Highly Efficient Aggregation-Induced Room-Temperature Phosphorescence with Extremely Large Stokes Shift Emitted from Trinuclear Gold(I) Complex Crystals
Abstract
:1. Introduction
2. Results and Discussion
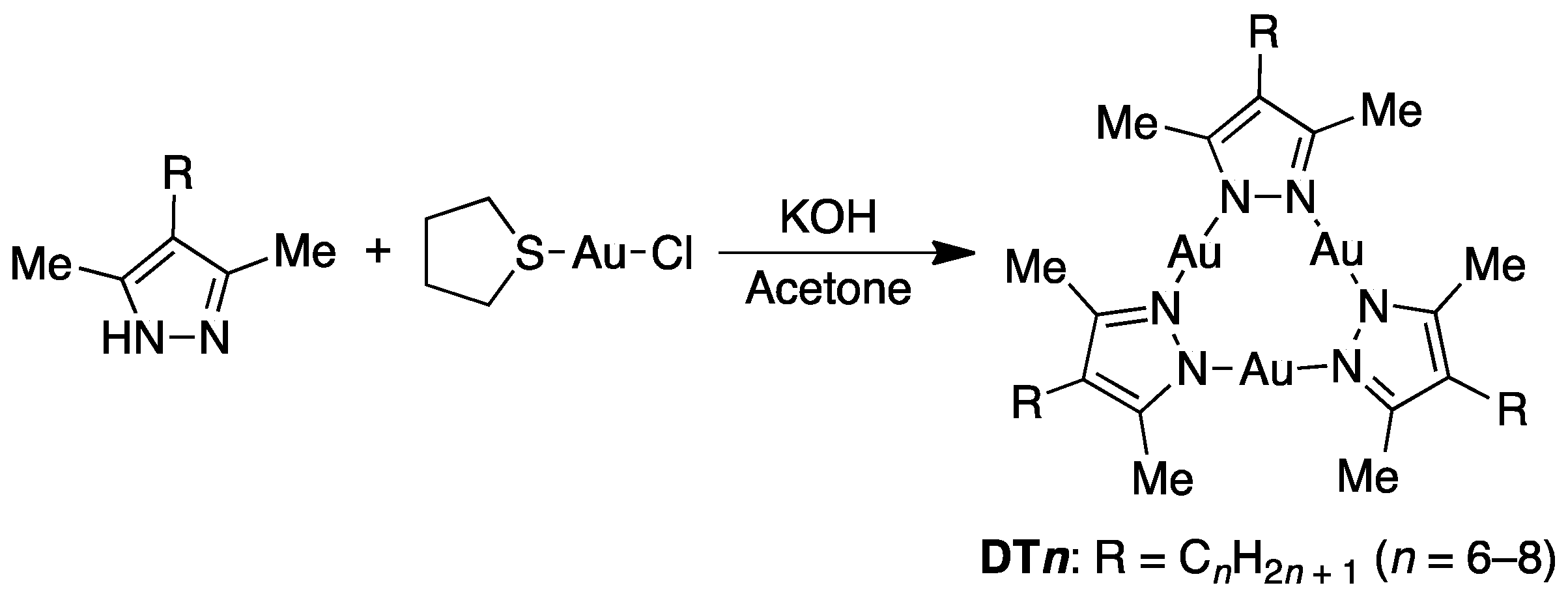
2.1. Synthesis and Characterization of Complexes
2.2. Photoluminescence Behavior of the Complexes
2.3. Relationship between the Room-Temperature Phosphorescence Properties and Aggregated Structure
3. Materials and Methods
3.1. Preparation of Materials
3.2. Single Crystal X-ray Structure
3.3. Photophysical Properties
3.4. Computational Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mei, J.; Leung, N.L.; Kwok, R.T.; Lam, J.W.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, Unite We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Qin, A.; Tang, B.Z. Aggregation-Induced Emission: Fundamentals; John Wiley& Sons, Ltd.: West Sussex, UK, 2014. [Google Scholar]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birks, J.B. Photophysics of Aromatic Molecules; Wiley: London, UK, 1970. [Google Scholar]
- Malkin, J. Photophysical and Photochemical Properties of Aromatic Compounds; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]
- Ronda, C.R. Luminescence: From Theory to Applications; Ronda, C.R., Ed.; Wiley-VCH Verlag GmbH &Co. KGaA: Weinheim, Germany, 2008; pp. 1–34. [Google Scholar]
- Leung, C.W.; Hong, Y.; Chen, S.; Zhao, E.; Lam, J.W.; Tang, B.Z. A photostable AIE luminogen for specific mitochondrial imaging and tracking. J. Am. Chem. Soc. 2013, 135, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.D.; Lam, J.W.Y.; Qin, W.; Li, J.; Xie, N.; Tang, B.Z. Molecular luminogens based on restriction of intramolecular motions through host-guest inclusion for cell imaging. Chem. Commun. 2014, 50, 1725–1727. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, J.; Liu, T.; Zhang, G.; Liu, S. Highly sensitive and selective fluorometric off-on K+ probe constructed via host-guest molecular recognition and aggregation-induced emission. J. Mater. Chem. 2012, 22, 8622–8628. [Google Scholar] [CrossRef]
- Huang, J.; Tang, R.; Zhang, T.; Li, Q.; Yu, G.; Xie, S.; Liu, Y.; Ye, S.; Qin, J.; Li, Z. A New Approch to prepare Efficient Blue AIE Emitters for Undoped OLEDs. Chemistry 2014, 20, 5317–5326. [Google Scholar] [CrossRef]
- Li, H.; Chi, Z.; Zhang, X.; Xu, B.; Liu, S.; Zhang, Y.; Xu, J. New Thermally Stable Aggregation-Induced Emission Enhancement Compounds for Non-Doped Red Light-Emitting Diodes. Chem. Commun. 2011, 47, 11273–11275. [Google Scholar] [CrossRef]
- Zhoa, W.; Cheung, T.S.; Jiang, N.; Huang, W.; Lam, J.W.Y.; Zhang, X.; He, Z.; Tang, B.Z. Boosting the Efficiency of Organic Persistent Room-Temperature Phosphorescence by Intramolecular Triplet-Triplet Energy Transfer. Nat. Commun. 2019, 10, 1595. [Google Scholar] [CrossRef] [Green Version]
- Crespo, O. Modern Supramolecular Gold Chemistry; Laguna, A., Ed.; Wiley-VCH Verlag GmbH &Co. KGaA: Weinheim, Germany, 2009; pp. 65–129. [Google Scholar]
