Menthol Increases Bendiocarb Efficacy Through Activation of Octopamine Receptors and Protein Kinase A
Abstract
:1. Introduction
2. Results
2.1. Electrophysiological Tests
2.2. Calcium Imaging
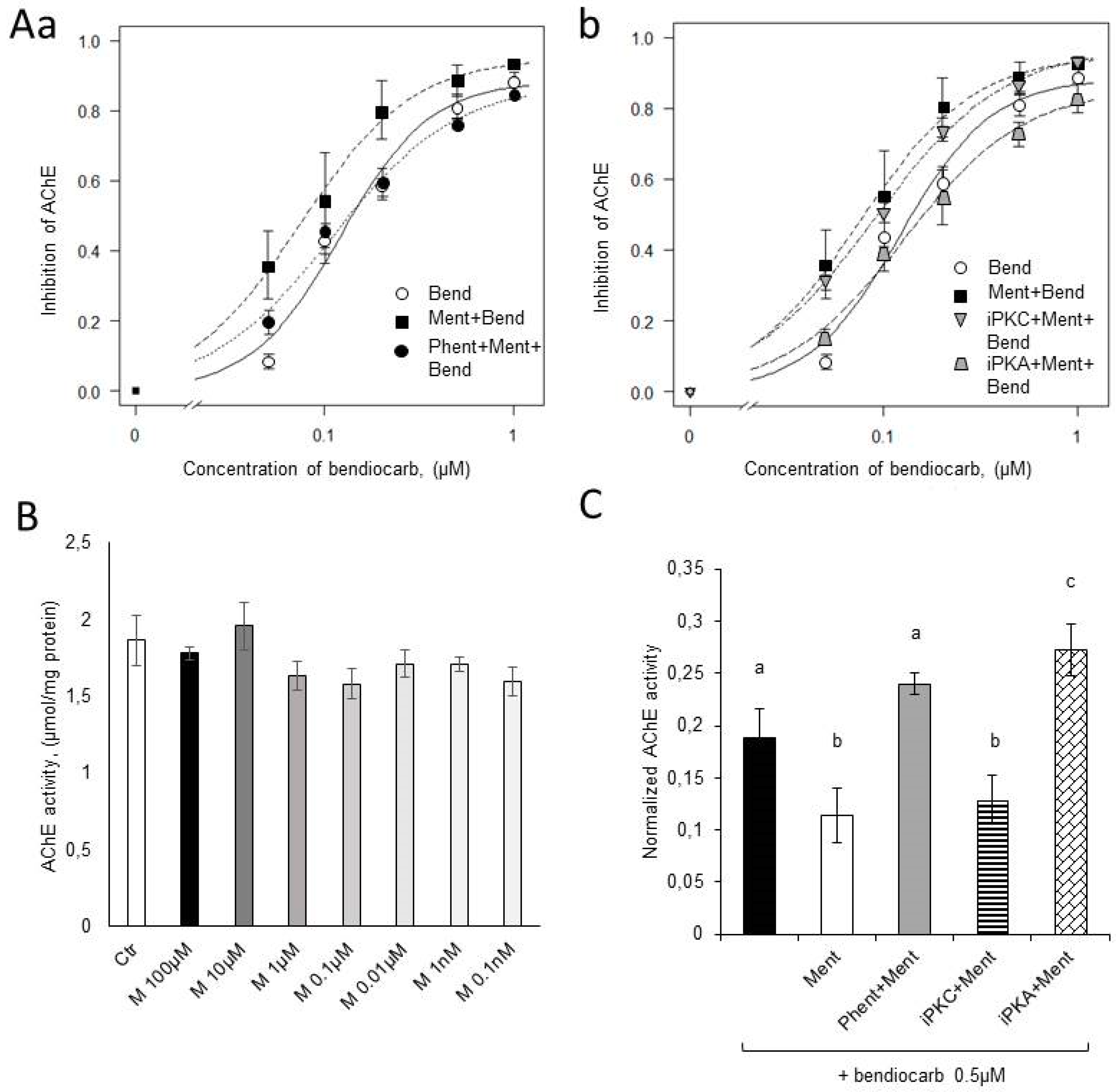
2.3. Acetylcholinesterase Activity
3. Discussion
4. Materials and Methods
4.1. Insects
4.2. Reagents
4.3. Electrophysiological Experiments
4.4. Acetylcholinesterase Activity—Biochemical Tests
4.5. Calcium Imaging
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sparks, T.C. Insecticide discovery: An evaluation and analysis. Pestic. Biochem. Physiol. 2013, 107, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Sudo, M.; Takahashi, D.; Andow, D.A.; Suzuki, Y.; Yamanaka, T. Optimal management strategy of insecticide resistance under various insect life histories: Heterogeneous timing of selection and interpatch dispersal. Evol. Appl. 2018, 11, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, X.; Shen, J.; Li, D.; Wan, H.; You, H.; Li, J. Cross-resistance and biochemical mechanisms of resistance to indoxacarb in the diamondback moth, Plutella xylostella. Pestic. Biochem. Physiol. 2017, 140, 85–89. [Google Scholar] [CrossRef]
- Messenger, L.A.; Shililu, J.; Irish, S.R.; Anshebo, G.Y.; Tesfaye, A.G.; Ye-Ebiyo, Y.; Chibsa, S.; Dengela, D.; Dissanayake, G.; Kebede, E.; et al. Insecticide resistance in Anopheles arabiensis from Ethiopia (2012–2016): A nationwide study for insecticide resistance monitoring. Malar. J. 2017, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Appel, A.G. Insecticide resistance of several field-collected german cockroach (Dictyoptera: Blattellidae) Strains. J. Econ. Entomol. 2017, 110, 1203–1209. [Google Scholar] [CrossRef]
- Ffrench-Constant, R.H. The molecular genetics of insecticide resistance. Genetics 2013, 194, 807–815. [Google Scholar] [CrossRef]
- Schmuck, R.; Lewis, G. Review of field and monitoring studies investigating the role of nitro-substituted neonicotinoid insecticides in the reported losses of honey bee colonies (Apis mellifera). Ecotoxicology 2016, 25, 1617–1629. [Google Scholar] [CrossRef]
- Burns, C.J.; Pastoor, T.P. Pyrethroid epidemiology: A quality-based review. Crit. Rev. Toxicol. 2018, 48, 297–311. [Google Scholar] [CrossRef]
- Corbel, V.; Chandre, F.; Darriet, F.; Lardeux, F.; Hougard, J.-M. Synergism between permethrin and propoxur against Culex quinquefasciatus mosquito larvae. Med. Vet. Entomol. 2003, 17, 158–164. [Google Scholar] [CrossRef]
- Corbel, V.; Stankiewicz, M.; Bonnet, J.; Grolleau, F.; Hougard, J.; Lapied, B. Synergism between insecticides permethrin and propoxur occurs through activation of presynaptic muscarinic negative feedback of acetylcholine release in the insect central nervous system. Neurotoxicology 2006, 27, 508–519. [Google Scholar] [CrossRef]
