Anti-Biofilm Effects of Synthetic Antimicrobial Peptides Against Drug-Resistant Pseudomonas aeruginosa and Staphylococcus aureus Planktonic Cells and Biofilm
Abstract
:1. Introduction
2. Results and Discussion
2.1. Growth Inhibition of Planktonic Cells by Peptides
2.2. Inhibitory Kinetics of Peptides on Bacterial Growth
2.3. Biofilm Formation Inhibition
2.4. Reductive Effects of Peptides on Preformed Biofilms
2.5. Effect of Peptides on Biofilm Components
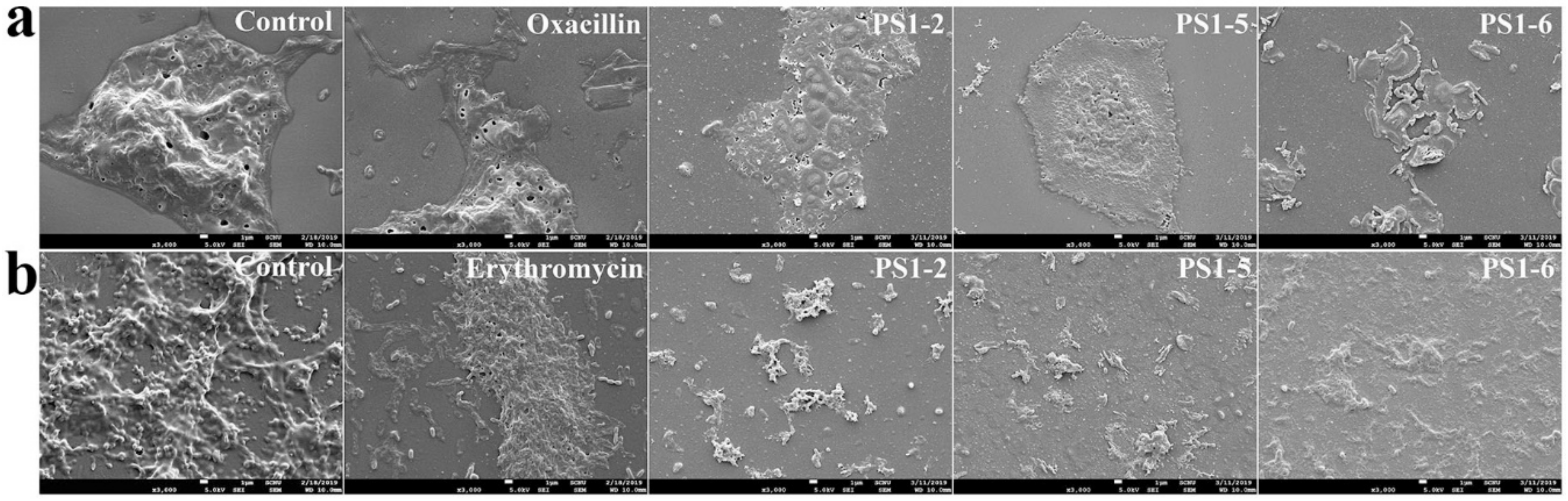
2.6. Biofilm Reduction in the Presence of Peptides
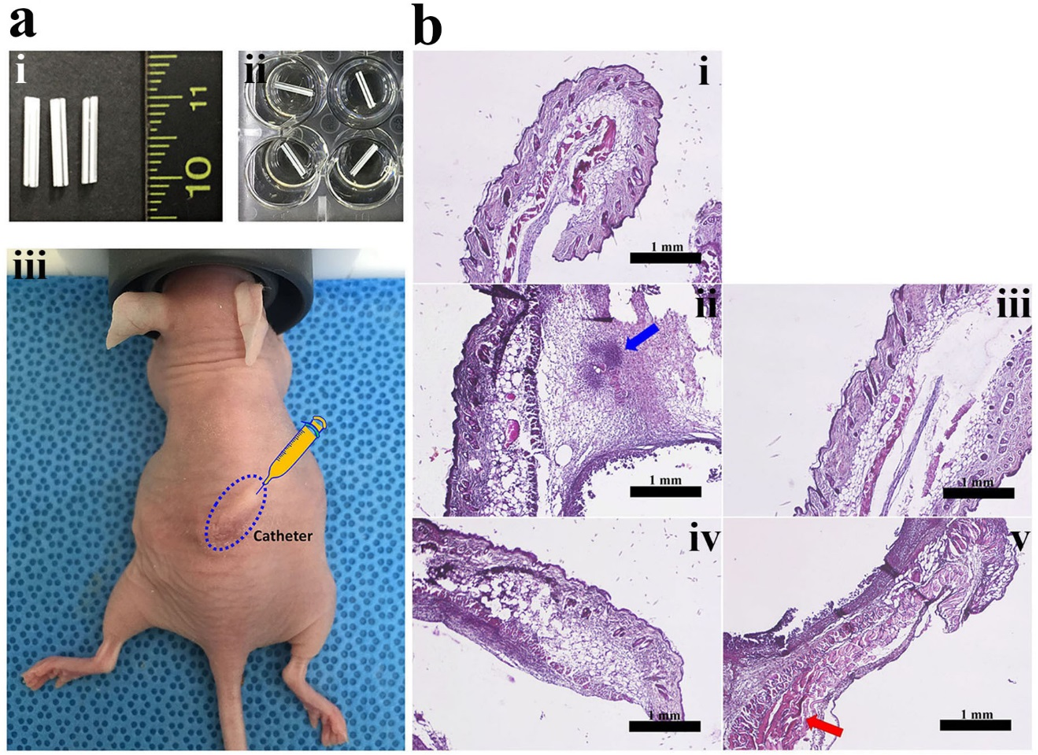
2.7. In Vivo Anti-Biofilm Action of PS Peptides
3. Materials and Methods
3.1. Materials
3.2. Peptide Synthesis by Solid-Phase Method
3.3. Bacterial Strains and Growth Conditions
3.4. Biofilm Susceptibility Assay
3.4.1. Growth Inhibition in Planktonic Bacterial Cells
3.4.2. Inhibition of Biofilm Formation Assay
3.4.3. Reductive Assay in Preformed Biofilm
3.5. Growth Inhibition Kinetics
3.6. Reductive EPS Analyses
3.7. Scanning Electron Microscopy
3.8. In Vivo Study
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gomes Von Borowski, R.; Gnoatto, S.C.B.; Macedo, A.J.; Gillet, R. Promising antibiofilm activity of peptidomimetics. Front. Microbiol. 2018, 9, 2157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petchiappan, A.; Chatterji, D. Antibiotic resistance: Current perspectives. ACS Omega 2017, 2, 7400–7409. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Willcox, M.D.P.; Dutta, D. Action of antimicrobial peptides against bacterial biofilms. Materials 2018, 11, 2468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Romling, U.; Kjelleberg, S.; Normark, S.; Nyman, L.; Uhlin, B.E.; Akerlund, B. Microbial biofilm formation: A need to act. J. Intern. Med. 2014, 276, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Montanaro, L.; Arciola, C.R. Biofilm in implant infections: Its production and regulation. Int. J. Art. Organs 2005, 28, 1062–1068. [Google Scholar] [CrossRef]
- Pelling, H.; Nzakizwanayo, J.; Milo, S.; Denham, E.L.; MacFarlane, W.M.; Bock, L.J.; Sutton, J.M.; Jones, B.V. Bacterial biofilm formation on indwelling urethral catheters. Lett. Appl. Microbiol. 2019, 68, 277–293. [Google Scholar] [CrossRef] [Green Version]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [Green Version]
