Cytisus scoparius and Ulex europaeus Produce Volatile Organic Compounds with Powerful Synergistic Herbicidal Effects
Abstract
:1. Introduction
2. Results
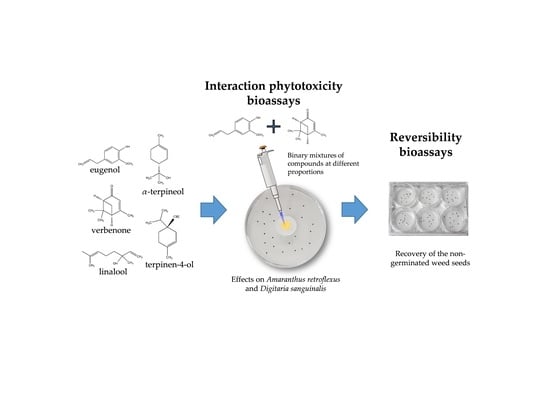
2.1. Phytotoxicity of the Volatile Compounds Applied in Pairs
2.2. Reversibility Bioassays
3. Discussion
4. Materials and Methods
4.1. Standard Compounds and Target Species
4.2. Phytotoxicity Bioassays of the Volatile Compounds Applied in Pairs
4.3. Reversibility Bioassays
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Soltys, D.; Krasuska, U.; Bogatek, R.; Gniazdowska, A. Allelochemicals as Bioherbicides-Present and Perspectives. In Herbicides-Current Research and Case Studies in Use; Price, A.J., Kelton, J.A., Eds.; InTech: Rijeka, Croatia, 2013; pp. 517–542. [Google Scholar] [CrossRef]
- Duke, S.O. Why have no new herbicide modes of action appeared in recent years? Pest. Manag. Sci. 2012, 68, 505–512. [Google Scholar] [CrossRef] [Green Version]
- Westwood, J.H.; Charudattan, R.; Duke, S.O.; Fennimore, S.A.; Marrone, P.; Slaughter, D.C.; Swanton, C.; Zollinger, R. Weed Management in 2050: Perspectives on the Future of Weed Science. Weed Sci. 2018, 66, 275–285. [Google Scholar] [CrossRef] [Green Version]
- Dayan, F.E. Current Status and Future Prospects in Herbicide Discovery. Plants 2019, 8, 341. [Google Scholar] [CrossRef] [Green Version]
- Heap, I. The International Survey of Herbicide Resistant Weeds. Available online: www.weedscience.com (accessed on 21 October 2019).
- Kudsk, P.; Streibig, J.C. Herbicides–a two-edged sword. Weed Res. 2003, 43, 90–102. [Google Scholar] [CrossRef]
- Dayan, F.E.; Duke, S.O. Natural compounds as next generation herbicides. Plant Physiol. 2014, 166, 1090–1105. [Google Scholar] [CrossRef] [Green Version]
- Benvenuti, S.; Cioni, P.L.; Flamini, G.; Pardossi, A. Weeds for weed control: Asteraceae essential oils as natural herbicides. Weed Res. 2017, 57, 342–353. [Google Scholar] [CrossRef]
- Brilli, F.; Loreto, F.; Baccelli, I. Exploiting plant volatile organic compounds (VOCs) in agriculture to improve sustainable defense strategies and productivity of crops. Front. Plant Sci. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Duke, S.O.; Romagni, J.G.; Dayan, F.E. Natural products as sources for new mechanisms of herbicidal action. Crop Prot. 2000, 19, 583–589. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kohli, R.K. Allelopathic interactions and allelochemicals: New possibilities for sustainable weed management. Crit Rev. Plant. Sci 2003, 22, 239–311. [Google Scholar] [CrossRef]
- Einhellig, F.A. Allelopathy: Current status and future goals. In Allelopathy: Organisms, Processes and Applications; Inderjit, Dakshini, K.M.M., Einhellig, F.A., Eds.; American Chemical Society: Washington, DC, USA, 1994; Volume 582, pp. 1–24. [Google Scholar] [CrossRef] [Green Version]
- Inderjit; Duke, S.O. Ecophysiological aspects of allelopathy. Planta 2003, 217, 529–539. [Google Scholar] [CrossRef]
- Reigosa, M.; Gomes, A.S.; Ferreira, A.G.; Borghetti, F. Allelopathic research in Brazil. Acta Bot. Brasilica 2013, 27, 629–646. [Google Scholar] [CrossRef] [Green Version]
