An Autophagy Inducing Triterpene Saponin Derived from Aster koraiensis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Elucidation of Astersaponin I
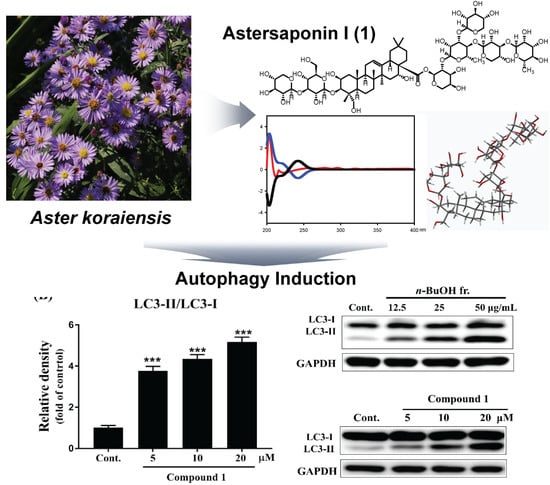
2.2. Bioactivities of Atersaponin I
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Materials
3.3. Extraction and Isolation
3.4. ECD Calculation
3.5. Autophagy Induction Assay
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lee, J.; Lee, Y.M.; Lee, B.W.; Kim, J.H.; Kim, J.S. Chemical constituents from the aerial parts of Aster koraiensis with protein glycation and aldose reductase inhibitory activities. J. Nat. Prod. 2012, 75, 267–270. [Google Scholar] [PubMed]
- Jung, H.J.; Min, B.S.; Park, J.Y.; Kim, Y.H.; Lee, H.K.; Bae, K.H. Gymnasterkoreaynes A-F, cytotoxic polyacetylenes from Gymnaster koraiensis. J. Nat. Prod. 2002, 65, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Dat, N.T.; Cai, X.F.; Shen, Q.; Lee, I.S.; Lee, E.J.; Park, Y.K.; Bae, K.; Kim, Y.H. Gymnasterkoreayne G, a new inhibitory polyacetylene against NFAT transcription factor from Gymnaster koraiensis. Chem. Pharm. Bull. (Tokyo) 2005, 53, 1194–1196. [Google Scholar] [CrossRef] [Green Version]
- Dat, N.T.; Van Kiem, P.; Cai, X.F.; Shen, Q.; Bae, K.; Kim, Y.H. Gymnastone, a new benzofuran derivative from Gymnaster koraiensis. Arch. Pharm. Res. 2004, 27, 1106–1108. [Google Scholar] [PubMed]
- Lee, I.-K.; Kim, K.-H.; Ryu, S.-Y.; Choi, S.-U.; Lee, K.-R. Phytochemical Constituents from the Flowers of Gymnaster koraiensis and Their Cytotoxic Activities in vitro. Bull. Korean Chem. Soc. 2010, 31, 227–229. [Google Scholar]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar]
- Johansen, T.; Lamark, T. Selective autophagy mediated by autophagic adapter proteins. Autophagy 2011, 7, 279–296. [Google Scholar] [CrossRef]
- Parzych, K.R.; Klionsky, D.J. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid. Redox Signal 2014, 20, 460–473. [Google Scholar] [CrossRef] [Green Version]
- Choi, A.M.; Ryter, S.W.; Levine, B. Autophagy in human health and disease. N. Engl. J. Med. 2013, 368, 651–662. [Google Scholar]
- Towers, C.G.; Thorburn, A. Therapeutic Targeting of Autophagy. EBioMedicine 2016, 14, 15–23. [Google Scholar]
- Saha, S.; Panigrahi, D.P.; Patil, S.; Bhutia, S.K. Autophagy in health and disease: A comprehensive review. Biomed. Pharmacother. 2018, 104, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Cherra, S.J., 3rd; Kulich, S.M.; Uechi, G.; Balasubramani, M.; Mountzouris, J.; Day, B.W.; Chu, C.T. Regulation of the autophagy protein LC3 by phosphorylation. J. Cell Biol. 2010, 190, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, S.R.; Mizushima, N. Monitoring and measuring autophagy. Int. J. Mol. Sci. 2017, 18, 1865. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, V.U.; Basha, A. Spectroscopic data of saponins—The Triterpenoid Glycosides; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Kasai, R.; Miyakoshi, M.; Nie, R.-L.; Zhou, J.; Matsumoto, K.; Morita, T.; Nishi, M.; Miyahara, K.; Tanaka, O. Saponins from Bolbostemma paniculatum: Cyclic bisdesmosides, tubeimosides II and III. Phytochemistry 1988, 27, 1439–1446. [Google Scholar] [CrossRef]