- Yam, V.W.W.; Au, V.K.; Leung, S.Y. Light-Emission Self-Assembled Materials Based on d8 and d10 Transition Metal Complexes. Chem. Rev. 2015, 115, 7589–7728. [Google Scholar] [CrossRef]
- Yang, C.; Messerschmidt, M.; Coppens, P.; Omary, M.A. Trinuclear Gold(I) Triazolates: A New Class of Wide-Band Phosphors and Sensors. Inorg. Chem. 2006, 45, 6592–6594. [Google Scholar] [CrossRef]
- Schmidbaur, H. The Aurophilicity Phenomenon: A Decade of Experimental Findings, Theoretical Concepts and Emerging Applications. Gold Bull. 2000, 33, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Schmidbaur, H.; Schier, A. Aurophilic Interactions as a Subject of Current Research: An Up-Date. Chem. Soc. Rev. 2012, 41, 370–412. [Google Scholar] [CrossRef] [PubMed]
- Baron, M.; Tubaro, C.; Biffis, A.; Basato, M.; Graiff, C.; Poater, A.; Cavallo, L.; Armaroli, N.; Accorsi, G. Blue-Emitting Dinuclear N-heterocyclic Dicarbene Gold(I) Complex Featuring a Nearly Unit Quantum Yield. Inorg. Chem. 2012, 51, 1178–1784. [Google Scholar] [CrossRef] [PubMed]
- Kishimura, A.; Yamasita, T.; Aida, T. Phosphorescent Organogels via “Mettalophilic” Interactions for Reversible RGB-Color Switching. J. Am. Chem. Soc. 2005, 127, 179–183. [Google Scholar] [CrossRef] [PubMed]
- White-Morris, R.L.; Olmstead, M.M.; Attar, S.; Balch, A.L. Intermolecular Interactions in Polymorphs of Trinuclear Gold(I) Complexes: Insight into the Solvoluminescence of AuI3(MeN=COMe)3. Inorg. Chem. 2005, 44, 5021–5029. [Google Scholar] [CrossRef] [PubMed]
- Balch, A.L.; Olmstead, M.M.; Vickery, J.C. Gold(I) Compounds without Significant Aurophilic Intermolecular Interactions: Synthesis, Structure, and Electronic Properties of Ph3PAuC(O)NHMe and Au3(PhCH2N=COMe)3: Comparative Monomeric and Trimeric Analogues of the Solvoluminescent Trimer, Au3(MeN=COMe)3. Inorg. Chem. 1999, 38, 3494–3499. [Google Scholar]
- Vickery, J.C.; Olmstead, M.M.; Fung, E.Y.; Balch, A.L. Solvent-Stimulated Luminescence from the Supramolecular Aggregation of a Trinuclear Gold (i) Complex that Displays Extensive Intermolecular AuċAu Interactions. Angew. Chem. Int. Ed. Engl. 1997, 36, 1179–1181. [Google Scholar] [CrossRef]
- Ito, H.; Saito, T.; Oshima, N.; Kitamura, N.; Ishizaka, S.; Hinatsu, Y.; Wakeshima, M.; Tsuge, K.; Sawamura, M. Reversible Mechanochromic Luminescence of [(C6F5Au]2(µ-1,4-diisocyanobenzene)]. J. Am. Chem. Soc. 2008, 130, 10044–10045. [Google Scholar] [CrossRef]
- Ito, H.; Muromoto, M.; Kurenuma, S.; Ishizaka, S.; Kitamura, N.; Sato, H.; Seki, T. Mechanical Stimulation and Solid Seeding Trigger Single-Crystal-to-Single-Crystal Molecular Domino Transformations. Nat. Commun. 2013, 4, 2009. [Google Scholar] [CrossRef] [Green Version]
- Seki, T.; Sakurada, K.; Muromoto, M.; Ito, H. Photoinduced Single-Crystal-to-Single-Crystal Phase Transition and Photosalient Effect of a Gold(I) Isocyanide Complex with Shortening of Intermolecular Aurophilic Bonds. Chem. Sci. 2015, 6, 1491–1497. [Google Scholar] [CrossRef] [Green Version]