- Xuan, T.D.; Shinkichi, T.; Khanh, T.D.; Chung, I.M. Biological control of weeds and plant pathogens in paddy rice by exploiting plant allelopathy: An overview. Crop Prot. 2005, 24, 197–206. [Google Scholar] [CrossRef]
- Puig, C.G.; Gonçalves, R.F.; Valentão, P.; Andrade, P.B.; Reigosa, M.J.; Pedrol, N. The consistency between phytotoxic effects and the dynamics of allelochemicals release from Eucalyptus globulus leaves used as bioherbicide green manure. J. Chem. Ecol. 2018, 44, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Puig, C.G.; Revilla, P.; Barreal, M.E.; Reigosa, M.J.; Pedrol, N. On the suitability of Eucalyptus globulus green manure for field weed control. Crop Prot. 2019, 121, 57–65. [Google Scholar] [CrossRef]
- Basanta, M.; Diaz Vizcaino, E.; Casal, M.; Morey, M. Diversity measurements in shrubland communities of Galicia (NW Spain). Plant Ecol. 1989, 82, 105–112. [Google Scholar] [CrossRef]
- Clements, D.R.; Peterson, D.J.; Prasad, R. The biology of Canadian weeds. 112. Ulex europaeus L. Can. J. Plant Sci. 2001, 81, 325–337. [Google Scholar]
- Peterson, D.J.; Prasad, R. The biology of Canadian weeds. 109. Cytisus scoparius (L.) Link. Can. J. Plant Sci. 1998, 78, 497–504. [Google Scholar] [CrossRef]
- Sundararajan, R.; Koduru, R. Cytisus scoparius: A review of ethnomedical, phytochemical and pharmacological information. Indo Am. J. Pharm. Res. 2014, 4, 2151–2169. [Google Scholar]
- Lores, M.; Pájaro, M.; Álvarez-Casas, M.; Domínguez, J.; García-Jares, C. Use of ethyl lactate to extract bioactive compounds from Cytisus scoparius: Comparison of pressurized liquid extraction and medium scale ambient temperature systems. Talanta 2015, 140, 134–142. [Google Scholar] [CrossRef]
- Pinela, J.; Barros, L.; Carvalho, A.M.; Ferreira, I.C. Influence of the drying method in the antioxidant potential and chemical composition of four shrubby flowering plants from the tribe Genisteae (Fabaceae). Food Chem. Toxicol. 2011, 49, 2983–2989. [Google Scholar] [CrossRef]
- López-Hortas, L.; Conde, E.; Falqué, E.; Domínguez, H. Flowers of Ulex europaeus L.–Comparing two extraction techniques (MHG and distillation). C. R. Chimi. 2016, 19, 718–725. [Google Scholar] [CrossRef]
- Máximo, P.; Lourenço, A.; Feio, S.S.; Roseiro, J.C. Flavonoids from Ulex airensis and Ulex europaeus ssp. europaeus. J. Nat. Prod. 2002, 65, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Spínola, V.; Llorent-Martínez, E.J.; Gouveia-Figueira, S.; Castilho, P.C. Ulex europaeus: From noxious weed to source of valuable isoflavones and flavanones. Ind. Crops Prod. 2016, 90, 9–27. [Google Scholar] [CrossRef]
- Pardo-Muras, M.; Puig, C.G.; López-Nogueira, A.; Cavaleiro, C.; Pedrol, N. On the bioherbicide potential of Ulex europaeus and Cytisus scoparius: Profiles of volatiles organic compounds and their phytotoxic effects. PLoS ONE 2018, 13, e0205997. [Google Scholar] [CrossRef] [PubMed]
- Meissle, M.; Mouron, P.; Musa, T.; Bigler, F.; Pons, X.; Vasileiadis, V.P.; Otto, S.; Antichi, D.; Kiss, J.; Pálinkás, Z.; et al. Pests, pesticide use and alternative options in European maize production: Current status and future prospects. J. Appl. Entomol. 2010, 134, 357–375. [Google Scholar] [CrossRef]
- Vokou, D.; Douvli, P.; Blionis, G.J.; Halley, J.M. Effects of monoterpenoids, acting alone or in pairs, on seed germination and subsequent seedling growth. J. Chem. Ecol. 2003, 29, 2281–2301. [Google Scholar] [CrossRef]