- Oxborough, R.M.; N’Guessan, R.; Kitau, J.; Tungu, P.K.; Malone, D.; Mosha, F.W.; Rowland, M.W. A new class of insecticide for malaria vector control: Evaluation of mosquito nets treated singly with indoxacarb (oxadiazine) or with a pyrethroid mixture against Anopheles gambiae and Culex quinquefasciatus. Malar. J. 2015, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ngufor, C.; Fongnikin, A.; Rowland, M.; N’Guessan, R. Indoor residual spraying with a mixture of clothianidin (a neonicotinoid insecticide) and deltamethrin provides improved control and long residual activity against pyrethroid resistant Anopheles gambiae sl in Southern Benin. PLoS ONE 2017, 12, e0189575. [Google Scholar] [CrossRef] [PubMed]
- Lavialle-Defaix, C.; Moignot, B.; Legros, C.; Lapied, B. How does calcium-dependent intracellular regulation of voltage-dependent sodium current increase the sensitivity to the oxadiazine insecticide indoxacarb metabolite decarbomethoxylated JW062 (DCJW) in insect pacemaker neurons? J. Pharmacol. Exp. Ther. 2010, 333, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Apaire-Marchais, V.; Ogliastro, M.; Chandre, F.; Pennetier, C.; Raymond, V.; Lapied, B. Virus and calcium: An unexpected tandem to optimize insecticide efficacy. Environ. Microbiol. Rep. 2016, 8, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Raymond, V.; Goven, D.; Benzidane, Y.; List, O.; Lapied, B. Influence of cellular and molecular factors on membrane target sensitivity to insecticides. Curr. Med. Chem. 2017, 24, 2974–2987. [Google Scholar] [CrossRef] [PubMed]
- Deshayes, C.; Moreau, E.; Pitti-Caballero, J.; Froger, J.-A.; Apaire-Marchais, V.; Lapied, B. Synergistic agent and intracellular calcium, a successful partnership in the optimization of insecticide efficacy. Curr. Opin. Insect Sci. 2018, 30, 52–58. [Google Scholar] [CrossRef]
- Abd-Ella, A.; Stankiewicz, M.; Mikulska, K.; Nowak, W.; Pennetier, C.; Goulu, M.; Fruchart-Gaillard, C.; Licznar, P.; Apaire-Marchais, V.; List, O.; et al. The repellent DEET potentiates carbamate effects via insect muscarinic receptor interactions: An alternative strategy to control insect vector-borne diseases. PLoS ONE 2015, 10, e0126406. [Google Scholar] [CrossRef]
- Jankowska, M.; Lapied, B.; Jankowski, W.; Stankiewicz, M. The unusual action of essential oil component, menthol, in potentiating the effect of the carbamate insecticide, bendiocarb. Pestic. Biochem. Physiol. 2019, 158, 101–111. [Google Scholar] [CrossRef]
- Pavela, R. History, presence and perspective of using plant extracts as commercial botanical insecticides and farm products for protection against insects—A review. Plant Prot. Sci. 2016, 52, 229–241. [Google Scholar]
- Pavela, R. Acute toxicity and synergistic and antagonistic effects of the aromatic compounds of some essential oils against Culex quinquefasciatus Say larvae. Parasitol. Res. 2015, 114, 3835–3853. [Google Scholar] [CrossRef]