- Nielsen, S.E.; Anthoni, U.; Christophersen, C.; Cornett, C. Triterpenoid saponins from Phytolacca rivinoides and Phytolacca bogotensis. Phytochemistry 1995, 39, 625–630. [Google Scholar] [CrossRef]
- Renwick, J.; Scopes, P.M. Optical rotatory dispersion and circular dichroism. Part LXIII. Unsaturated triterpene 28-carboxylic acids and related compounds. J. Chem. Soc. C 1969, 18, 2544–2549. [Google Scholar] [CrossRef]
- Su, Y.; Koike, K.; Nikaido, T.; Liu, J.; Zheng, J.; Guo, D. Conyzasaponins I-Q, Nine New Triterpenoid Saponins from Conyza blinii. J. Nat. Prod. 2003, 66, 1593–1599. [Google Scholar] [CrossRef]
- Mizushima, N. Methods for monitoring autophagy. Int. J. Biochem. Cell. Biol. 2004, 36, 2491–2502. [Google Scholar] [CrossRef]
- Jiang, S.L.; Guan, Y.D.; Chen, X.S.; Ge, P.; Wang, X.L.; Lao, Y.Z.; Xiao, S.S.; Zhang, Y.; Yang, J.M.; Xu, X.J.; et al. Tubeimoside-1, a triterpenoid saponin, induces cytoprotective autophagy in human breast cancer cells in vitro via Akt-mediated pathway. Acta Pharmacol. Sin. 2019, 40, 919–928. [Google Scholar] [CrossRef]
- Mai, T.T.; Moon, J.; Song, Y.; Viet, P.Q.; Phuc, P.V.; Lee, J.M.; Yi, T.H.; Cho, M.; Cho, S.K. Ginsenoside F2 induces apoptosis accompanied by protective autophagy in breast cancer stem cells. Cancer Lett. 2012, 321, 144–153. [Google Scholar] [CrossRef]
- Kwon, J.; Lee, H.; Yoon, Y.D.; Hwang, B.Y.; Guo, Y.; Kang, J.S.; Kim, J.J.; Lee, D. Lanostane Triterpenes Isolated from Antrodia heteromorpha and Their Inhibitory Effects on RANKL-Induced Osteoclastogenesis. J. Nat. Prod. 2016, 79, 1689–1693. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compound are available from the authors. |





| Position | δC | δH (J in Hz) | Intensities | Position | δC | δH (J in Hz) | |
|---|---|---|---|---|---|---|---|
| Aglycone | Sugar Moiety | ||||||
| 1 | 44.6 | 2.09 (dd) J = 14.0 Hz, 2.0 Hz | 2H | Glc | 1′ | 105.3 | 4.49 (d) J = 7.5 Hz |
| 1.18 (dd) J = 14.0 Hz, 3.5 Hz | 2′ | 74.7 | 3.48 (m) | ||||
| 2 | 71.3 | 4.33 (m) | 1H | 3′ | 88.1 | 3.52 (m) | |
| 3 | 84.2 | 3.63 (m) | 1H | 4′ | 71.1 | 3.51 (m) | |
| 4 | 43.3 | 5′ | 77.5 | 3.31 (m) | |||
| 5 | 48.5 | 1.33 (m) | 1H | 6′ | 62.3 | 3.81 (m) | |
| 6 | 18.9 | 1.50 (m) | 2H | 3.71 (m) | |||
| 7 | 34.0 | 1.67 (m) | 2H | Xyl I | 1′′ | 106.2 | 4.51(d) J = 7.5 Hz |
| 1.35 (m) | 2′′ | 73.2 | 3.64 (m) | ||||
| 8 | 41.0 | 1H | 3′′ | 76.3 | 3.23 (m) | ||