- Araniti, F.; Graña, E.; Reigosa, M.J.; Sanchez-Moreiras, A.M.; Abenavoli, M.R. Individual and joint activity of terpenoids, isolated from Calamintha nepeta extract, on Arabidopsis thaliana. Nat. Prod. Res. 2013, 27, 2297–2303. [Google Scholar] [CrossRef]
- Silva, E.A.D.; Lobo, L.T.; Silva, G.A.; Souza Filho, A.P.D.S.; Silva, M.N.; Arruda, A.C.; Guilhon, G.M.S.P.; Santos, L.S.; Arruda, M.S.P. Flavonoids from leaves of Derris urucu: Assessment of potential effects on seed germination and development of weeds. An. Acad. Bras. Cienc. 2013, 85, 881–889. [Google Scholar] [CrossRef]
- Feng, G.; Chen, M.; Ye, H.C.; Zhang, Z.K.; Li, H.; Chen, L.L.; Chen, X.L.; Yan, C.; Zhang, J. Herbicidal activities of compounds isolated from the medicinal plant Piper sarmentosum. Ind. Crops Prod. 2019, 132, 41–47. [Google Scholar] [CrossRef]
- Asplund, R.O. Some quantitative aspects of the phytotoxicity of monoterpenes. Weed Sci. 1969, 17, 454–455. [Google Scholar] [CrossRef]
- Dudai, N.; Poljakoff-Maybe, A.; Mayer, A.M.; Putievsky, E.; Lerner, H.R. Essential oils as allelochemicals and their potential use as bioherbicides. J. Chem. Ecol. 1999, 25, 1079–1089. [Google Scholar] [CrossRef]
- Sobrero, M.C.; Ronco, A. Ensayo de toxicidad aguda con semillas de lechuga (Lactuca sativa L.). In Ensayos toxicológicos y métodos de evaluación de calidad de aguas. Estandarización, intercalibración, resultados y aplicaciones, 1st ed.; Castillo, G., Ed.; IDRC: Ottawa, ON, Canada, 2004; Volume 1, pp. 71–79. [Google Scholar]
- Sturchio, E.; Boccia, P.; Zanellato, M.; Meconi, C.; Donnarumma, L.; Mercurio, G.; Mecozzi, M. Molecular and structural changes induced by essential oils treatments in Vicia faba roots detected by genotoxicity testing. J. Toxicol. Environ. Health, Part. A 2016, 79, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Muras, M.; Puig, C.G.; Souto, C.; Pedrol, N. More about the bioherbicide potential of Ulex europaeus and Cytisus scoparius: Profiles of water-soluble compounds and their phytotoxic effects. J. Chem. Ecol. under review.
- Mayer, A.M.; Poljakoff-Mayber, A. The Germination of Seeds, 1st ed.; Pergamon Press: Oxford, UK, 1963; p. 244. [Google Scholar]
- Chiapusio, G.; Sanchez, A.M.; Reigosa, M.J.; Gonzalez, L.; Pellissier, F. Do germination indices adequately reflect allelochemical effects on the germination process? J. Chem. Ecol. 1997, 23, 2445–2453. [Google Scholar] [CrossRef]
- De Bertoldi, C.; De Leo, M.; Braca, A.; Ercoli, L. Bioassay-guided isolation of allelochemicals from Avena sativa L.: Allelopathic potential of flavone C-glycosides. Chemoecology 2009, 19, 169–176. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds eugenol, verbenone, α-terpineol, terpinen-4-ol, and linalool are available from the authors. |





| Pair | Pre-Treatment Participation of the First Compound (%) | Germination (% ± SD) | |||||
|---|---|---|---|---|---|---|---|
| Amaranthus retroflexus | p-value | Digitaria sanguinalis | p-value | ||||
| Eugenol/verbenone | 0 | 10.0 ± 8.17 | b | 0.022 | 25.0 ± 19.1 | a | 0.412 |
| 25 | 45.0 ± 10.0 | a | 45.0 ± 34.2 | a | |||
| 50 | 50.0 ± 25.8 | a | 20.0 ± 16.3 | a | |||