- Seki, T.; Takamatsu, Y.; Ito, H. A Screening Approach for the Discover of Mechanochromic Gold(I) Isocyanide Complexes with Crystal-to-Crystal Phase Transitions. J. Am. Chem. Soc. 2016, 138, 6252–6260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sathyanarayana, A.; Nakamura, S.; Hisano, K.; Tsutsumi, O.; Srinivas, K.; Prabusankar, G. Controlling the Solid-State Luminescence of Gold(I) N-Heterocyclic Carbene Complexes through Change in the Structure of Molecular Aggregates. Sci. China Chem. 2018, 61, 957–965. [Google Scholar] [CrossRef]
- Yamada, S.; Rokusha, Y.; Kawano, R.; Fujisawa, K.; Tsutsumi, O. Mesogenic Gold Complexes Showing Aggregation-Induced Enhancement of Phosphorescence in Both Crystalline and Liquid-Crystalline Phases. Faraday Discuss. 2017, 196, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Kawano, R.; Younis, O.; Ando, A.; Rokusha, Y.; Yamada, S.; Tsutsumi, O. Photoluminescence from Au(I) Complexes Exhibiting Color Sensitivity to the Structure of the Molecular Aggregates. Chem. Lett. 2016, 45, 66–68. [Google Scholar] [CrossRef] [Green Version]
- Fujisawa, K.; Yamada, S.; Yanagi, Y.; Yoshioka, Y.; Kiyohara, A.; Tsutsumi, O. Tunning the Photoluminescence of Condensed-Phase Cyclic Trinuclear Au(I) Complexes through Control of Their Aggregated Structures by External Stimuli. Sci. Rep. 2015, 5, 7934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujisawa, K.; Okuda, Y.; Izumi, Y.; Nagamatsu, A.; Rokusha, Y.; Sadaike, Y.; Tsutsumi, O. Reversible Thermal-Mode Control of Luminescence fromLiquid-Crystalline Gold(I) Complexes. J. Mater. Chem. C 2014, 2, 3549–3555. [Google Scholar] [CrossRef]
- Fujisawa, K.; Kawakami, N.; Onishi, Y.; Izumi, Y.; Tamai, S.; Sugimoto, N.; Tsutsumi, O. Photoluminescent Properties of Liquid Crystalline Gold(I) Isocyanide Complexes with a Rod-Like Molecular Structure. J. Mater. Chem. C 2013, 1, 5359–5366. [Google Scholar] [CrossRef]
- Baeberá, J.; Elduque, A.; Giménez, R.; Ora, L.A.; Serrano, J.L. Pyrazolate “Golden” Rings: Trinuclear Complexes That Form Columnar Mesophases at Room Temperature. Angew. Chem. Int. Ed. Engl. 1996, 35, 2832–2835. [Google Scholar] [CrossRef]
- Kim, S.J.; Kang, S.H.; Park, K.M.; Kim, H.; Zin, W.C.; Choi, M.G.; Kim, K. Trinuclear Gold(I) Pyrazolate Complexes. Exhibiting Heaxagonal Columnar Mesophases with Only Three Side-Chains. Chem. Mater. 1998, 10, 1889–1893. [Google Scholar] [CrossRef]
- Cored, J.; Crespo, O.; Serrano, J.L.; Elduque, A.; Giménez, R. Decisive Influence of the Metal in Multifunctional Gold, Silver, and Copper Metallacycles: High Quantum Yield Phosphorescence, Color Switching, and Liquid Crystalline Behavior. Inorg. Chem. 2018, 57, 12632–12640. [Google Scholar] [CrossRef]
- Tiekink, E.R.T.; Zukerman-Schpector, J. Gold⋅⋅⋅π Aryl Interactions as Supramolecular Synthons. CrystEngComm 2009, 11, 1176–1186. [Google Scholar] [CrossRef]