- Pavela, R.; Bartolucci, F.; Desneux, N.; Lavoir, A.-V.; Canale, A.; Maggi, F.; Benelli, G. Chemical profiles and insecticidal efficacy of the essential oils from four Thymus taxa growing in central-southern Italy. Ind. Crops Prod. 2019, 138, 111460. [Google Scholar] [CrossRef]
- Mansour, S.A.; El-Sharkawy, A.Z.; Abdel-Hamid, N.A. Toxicity of essential plant oils, in comparison with conventional insecticides, against the desert locust, Schistocerca gregaria (Forskål). Ind. Crops Prod. 2015, 63, 92–99. [Google Scholar] [CrossRef]
- Kostyukovsky, M.; Rafaeli, A.; Gileadi, C.; Demchenko, N.; Shaaya, E. Activation of octopaminergic receptors by essential oil constituents isolated from aromatic plants: Possible mode of action against insect pests. Pest Manag. Sci. 2002, 58, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, M.; Rogalska, J.; Wyszkowska, J.; Stankiewicz, M. Molecular targets for components of essential oils in the insect nervous system—A review. Molecules 2017, 23, 34. [Google Scholar] [CrossRef] [PubMed]
- Price, D.N.; Berry, M.S. Comparison of effects of octopamine and insecticidal essential oils on activity in the nerve cord, foregut, and dorsal unpaired median neurons of cockroaches. J. Insect Physiol. 2006, 52, 309–319. [Google Scholar] [CrossRef]
- Farooqui, T. Review of octopamine in insect nervous systems. Open Access Insect Physiol. 2012, 4, 1–17. [Google Scholar] [CrossRef]
- Farooqui, T. Octopamine-mediated neuromodulation of insect senses. Neurochem. Res. 2007, 32, 1511–1529. [Google Scholar] [CrossRef]
- Maqueira, B.; Chatwin, H.; Evans, P.D. Identification and characterization of a novel family of Drosophila β-adrenergic-like octopamine G-protein coupled receptors. J. Neurochem. 2005, 94, 547–560. [Google Scholar] [CrossRef]
- Bischof, L.J.; Enan, E.E. Cloning, expression and functional analysis of an octopamine receptor from Periplaneta americana. Insect Biochem. Mol. Biol. 2004, 34, 511–521. [Google Scholar] [CrossRef]
- Casida, J.E.; Durkin, K.A. Anticholinesterase insecticide retrospective. Chem. Biol. Interact. 2013, 203, 221–225. [Google Scholar] [CrossRef]
- Thapa, S.; Lv, M.; Xu, H. Acetylcholinesterase: A primary target for drugs and insecticides. Mini Rev. Med. Chem. 2017, 17, 1665–1676. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Indoor Residual Spraying. An Operational Manual for Indoor Residual Spraying (IRS) for Malaria Transmission Control and Elimination; World Health Organization: Copenhagen, Denmark, 2015. [Google Scholar]
- The European Commission. Commission Directive 2012/3/EU of 9 February 2012 amending Directive 98/8/EC of the European Parliament and of the Council to include bendiocarb as an active substance in Annex I thereto. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32012L0003 (accessed on 20 October 2019).