- Hao, Y.; Huang, X.; Zhou, X.; Li, M.; Ren, B.; Peng, X.; Cheng, L. Influence of dental prosthesis and restorative materials interface on oral biofilms. Int. J. Mol. Sci. 2018, 19, 3157. [Google Scholar] [CrossRef] [Green Version]
- Zimmerli, W.; Sendi, P. Orthopaedic biofilm infections. Apmis 2017, 125, 353–364. [Google Scholar] [CrossRef] [Green Version]
- Rupp, M.E.; Majorant, D. Prevention of vascular catheter-related bloodstream infections. Infect. Dis. Clin. North Am. 2016, 30, 853–868. [Google Scholar] [CrossRef]
- Hardy, L.; Cerca, N.; Jespers, V.; Vaneechoutte, M.; Crucitti, T. Bacterial biofilms in the vagina. Res. Microbiol. 2017, 168, 865–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kriebel, K.; Hieke, C.; Müller-Hilke, B.; Nakata, M.; Kreikemeyer, B. Oral Biofilms from symbiotic to pathogenic interactions and associated disease-Connection of periodontitis and rheumatic arthritis by peptidylarginine deiminase. Front. Microbiol. 2018, 9, 53. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; Jr Palmer, R.J.; Periasamy, S.; Jakubovics, N.S. Oral multispecies biofilm development and the key role of cell-cell distance. Nat. Rev. Microbiol. 2010, 8, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Park, S.C.; Park, Y.; Hahm, K.S. The role of antimicrobial peptides in preventing multidrug-resistant bacterial infections and biofilm formation. Int. J. Mol. Sci. 2011, 12, 5971–5992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrova, O.E.; Sauer, K. Sticky situations: Key components that control bacterial surface attachment. J. Bacteriol. 2012, 194, 2413–2425. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Q.; Chen, J.; Yang, C.; Yin, Y.; Yao, K. Quorum Sensing: A Prospective Therapeutic Target for Bacterial Diseases. Biomed. Res. Int. 2019, 2019, 2015978. [Google Scholar] [CrossRef] [Green Version]
- Senadheera, D.; Cvitkovitch, D.G. Quorum sensing and biofilm formation by Streptococcus mutans. Adv. Exp. Med. Biol. 2008, 631, 178–188. [Google Scholar]
- Overhage, J.; Campisano, A.; Bains, M.; Torfs, E.C.; Rehm, B.H.; Hancock, R.E. Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect. Immun. 2008, 76, 4176–4182. [Google Scholar] [CrossRef] [Green Version]
- Saporito, P.; Vang Mouritzen, M.; Løbner-Olesen, A.; Jenssen, H. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell Microbiol. 2004, 6, 269–275. [Google Scholar]
