37.67 ± 1.88a | 16.26 ± 0.81d | 19.59 ± 0.98c |
| d-Limonene | 8.434 | 1018 | 13.03 ± 0.65a | 13.53 ± 0.68a | 7.07 ± 0.35b | 6.67 ± 0.33b |
| γ-Terpinene | 9.452 | 998 | 2.83 ± 0.14a | 2.83 ± 0.14a | 1.62 ± 0.08c | 2.32 ± 0.12b |
| o-Cymene | 9.888 | 1042 | 5.84 ± 0.29a | 5.76 ± 0.29a | 2.93 ± 0.15c | 4.04 ± 0.2b |
| β-Myrcene | 7.796 | 958 | 8.28 ± 0.41a | 12.93 ± 0.65b | 5.25 ± 0.26c | 5.15 ± 0.26c |
| Phellandrene | 7.685 | 969 | 16.06 ± 0.8a | 15.15 ± 0.76a | 6.67 ± 0.33c | 8.79 ± 0.44b |
| β-Ocimene | 9.65 | 976 | 3.33 ± 0.17b | 4.04 ± 0.2a | – | 1.62 ± 0.08c |
| Caryophyllene | 15.637 | 1494 | 1.21 ± 0.06a | – | – | – |
| α-Pinene | 4.411 | 948 | 1.82 ± 0.09a | 1.92 ± 0.1a | – | – |
| α-Copaene | 14.05 | 1221 | 1.11 ± 0.06a | – | – | 0.61 ± 0.03b |
| Subtotal | 604.47 | 629.33 | 303.7 | 324.41 | ||
| Alcohols | ||||||
| Ethanol | 2.829 | 463 | – | 22.56 ± 1.13c | 248.22 ± 12.41a | 141.84 ± 7.09b |
| 2-Penten-1-ol | 10.871 | 769 | 9.22 ± 0.46a | 1.65 ± 0.08b | – | 0.4 ± 0.02c |
| 1-Hexanol | 11.52 | 860 | 0.54 ± 0.03c | 1.35 ± 0.07a | – | 0.64 ± 0.03b |
| (Z)-3-Hexen-1-ol | 12.062 | 868 | – | 1.58 ± 0.08a | – | 0.3 ± 0.02b |
| 1-Octanol | 14.979 | 1059 | 0.64 ± 0.03b | 1.68 ± 0.08a | – | 1.78 ± 0.09a |
| (E,Z)-3,6-Nonadien-1-ol | 17.564 | 1175 | 1.68 ± 0.08a | 1.82 ± 0.09a | 0.57 ± 0.03c | 1.01 ± 0.05b |
| 3-methyl-1-Butanol | 8.72 | 697 | – | – | 89.18 ± 4.46a | 52.18 ± 2.61b |
| Phenylethyl Alcohol | 19.359 | 1136 | – | 1.92 ± 0.1c | 53.6 ± 2.68a | 29.36 ± 1.47b |
| 2-methyl-1-Propanol | 6.289 | 597 | – | – | 14.85 ± 0.74a | 6.13 ± 0.31b |
| cis-p-Mentha-2,8-dien-1-ol | 18.55 | 1140 | – | 7.37 ± 0.37a | 1.65 ± 0.08c | 4.38 ± 0.22b |
| [R-(R*,R*)]-2,3-Butanediol | 14.564 | 743 | – | – | 4.41 ± 0.22a | 1.14 ± 0.06b |
| 3,7-dimethyl-1,6-Octadien-3-ol | 14.835 | 1082 | 0.77 ± 0.04b | 1.11 ± 0.06a | 0.57 ± 0.03d | 0.67 ± 0.03c |
| α-Terpineol | 16.889 | 1143 | 0.54 ± 0.03a | 0.57 ± 0.03a | – | – |
| Benzyl alcohol | 18.957 | 1036 | – | 1.38 ± 0.07a | – | 0.84 ± 0.04b |
| 2,4-bis(1,1-dimethylethyl)-Phenol | 23.362 | 1555 | 4.48 ± 0.22a | 1.52 ± 0.08c | 2.93 ± 0.15b | 1.11 ± 0.06d |
| 6-Nonen-1-ol | 17.155 | 1167 | – | – | 2.26 ± 0.11a | 2.42 ± 0.12a |
| Eugenol | 21.995 | 1392 | – | 1.18 ± 0.06b | – | 0.84 ± 0.04c |
| Subtotal | 17.88 | 45.69 | 418.24 | 245.06 | ||
| Esters | ||||||
| Ethyl Acetate | 2.35 | 586 | 33.03 ± 1.65a | 5.76 ± 0.29d | 22.12 ± 1.11b | 9.49 ± 0.47c |
| Octanoic acid, ethyl ester | 13.079 | 1183 | 5.05 ± 0.25d | 37.64 ± 1.88c | 317.14 ± 15.86a | 193.22 ± 9.66b |
| Butanoic acid, ethyl ester | 4.816 | 785 | 7.17 ± 0.36b | 7.17 ± 0.36b | 8.59 ± 0.43a | 4.14 ± 0.21c |
| Decanoic acid, ethyl ester | 16.239 | 1381 | 2.22 ± 0.11d | 13.54 ± 0.68c | 116.55 ± 5.83a | 77.17 ± 3.86b |
| Hexanoic acid, ethyl ester | 9.265 | 984 | – | – | 77.87 ± 3.89a | 43.43 ± 2.17b |