- Stankiewicz, M.; Dąbrowski, M.; de Lima, M.E. Nervous System of Periplaneta americana Cockroach as a Model in Toxinological Studies: A Short Historical and Actual View. J. Toxicol. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bräunig, P.; Pflüger, H.-J. The unpaired median neurons of insects. Adv. Insect Phys. 2001, 28, 185–266. [Google Scholar]
- Grolleau, F.; Lapied, B. Dorsal unpaired median neurones in the insect central nervous system: Towards a better understanding of the ionic mechanisms underlying spontaneous electrical activity. J. Exp. Biol. 2000, 203, 1633–1648. [Google Scholar]
- Gross, A.D.; Norris, E.J.; Kimber, M.J.; Bartholomay, L.C.; Coats, J.R. Essential oils enhance the toxicity of permethrin against Aedes aegypti and Anopheles gambiae. Med. Vet. Entomol. 2017, 31, 55–62. [Google Scholar] [CrossRef]
- Norris, E.; Johnson, J.; Gross, A.; Bartholomay, L.; Coats, J. Plant essential oils enhance diverse pyrethroids against multiple strains of mosquitoes and inhibit detoxification enzyme processes. Insects 2018, 9, 132. [Google Scholar] [CrossRef]
- Faraone, N.; Hillier, N.K.; Cutler, G.C. Plant essential oils synergize and antagonize toxicity of different conventional insecticides against Myzus persicae (Hemiptera: Aphididae). PLoS ONE 2015, 10, e0127774. [Google Scholar] [CrossRef]
- Verlinden, H.; Vleugels, R.; Marchal, E.; Badisco, L.; Pflüger, H.-J.; Blenau, W.; Broeck, J. Vanden The role of octopamine in locusts and other arthropods. J. Insect Physiol. 2010, 56, 854–867. [Google Scholar] [CrossRef]
- Li, Y.; Hoffmann, J.; Li, Y.; Stephano, F.; Bruchhaus, I.; Fink, C.; Roeder, T. Octopamine controls starvation resistance, life span and metabolic traits in Drosophila. Sci. Rep. 2016, 6, 35359. [Google Scholar] [CrossRef] [PubMed]
- Balfanz, S.; Jordan, N.; Langenstück, T.; Breuer, J.; Bergmeier, V.; Baumann, A. Molecular, pharmacological, and signaling properties of octopamine receptors from honeybee (Apis mellifera) brain. J. Neurochem. 2014, 129, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.D.; Maqueira, B. Insect octopamine receptors: A new classification scheme based on studies of cloned Drosophila G-protein coupled receptors. Invertebr. Neurosci. 2005, 5, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Zucchi, R.; Chiellini, G.; Scanlan, T.S.; Grandy, D.K. Trace amine-associated receptors and their ligands. Br. J. Pharmacol. 2009, 149, 967–978. [Google Scholar] [CrossRef]
- Blenau, W.; Rademacher, E.; Baumann, A. Plant essential oils and formamidines as insecticides/acaricides: What are the molecular targets? Apidologie 2012, 43, 334–347. [Google Scholar] [CrossRef]
- Achenbach, H.; Walther, C.; Wicher, D. Octopamine modulates ionic currents and spiking in dorsal unpaired median (DUM) neurons. Neuroreport 1997, 8, 3737–3741. [Google Scholar] [CrossRef]
- Wicher, D.; Penzlin, H. Ca2+ currents in central insect neurons: Electrophysiological and pharmacological properties. J. Neurophysiol. 1997, 77, 186–199. [Google Scholar] [CrossRef]