- Haisma, E.M.; de Breij, A.; Chan, H.; van Dissel, J.T.; Drijfhout, J.W.; Hiemstra, P.S.; El Ghalbzouri, A.; Nibbering, P.H. LL-37-derived peptides eradicate multidrug-resistant Staphylococcus aureus from thermally wounded human skin equivalents. Antimicrob. Agents Chemother. 2014, 58, 4411–4419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mataraci, E.; Dosler, S. In vitro activities of antibiotics and antimicrobial cationic peptides alone and in combination against methicillin-resistant Staphylococcus aureus biofilms. Antimicrob. Agents Chemother. 2012, 56, 6366–6371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, S.N.; Dias, S.A.; Cruz, A.F.; Mil-Homens, D.; Fernandes, F.; Valle, J.; Andreu, D.; Prieto, M.; Castanho, M.A.R.B.; Coutinho, A.; et al. The mechanism of action of pepR, a viral-derived peptide, against Staphylococcus aureus biofilms. J. Antimicrob. Chemother. 2019, 74, 2617–2625. [Google Scholar] [CrossRef] [PubMed]
- Brancatisano, F.L.; Maisetta, G.; Di Luca, M.; Esin, S.; Bottai, D.; Bizzarri, R.; Campa, M.; Batoni, G. Inhibitory effect of the human liver-derived antimicrobial peptide hepcidin 20 on biofilms of polysaccharide intercellular adhesin (PIA)-positive and PIA-negative strains of Staphylococcus epidermidis. Biofouling 2014, 30, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Quiles, F.; Saadi, S.; Francius, G.; Bacharouche, J.; Humbert, F. In situ and real time investigation of the evolution of a Pseudomonas fluorescens nascent biofilm in the presence of an antimicrobial peptide. BBA-biomembranes 2016, 1858, 75–84. [Google Scholar] [CrossRef]
- Åberg, A.; Shingler, V.; Balsalobre, C. (p)ppGpp regulates type 1 fimbriation of Escherichia coli by modulating the expression of the site-specific recombinase FimB. Mol. Microbiol. 2006, 6, 1520–1533. [Google Scholar] [CrossRef]
- Hinsa, S.M.; Espinosa-Urgel, M.; Ramos, J.L.; O’Toole, G.A. Transition from reversible to irreversible attachment during biofilm formation by Pseudomonas fluorescens WCS365 requires an ABC transporter and a large secreted protein. Mol. Microbiol. 2003, 49, 905–918. [Google Scholar] [CrossRef]
- Lee, M.Y.; Park, S.C.; Jung, M.; Shin, M.K.; Kang, H.L.; Baik, S.C.; Cheong, G.W.; Jang, M.K.; Lee, W.K. Cell-selectivity of tryptophan and tyrosine in amphiphilic α-helical antimicrobial peptides against drug-resistant bacteria. Biochem. Biophys. Res. Commun. 2018, 505, 478–484. [Google Scholar] [CrossRef]