| Dodecanoic acid, ethyl ester | 18.801 | 1580 | 1.72 ± 0.09c | – | 15.35 ± 0.77a | 4.04 ± 0.2b |
| Isoamyl acetate | 6.779 | 820 | – | – | 28.58 ± 1.43a | 16.36 ± 0.82b |
| Acetic acid,2-phenylethyl ester | 18.348 | 1259 | – | – | 27.98 ± 1.4a | 9.8 ± 0.49b |
| Octanoic acid, methyl ester | 12.267 | 1083 | – | – | 5.86 ± 0.29a | 5.96 ± 0.3a |
| Ethyl 9-decenoate | 16.913 | 1371 | – | – | 20.81 ± 1.04a | – |
| Tetradecanoic acid, ethyl ester | 21.017 | 1779 | – | – | 4.55 ± 0.23a | 0.61 ± 0.03b |
| Decanoic acid, methyl ester | 15.618 | 1282 | – | – | 5.45 ± 0.27a | 2.93 ± 0.15b |
| Hexanoic acid, methyl ester | 8.229 | 884 | – | 1.21 ± 0.06b | 1.82 ± 0.06a | 1.82 ± 0.09a |
| 4-Terpinenyl acetate | 6.901 | 1327 | – | – | – | 2.83 ± 0.14a |
| Formic acid, butyl ester | 7.261 | 783 | 1.62 ± 0.08b | 2.02 ± 0.11a | – | – |
| (S)-1-Alanine ethylamide | 1.283 | 864 | – | 2.53 ± 0.12b | – | 2.83 ± 0.15a |
| Formic acid, heptyl ester | 13.33 | 1081 | – | 2.42 ± 0.12a | – | – |
| Subtotal | 50.81 | 72.29 | 652.67 | 374.63 | ||
| Acids | ||||||
| Butanoic acid | 15.839 | 775 | 34.64 ± 1.53b | 37.02 ± 1.85a | 14.04 ± 0.7d | 16.82 ± 0.84c |
| Hexanoic acid | 23.507 | 974 | 64.99 ± 3.25a | 22.67 ± 1.13b | 13.58 ± 0.68d | 16.51 ± 0.83c |
| Acetic acid | 13.037 | 576 | – | 55.2 ± 2.76a | – | 17.07 ± 0.85b |
| Octanoic acid | 20.917 | 1173 | 2.88 ± 0.14d | 4.34 ± 0.22c | 20.91 ± 1.05a | 15.91 ± 0.8b |
| n-Decanoic acid | 22.978 | 1372 | 0.91 ± 0.05c | 0.4 ± 0.02d | 13.79 ± 0.69a | 4.75 ± 0.24b |
| Octadecanoic acid | 24.14 | 2167 | 3.23 ± 0.16c | 17.57 ± 0.88b | 27.72 ± 1.39a | 1.46 ± 0.07d |
| Dodecanoic acid | 24.845 | 1570 | 1.31 ± 0.07d | 3.43 ± 0.17a | 1.72 ± 0.09c | 3.08 ± 0.15b |
| n-Hexadecanoic acid | 24.718 | 1968 | – | 9.04 ± 0.45a | – | 2.53 ± 0.13b |
| Subtotal | 107.96 | 149.67 | 91.76 | 78.13 | ||
| Aldehydes | ||||||
| (E,Z)-2,6-Nonadienal | 15.366 | 1120 | 9.29 ± 0.46a | – | – | – |
| Furfural | 13.331 | 831 | 3.74 ± 0.19a | – | – | – |
| (E,E)-2,4-Heptadienal | 13.865 | 921 | 4.24 ± 0.21a | – | – | – |
| Nonanal | 12.286 | 1104 | 3.74 ± 0.19a | – | – | – |
| Citral | 16.762 | 1174 | 7.78 ± 0.39b | 10.5 ± 0.53a | – | 11.51 ± 0.58a |
| 2-Hexenal | 8.754 | 814 | 2.63 ± 0.13a | – | – | – |
| Subtotal | 31.42 | 10.5 | 0 | 11.51 | ||
| Ketones | ||||||
| 3-Penten-2-one | 6.686 | 662 | 17.17 ± 0.86b | 28.89 ± 1.44a | – | – |
| 4-hydroxy-2-Pentanone | 13.22 | 817 | 10.2 ± 0.51a | 9.7 ± 0.48a | 3.13 ± 0.16b | 3.03 ± 0.15b |
| 5-ethyldihydro-2(3H)-Furanone | 16.831 | 986 | 10.4 ± 0.52a | 7.68 ± 0.38b | 2.83 ± 0.14d | 4.75 ± 0.24c |
| 5-butyldihydro-2(3H)-Furanone | 19.425 | 1184 | 3.54 ± 0.18a | 1.62 ± 0.08b | – | – |
| trans-β-Ionone | 19.765 | 1457 | 3.03 ± 0.15a | 1.01 ± 0.05d | 2.32 ± 0.12b | 1.82 ± 0.09c |
| 2-Heptanone | 8.068 | 853 | – | 4.55 ± 0.23a | – | 1.01 ± 0.05b |