- Grolleau, F.; Lapied, B. Two distinct low-voltage-activated Ca2+ currents contribute to the pacemaker mechanism in cockroach dorsal unpaired median neurons. J. Neurophysiol. 1996, 76, 963–976. [Google Scholar] [CrossRef]
- Defaix, A.; Lapied, B. Role of a novel maintained low-voltage-activated inward current permeable to sodium and calcium in pacemaking of insect neurosecretory neurons. Invertebr. Neurosci. 2005, 5, 135–146. [Google Scholar] [CrossRef]
- Lapied, B.; Defaix, A.; Stankiewicz, M.; Moreau, E.; Raymond, V. Modulation of low-voltage-activated inward current permeable to sodium and calcium by DARPP-32 drives spontaneous firing of insect octopaminergic neurosecretory cells. Front. Syst. Neurosci. 2017, 11, 31:1–31:12. [Google Scholar] [CrossRef]
- Wicher, D.; Penzlin, H. Ca2+ currents in cockroach neurones. Neuroreport 1994, 5, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Grohmann, L.; Blenau, W.; Erber, J.; Ebert, P.R.; Strünker, T.; Baumann, A. Molecular and functional characterization of an octopamine receptor from honeybee (Apis mellifera) brain. J. Neurochem. 2003, 86, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Enan, E. Insecticidal activity of essential oils: Octopaminergic sites of action. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2001, 130, 325–337. [Google Scholar] [CrossRef]
- Huang, J.; Wu, S.-F.; Li, X.-H.; Adamo, S.A.; Ye, G.-Y. The characterization of a concentration-sensitive α-adrenergic-like octopamine receptor found on insect immune cells and its possible role in mediating stress hormone effects on immune function. Brain Behav. Immun. 2012, 26, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Beggs, K.T.; Tyndall, J.D.A.; Mercer, A.R. Honey bee dopamine and octopamine receptors linked to intracellular calcium signaling have a close phylogenetic and pharmacological relationship. PLoS ONE 2011, 6, e26809. [Google Scholar] [CrossRef]
- Balfanz, S.; Strünker, T.; Frings, S.; Baumann, A. A family of octapamine receptors that specifically induce cyclic AMP production or Ca2+ release in Drosophila melanogaster. J. Neurochem. 2005, 93, 440–451. [Google Scholar] [CrossRef]
- Yau, K.W. Cyclic nucleotide-gated channels: An expanding new family of ion channels. Proc. Natl. Acad. Sci. USA 1994, 91, 3481–3483. [Google Scholar] [CrossRef]
- Kaupp, U.B.; Seifert, R. Cyclic nucleotide-gated ion channels. Physiol. Rev. 2002, 82, 769–824. [Google Scholar] [CrossRef]
- Cooper, D.M.F.; Mons, N.; Karpen, J.W. Adenylyl cyclases and the interaction between calcium and cAMP signalling. Nature 1995, 374, 421–424. [Google Scholar] [CrossRef]
- Colvin, R.A.; Oibo, J.A.; Allen, R.A. Calcium inhibition of cardiac adenylyl cyclase. Evidence for two distinct sites of inhibition. Cell Calcium 1991, 12, 19–27. [Google Scholar] [CrossRef]