- Cutrona, K.J.; Kaufman, B.A.; Figueroa, D.M.; Elmore, D.E. Role of arginine and lysine in the antimicrobial mechanism of histone-derived antimicrobial peptides. FEBS Lett. 2015, 589, 3915–3920. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, D.J.; Kim, D.T.; Steinman, L.; Fathman, C.G.; Rothbard, J.B. Polyarginine enters cells more efficiently than other polycationic homopolymers. J. Pept. Res. 2000, 56, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Stanzl, E.G.; Trantow, B.M.; Vargas, J.R.; Wender, P.A. Fifteen years of cell-penetrating, guanidinium-rich molecular transporters: Basic science, research tools, and clinical applications. Accounts Chem. Res. 2013, 46, 2944–2954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.R.; Huang, Y.W.; Aronstam, R.S.; Lee, H.J. Comparative mechanisms of protein transduction mediated by cell-penetrating peptides in prokaryotes. J. Membr. Biol. 2015, 248, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Park, S.C.; Kim, J.Y.; Kim, E.J.; Cheong, G.W.; Lee, Y.; Choi, W.; Lee, J.R.; Jang, M.K. Hydrophilic linear peptide with histidine and lysine residues as a key factor affecting antifungal activity. Int. J. Mol. Sci. 2018, 19, 3781. [Google Scholar] [CrossRef] [Green Version]
- Park, S.C.; Kim, J.Y.; Jeong, C.; Yoo, S.; Hahm, K.S.; Park, Y. A plausible mode of action of pseudin-2, an antimicrobial peptide from Pseudis paradoxa. BBA-biomembranes 2011, 1808, 171–182. [Google Scholar] [CrossRef] [Green Version]
- Johnsen, A.R.; Hausner, M.; Schnell, A.; Wuertz, S. Evaluation of fluorescently labeled lectins for noninvasive localization of extracellular polymeric substances in Sphingomonas biofilms. Appl. Environ. Microbiol. 2000, 66, 3487–3491. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Sreenivasan, P.K.; Subramanyam, R.; Cummins, D. Multiparameter assessments to determine the effects of sugars and antimicrobials on a polymicrobial oral biofilm. Appl. Environ. Microbiol. 2006, 72, 6734–6742. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Lee, D.; Tay, J.; Show, K. Staining of extracellular polymeric substances and cells in bioaggregates. Appl. Microb. Biotechnol. 2007, 75, 467–474. [Google Scholar] [CrossRef]