| 75 | 40.0 ± 16.3 | a | 35.0 ± 10.0 | a | |||
| 100 | # | # | |||||
| Eugenol/terpinen-4-ol | 0 | # | 0.493 | # | 0.064 | ||
| 25 | 55.0 ± 19.1 | 5.0 ± 10.0 | a | ||||
| 50 | 70.0 ± 34.6 | 30.0 ± 11.5 | a | ||||
| 75 | 50.0 ± 11.5 | 20.0 ± 16.3 | a | ||||
| 100 | # | # | |||||
| Eugenol/α-terpineol | 0 | 50.0 ± 20.0 | ab | 0.022 | 15.0 ± 10.0 | a | 0.538 |
| 25 | 30.0 ± 25.8 | b | 20.0 ± 16.3 | a | |||
| 50 | 80.0 ± 16.3 | a | 15.0 ± 19.1 | a | |||
| 75 | 40.0 ± 16.3 | b | 5.0 ± 10.0 | a | |||
| 100 | # | # | |||||
| Eugenol/linalool | 0 | # | 0.704 | 13.2 ± 5.3 | a | 0.888 | |
| 25 | 80.0 ± 28.3 | a | 15.0 ± 10.0 | a | |||
| 50 | 60.0 ± 43.2 | a | 15.0 ± 19.1 | a | |||
| 75 | 75.0 ± 30.0 | a | 20.0 ± 16.3 | a | |||
| 100 | # | # | |||||
| Verbenone/α-terpineol | 0 | 50.0 ± 20.0 | a | 0.009 | 15.0 ± 10.0 | a | 0.749 |
| 25 | 50.0 ± 24.6 | a | 15.0 ± 10.0 | a | |||
| 50 | 50.0 ± 25.8 | a | 25.0 ± 25.2 | a | |||
| 75 | 20.0 ± 11.3 | b | 30.0 ± 25.8 | a | |||
| 100 | 10.0 ± 8.17 | b | 25.0 ± 19.1 | a | |||
| Verbenone/linalool | 0 | # | 0.023 | 13.2 ± 5.3 | a | 0.315 | |
| 25 | 55.0 ± 10.0 | a | 20.0 ± 16.3 | a | |||
| 50 | 40.0 ± 16.3 | ab | 25.0 ± 10.0 | a | |||
| 75 | 40.0 ± 28.3 | ab | 40.0 ± 28.3 | a | |||
| 100 | 10.0 ± 8.17 | b | 25.0 ± 19.1 | a | |||
| α-Terpineol/linalool | 0 | # | 0.714 | 13.2 ± 5.3 | a | 0.720 | |
| 25 | 45.0 ± 19.1 | a | 25.0 ± 10.0 | a | |||
| 50 | 65.0 ± 19.1 | a | 30.0 ± 11.5 | a | |||
| 75 | 50.0 ± 38.3 | a | 20.0 ± 40.0 | a | |||
| 100 | 50.0 ± 20.0 | a | 15.0 ± 10.0 | a | |||
| Verbenone/terpinen-4-ol | 0 | # | 0.039 | # | 0.227 | ||
| 25 | 25.0 ± 10.0 | b | 30.0 ± 25.8 | a | |||
| 50 | 45.0 ± 11.23 | a | 15.0 ± 10.0 | a | |||
| 75 | 55.0 ± 14.3 | a | 45.0 ± 19.1 | a | |||
| 100 | 10.0 ± 8.17 | b | 25.0 ± 19.1 | a | |||
| α-Terpineol/terpinen-4-ol | 0 | # | 0.292 | # | 0.726 | ||
| 25 | 50.0 ± 25.8 | a | 10.0 ± 11.5 | a | |||
| 50 | 70.0 ± 25.8 | a | 20.0 ± 16.3 | a | |||
| 75 | 35.0 ± 25.2 | a | 15.0 ± 10.0 | a | |||
| 100 | 50.0 ± 20.0 | a | 15.0 ± 10.0 | a | |||
| Terpinen-4-ol/linalool | 0 | # | 0.943 | 13.2 ± 5.3 | a | 0.906 | |
| 25 | 45.0 ± 19.1 | a | # | ||||
| 50 | 50.0 ± 25.8 | a | 15.0 ± 19.1 | a | |||
| 75 | 50.0 ± 25.8 | a | # | ||||
| 100 | # | # | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pardo-Muras, M.; G. Puig, C.; Pedrol, N. Cytisus scoparius and Ulex europaeus Produce Volatile Organic Compounds with Powerful Synergistic Herbicidal Effects. Molecules 2019, 24, 4539. https://doi.org/10.3390/molecules24244539
Pardo-Muras M, G. Puig C, Pedrol N. Cytisus scoparius and Ulex europaeus Produce Volatile Organic Compounds with Powerful Synergistic Herbicidal Effects. Molecules. 2019; 24(24):4539. https://doi.org/10.3390/molecules24244539
Chicago/Turabian StylePardo-Muras, María, Carolina G. Puig, and Nuria Pedrol. 2019. "Cytisus scoparius and Ulex europaeus Produce Volatile Organic Compounds with Powerful Synergistic Herbicidal Effects" Molecules 24, no. 24: 4539. https://doi.org/10.3390/molecules24244539
APA StylePardo-Muras, M., G. Puig, C., & Pedrol, N. (2019). Cytisus scoparius and Ulex europaeus Produce Volatile Organic Compounds with Powerful Synergistic Herbicidal Effects. Molecules, 24(24), 4539. https://doi.org/10.3390/molecules24244539