- Iourgenko, V.; Levin, L.R. A calcium-inhibited Drosophila adenylyl cyclase. Biochim. Biophys. Acta 2000, 1495, 125–139. [Google Scholar] [CrossRef]
- Guillou, J.L.; Nakata, H.; Cooper, D.M. Inhibition by calcium of mammalian adenylyl cyclases. J. Biol. Chem. 1999, 274, 35539–35545. [Google Scholar] [CrossRef] [PubMed]
- Wicher, D. Peptidergic modulation of insect voltage-gated Ca2+ currents: Role of resting Ca2+ current and protein kinases A and C. J. Neurophysiol. 2001, 86, 2353–2362. [Google Scholar] [CrossRef] [PubMed]
- Nemer, A.; Azab, A.N.; Rimon, G.; Lamprecht, S.; Ben-Menahem, D. Different roles of cAMP/PKA and PKC signaling in regulating progesterone and PGE2 levels in immortalized rat granulosa cell cultures. Gen. Comp. Endocrinol. 2018, 269, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Guo, S.-Z.; Lu, K.-H.; Li, H.-Y.; Li, X.-D.; Zhang, L.-X.; Yang, L. Different roles of PKC and PKA in effect of interferon-gamma on proliferation and collagen synthesis of fibroblasts. Acta Pharmacol. Sin. 2004, 25, 1320–1326. [Google Scholar] [PubMed]
- Bohnsack, J.P.; Carlson, S.L.; Morrow, A.L. Differential regulation of synaptic and extrasynaptic α4 GABA(A) receptor populations by protein kinase A and protein kinase C in cultured cortical neurons. Neuropharmacology 2016, 105, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Michel, L.Y.M.; Verkaart, S.; Latta, F.; Hoenderop, J.G.J.; Bindels, R.J.M. Differential regulation of the Na+ -Ca2+ exchanger 3 (NCX3) by protein kinase PKC and PKA. Cell Calcium 2017, 65, 52–62. [Google Scholar] [CrossRef]
- Ochi, H.; Kume, N.; Nishi, E.; Kita, T. Elevated levels of cAMP inhibit protein kinase C– independent mechanisms of endothelial platelet-derived growth factor–B chain and intercellular adhesion molecule-1 gene induction by lysophosphatidylcholine. Circ. Res. 1995, 77, 530–535. [Google Scholar] [CrossRef]
- Rahamim Ben-Navi, L.; Almog, T.; Yao, Z.; Seger, R.; Naor, Z. A-kinase anchoring protein 4 (AKAP4) is an ERK1/2 substrate and a switch molecule between cAMP/PKA and PKC/ERK1/2 in human spermatozoa. Sci. Rep. 2016, 6, 37922. [Google Scholar] [CrossRef]
- Bodereau-Dubois, B.; List, O.; Calas-List, D.; Marques, O.; Communal, P.-Y.; Thany, S.H.; Lapied, B. Transmembrane potential polarization, calcium influx, and receptor conformational state modulate the sensitivity of the imidacloprid-insensitive neuronal insect nicotinic acetylcholine receptor to neonicotinoid insecticides. J. Pharmacol. Exp. Ther. 2012, 341, 326–339. [Google Scholar] [CrossRef]