| Acetoin | 10.085 | 717 | – | 19.38 ± 0.97a | 9.59 ± 0.48c | 16.16 ± 0.81b |
| 2,3-Butanedione | 3.523 | 691 | – | 6.16 ± 0.31a | – | – |
| 1-(3-methylphenyl)-Ethanone | 17.797 | 1142 | 5.56 ± 0.28a | – | – | – |
| 4-methyl-4-Hexen-3-one | 6.761 | 838 | 4.55 ± 0.23a | – | – | – |
| tetrahydro-6-methyl-2H-Pyran-2-one | 17.936 | 1006 | 13.94 ± 0.7c | 8.32 ± 0.42d | 16.78 ± 0.64a | 15.08 ± 0.75b |
| Subtotal | 68.39 | 87.31 | 34.65 | 41.85 | ||
| Alkanes | ||||||
| (2-methyl-1-propenyl)-Benzene | 12.993 | 1077 | 14.04 ± 0.7a | 12.63 ± 0.63ab | 11.92 ± 0.6b | 10.2 ± 0.51c |
| 1,3,8-p-Menthatriene | 12.243 | 1029 | – | 2.32 ± 0.12a | – | – |
| bis(1-methylethylidene)-Cyclobutene | 12.81 | 983 | 2.63 ± 0.13a | 2.02 ± 0.1b | 1.52 ± 0.08c | 1.01 ± 0.05d |
| Styrene | 9.588 | 883 | – | – | 11.41 ± 0.57a | 2.12 ± 0.11b |
| 2,6,10,14-tetramethyl-Pentadecane | 20.42 | 1653 | – | – | 11.51 ± 0.58a | 7.07 ± 0.35b |
| Heneicosane | 18.106 | 2109 | 12.83 ± 0.64b | 10.91 ± 0.55c | 20.5 ± 1.03a | 18.28 ± 0.91a |
| 2-methyloctacosane | 18.397 | 2840 | 2.32 ± 0.12a | – | – | – |
| Subtotal | 31.82 | 27.88 | 56.86 | 38.68 | ||
| Others | ||||||
| trans-2-(2-Pentenyl)furan | 10.603 | 1048 | 1.82 ± 0.09b | 2.43 ± 0.09a | – | 0.91 ± 0.05c |
| 1,1-diethoxy-Ethane | 2.439 | 705 | – | – | 13.23 ± 0.66a | 6.77 ± 0.34b |
| 2,3-dihydro-Benzofuran | 23.969 | 1036 | – | 3.74 ± 0.19a | 0.81 ± 0.03b | 0.81 ± 0.04b |
| 2,4,5-trimethyl-1,3-Dioxolane | 3.022 | 761 | – | – | 12.02 ± 0.47b | 13.53 ± 0.38a |
| Subtotal | 1.82 | 6.17 | 26.06 | 22.02 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, X.; Chen, W.; Chen, H.; Chen, W.; Zhong, Q. Combination of Lactobacillus plantarum and Saccharomyces cerevisiae DV10 as Starter Culture to Produce Mango Slurry: Microbiological, Chemical Parameters and Antioxidant Activity. Molecules 2019, 24, 4349. https://doi.org/10.3390/molecules24234349
Jin X, Chen W, Chen H, Chen W, Zhong Q. Combination of Lactobacillus plantarum and Saccharomyces cerevisiae DV10 as Starter Culture to Produce Mango Slurry: Microbiological, Chemical Parameters and Antioxidant Activity. Molecules. 2019; 24(23):4349. https://doi.org/10.3390/molecules24234349
Chicago/Turabian StyleJin, Xiaofan, Wenxue Chen, Haiming Chen, Weijun Chen, and Qiuping Zhong. 2019. "Combination of Lactobacillus plantarum and Saccharomyces cerevisiae DV10 as Starter Culture to Produce Mango Slurry: Microbiological, Chemical Parameters and Antioxidant Activity" Molecules 24, no. 23: 4349. https://doi.org/10.3390/molecules24234349
APA StyleJin, X., Chen, W., Chen, H., Chen, W., & Zhong, Q. (2019). Combination of Lactobacillus plantarum and Saccharomyces cerevisiae DV10 as Starter Culture to Produce Mango Slurry: Microbiological, Chemical Parameters and Antioxidant Activity. Molecules, 24(23), 4349. https://doi.org/10.3390/molecules24234349