| 9 | 48.7, | 1.63 (m) | 1H | 4′′ | 70.2 | 3.81 (m) | |
| 10 | 37.7 | 5′′ | 67.6 | 3.87 (d) J = 11.5 Hz | |||
| 11 | 24.8 | 2.00, (m) | 2H | 3.57 (d) J = 11.5 Hz | |||
| 1.96 (m) | Ara | 1′′ | 94.1 | 5.63 (br d) J = 3.0 Hz | |||
| 12 | 123.9 | 5.38, (br t) J = 3.5 Hz | 1H | 2′′′ | 75.6 | 3.78 (dd) J = 5.0 Hz, 3.0 Hz | |
| 13 | 144.9 | 3′′′ | 70.6 | 3.91 (m) | |||
| 14 | 43.1 | 4′′′ | 66.8 | 3.84 (m) | |||
| 15 | 36.5 | 1.78 (m) | 2H | 5′′′ | 63.4 | 3.92 (m) | |
| 1.39 (m) | 3.49 (m) | ||||||
| 16 | 74.8 | 4.49 (d) J = 5.0 Hz | 1H | Rha I | 1′′′′ | 101.0 | 5.00 (br d) J = 1.5 Hz |
| 17 | 50.5 | 2′′′′ | 72.3 | 4.07 (m) | |||
| 18 | 42.3 | 3.06 (br dd) J=14.0 Hz, 4.0 Hz | 1H | 3′′′′ | 82.7 | 3.87 (m) | |
| 19 | 47.8 | 2.28 (br dd) J = 14.0 Hz, 12.5 Hz | 2H | 4′′′′ | 78.9 | 3.69 (m) | |
| 1.04 (br dd) J = 12.5Hz, 3.5 Hz | 5′′′′ | 69.2 | 3.71 (m) | ||||
| 20 | 31.5 | 6′′′′ | 18.5 | 1.27 (d) J = 6.0 Hz | |||
| 21 | 36.6, | 1.93 (m) | 2H | Xyl II | 1′′′′′ | 105.0 | 4.74 (d) J = 8.0 Hz |
| 1.16 (m) | 2′′′′′ | 75.4 | 3.29 (m) | ||||
| 22 | 32.1 | 1.92 (m) | 2H | 3′′′′′ | 84.5 | 3.41 (m) | |
| 1.80 (m) | 4′′′′′ | 70.4 | 3.50 (m) | ||||
| 23 | 66.0 | 3.63 (m) | 2H | 5′′′′′ | 67.1 | 3.86 (m) | |
| 3.24 (m) | 3.20 (m) | ||||||
| 24 | 15.0 | 0.95 (s) | 3H | Rha II | 1′′′′′′ | 102.8 | 5.14 (br d) J = 1.5 Hz |
| 25 | 17.8 | 1.31 (s) | 3H | 2′′′′′′ | 72.4 | 3.93 (m) | |
| 26 | 18.2 | 0.80 (s) | 3H | 3′′′′′′ | 72.4 | 3.70 (m) | |
| 27 | 27.5 | 1.38 (s) | 3H | 4′′′′′′ | 74.1 | 3.40 (m) | |
| 28 | 177.2 | 5′′′′′′ | 70.1 | 4.02 (m) | |||
| 29 | 25.3 | 0.98 (s) | 3H | 6′′′′′′ | 18.0 | 1.24 (d) J = 6.0 Hz | |
| 30 | 33.5 | 0.89 (s) | 3H | Xyl III | 1′′′′′′′ | 106.3 | 4.50 (d) J = 7.5 Hz |
| 2′′′′′′′ | 75.4 | 3.28 (m) | |||||
| 3′′′′′′′ | 77.8 | 3.32 (m) | |||||
| 4′′′′′′′ | 71.1 | 3.50 (m) | |||||
| 5′′′′′′′ | 67.3 | 3.91 (m) | |||||
| 3.25 (m) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, J.; Ko, K.; Zhang, L.; Zhao, D.; Yang, H.O.; Kwon, H.C. An Autophagy Inducing Triterpene Saponin Derived from Aster koraiensis. Molecules 2019, 24, 4489. https://doi.org/10.3390/molecules24244489
Kwon J, Ko K, Zhang L, Zhao D, Yang HO, Kwon HC. An Autophagy Inducing Triterpene Saponin Derived from Aster koraiensis. Molecules. 2019; 24(24):4489. https://doi.org/10.3390/molecules24244489
Chicago/Turabian StyleKwon, Jaeyoung, Keebeom Ko, Lijun Zhang, Dong Zhao, Hyun Ok Yang, and Hak Cheol Kwon. 2019. "An Autophagy Inducing Triterpene Saponin Derived from Aster koraiensis" Molecules 24, no. 24: 4489. https://doi.org/10.3390/molecules24244489
APA StyleKwon, J., Ko, K., Zhang, L., Zhao, D., Yang, H. O., & Kwon, H. C. (2019). An Autophagy Inducing Triterpene Saponin Derived from Aster koraiensis. Molecules, 24(24), 4489. https://doi.org/10.3390/molecules24244489