- Licznar, P.; List, O.; Goven, D.; Ndong Nna, R.; Lapied, B.; Apaire-Marchais, V. A novel method using Autographa californica multiple nucleopolyhedrovirus for increasing the sensitivity of insecticide through calcium influx in insect cell line. J. Virol. Methods 2014, 195, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Pitti Caballero, J.; Murillo, L.; List, O.; Bastiat, G.; Flochlay-Sigognault, A.; Guerino, F.; Lefrançois, C.; Lautram, N.; Lapied, B.; Apaire-Marchais, V. Nanoencapsulated deltamethrin as synergistic agent potentiates insecticide effect of indoxacarb through an unusual neuronal calcium-dependent mechanism. Pestic. Biochem. Physiol. 2019, 157, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Grifman, M.; Arbel, A.; Ginzberg, D.; Glick, D.; Elgavish, S.; Shaanan, B.; Soreq, H. In vitro phosphorylation of acetylcholinesterase at non-consensus protein kinase A sites enhances the rate of acetylcholine hydrolysis. Brain Res. Mol. Brain Res. 1997, 51, 179–187. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing 2018. Available online: https://www.scirp.org/(S(lz5mqp453edsnp55rrgjct55))/reference/ReferencesPapers.aspx?ReferenceID=2342186 (accessed on 20 October 2019).
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef]
- Zeileis, A.; Hothorn, T. Diagnostic Checking in Regression Relationships. Available online: https://cran.r-project.org/web/packages/lmtest/vignettes/lmtest-intro.pdf (accessed on 20 October 2019).
- Zeileis, A. Econometric Computing with HC and HAC Covariance Matrix Estimators. J. Stat. Softw. 2004, 11, 1–17. [Google Scholar] [CrossRef]
Sample Availability: Not available. |


| Sample Name | Physiological Saline | Menthol (µM) | Bendiocarb (µM) | Phentolamine (µM) | iPKC (µM) | iPKA (µM) |
|---|---|---|---|---|---|---|
| Control | + | |||||
| Ment | 0.0001, 0.001, 0.01, 0.1, 1, 10, 100 | |||||
| Bend | 0.05, 0.1, 0.2, 0.5, 1 | |||||
| Ment + Bend | 0.1 | 0.05, 0.1, 0.2, 0.5, 1 | ||||
| Phent + Ment + Bend | 0.1 | 0.05, 0.1, 0.2, 0.5, 1 | 10 | |||
| iPKC + Ment + Bend | 0.1 | 0.05, 0.1, 0.2, 0.5, 1 | 1 | |||
| iPKA + Ment + Bend | 0.1 | 0.05, 0.1, 0.2, 0.5, 1 | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jankowska, M.; Wiśniewska, J.; Fałtynowicz, Ł.; Lapied, B.; Stankiewicz, M. Menthol Increases Bendiocarb Efficacy Through Activation of Octopamine Receptors and Protein Kinase A. Molecules 2019, 24, 3775. https://doi.org/10.3390/molecules24203775
Jankowska M, Wiśniewska J, Fałtynowicz Ł, Lapied B, Stankiewicz M. Menthol Increases Bendiocarb Efficacy Through Activation of Octopamine Receptors and Protein Kinase A. Molecules. 2019; 24(20):3775. https://doi.org/10.3390/molecules24203775
Chicago/Turabian StyleJankowska, Milena, Justyna Wiśniewska, Łukasz Fałtynowicz, Bruno Lapied, and Maria Stankiewicz. 2019. "Menthol Increases Bendiocarb Efficacy Through Activation of Octopamine Receptors and Protein Kinase A" Molecules 24, no. 20: 3775. https://doi.org/10.3390/molecules24203775
APA StyleJankowska, M., Wiśniewska, J., Fałtynowicz, Ł., Lapied, B., & Stankiewicz, M. (2019). Menthol Increases Bendiocarb Efficacy Through Activation of Octopamine Receptors and Protein Kinase A. Molecules, 24(20), 3775. https://doi.org/10.3390/molecules24203775