- Park, S.C.; Kim, I.R.; Hwang, J.E.; Kim, J.Y.; Jung, Y.J.; Choi, W.; Lee, L.; Jang, M.K.; Lee, J.R. Functional mechanisms underlying the antimicrobial activity of the Oryza sativa Trx-like protein. Int. J. Mol. Sci. 2019, 20, 1413. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Not available. |




| Bacteria | MIC (µM (µg/mL)) | |||||
|---|---|---|---|---|---|---|
| PS1-2 | PS1-5 | PS1-6 | Gentamicin | Oxacillin | Erythromycin | |
| P. aeruginosa | ||||||
| ATCC 15692 | 2 (3.67) | 2 (4) | 2 (3.37) | 1 (0.96) | 4 (3.21) | - |
| CCARM 2073 | 2 (3.67) | 2 (4) | 2 (3.37) | 256 (244) | 512 (411) | - |
| CCARM 2075 | 2 (3.67) | 2 (4) | 1 (1.68) | 256 (244) | 512 (411) | - |
| DRPa 4007 | 4 (7.34) | 2 (4) | 2 (3.37) | 512 (489) | 256 (206) | - |
| DRPa 3241 | 2 (3.67) | 2 (4) | 2 (3.37) | 512 (489) | 128 (103) | - |
| S. aureus | ||||||
| ATCC 25923 | 4 (7.34) | 32 (64) | 2 (3.37) | - | 8 (6.42) | 2 (1.47) |
| CCARM 3125 | 4 (7.34) | 16 (32) | 2 (3.37) | - | 512 (411) | 256 (188) |
| CCARM 3709 | 2 (3.67) | 16 (32) | 2 (3.37) | - | 32 (25.7) | 128 (93.9) |
| DRSa 3399 | 4 (7.34) | 2 (4) | 2 (3.37) | - | 512 (411) | 256 (188) |
| DRSa 3518 | 2 (3.67) | 2 (4) | 2 (3.37) | - | 256 (206) | 512 (376) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-C.; Lee, M.-Y.; Kim, J.-Y.; Kim, H.; Jung, M.; Shin, M.-K.; Lee, W.-K.; Cheong, G.-W.; Lee, J.R.; Jang, M.-K. Anti-Biofilm Effects of Synthetic Antimicrobial Peptides Against Drug-Resistant Pseudomonas aeruginosa and Staphylococcus aureus Planktonic Cells and Biofilm. Molecules 2019, 24, 4560. https://doi.org/10.3390/molecules24244560
Park S-C, Lee M-Y, Kim J-Y, Kim H, Jung M, Shin M-K, Lee W-K, Cheong G-W, Lee JR, Jang M-K. Anti-Biofilm Effects of Synthetic Antimicrobial Peptides Against Drug-Resistant Pseudomonas aeruginosa and Staphylococcus aureus Planktonic Cells and Biofilm. Molecules. 2019; 24(24):4560. https://doi.org/10.3390/molecules24244560
Chicago/Turabian StylePark, Seong-Cheol, Min-Young Lee, Jin-Young Kim, Hyeonseok Kim, Myunghwan Jung, Min-Kyoung Shin, Woo-Kon Lee, Gang-Won Cheong, Jung Ro Lee, and Mi-Kyeong Jang. 2019. "Anti-Biofilm Effects of Synthetic Antimicrobial Peptides Against Drug-Resistant Pseudomonas aeruginosa and Staphylococcus aureus Planktonic Cells and Biofilm" Molecules 24, no. 24: 4560. https://doi.org/10.3390/molecules24244560