- Yuan, W.Z.; Zhang, Y.; Tang, B.Z. Aggregation-Induced Emission: Applications; Tang, B.Z., Qin, A., Eds.; John Wiley & Sons: London, UK, 2013; pp. 43–60. [Google Scholar]
- Yaun, W.Z.; Shen, X.Y.; Zhao, H.; Lam, J.W.Y.; Tang, L.; Lu, P.; Wang, C.; Liu, Y.; Wang, Z.; Zheng, Q.; et al. Crystallization-Induced Phosphorescence of Pure Organic Luminescence at Room Temperature. J. Phys. Chem. C 2010, 114, 6090–6099. [Google Scholar] [CrossRef]
- Sathish, V.; Ramdass, A.; Thanasekaran, P.; Lu, K.L.; Rajagopal, S. Aggregation-Induced Phosphorescence Enhancement (AIPE) Base on Transition Metal Complexes—An overview. J. Photochem. Photobiol. C Photochem. Rev. 2015, 23, 25–44. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS-2014, Program for Crystal Structure Solution; University of Göttingen: Göttingen, Germany, 2014. [Google Scholar]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A Found Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A. Completion and Refinement of Crystal Structure with SIR92. J. Appl. Crystallogr. 1993, 26, 343–350. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGx Suite for Small-Molecule Single-Crystal Crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Vreven, J.T.; Kudin, K.N.; Burant, J.C.; Millam, J.M.; et al. Gaussian 03, Revision, E.01; Gaussian, Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Qin, W.; Alifu, N.; Cai, Y.; Lam, J.W.Y.; He, X.; Su, H.; Zhang, P.; Qian, J.; Tang, B.Z. Synthesis of an Efficient Far-Red/Near-Infrared Luminogen with AIE Characteristic for in vivo Bioimaging Applications. Chem. Commun. 2019, 55, 5615–5618. [Google Scholar] [CrossRef]
- Kanosue, K.; Ando, S. Polymides with Heavy Halogens Exhibiting Room-Temperature Phosphorescence with Very Large Stokes Shifts. ACS Macro Lett. 2016, 5, 1301–1305. [Google Scholar] [CrossRef]
- He, T.; Wang, Y.; Tian, X.; Gao, Y.; Zhao, X.; Grimsdale, A.C.; Lin, X.; Sun, H. An Organic Dye with Very Large Stockes-Shift and Broad Tunability of Fluorescence: Potentail Two-Photon Probe for Bioimaging and Ultra-Sensitive Solid-State Gas Sensor. Appl. Phys. Lett. 2016, 108, 011901. [Google Scholar] [CrossRef]
- Turrisi, R.; Sanguineti, A.; Sassi, M.; Savoie, B.; Takai, A.; Patriarca, G.E.; Salamone, M.M.; Ruffo, R.; Vaccaro, G.; Meinardi, F.; et al. Stokes Shift/Emission Efficiency Trade-Off in Donor-Acceptor Perylenemonoimides for Luminescenct Solar Concentrators. J. Mater. Chem. A 2015, 3, 8045–8054. [Google Scholar] [CrossRef]
- Zhu, Y.; Qiu, Z.; Ai, B.; Lin, Y.; Zhou, W.; Zhang, J.; Yu, L.; Mi, Q.; Lian, S. Significant Improved Quantum Yield of CaAl12O19: Mn4+ Red Phosphor by Co-Doping Bi3+ and B3+ Ions and Dual Application for Plant Cultivations. J. Lumin. 2018, 201, 314–320. [Google Scholar] [CrossRef]
Sample Availability: Not available. |



| Sample | λmaxlum[nm] a) | Φa,b) | τ [μs] | kr [s−1] c) | knr [s−1] c) |
|---|---|---|---|---|---|
| DT6 | 733 | 0.748 | 13 | 5.8 × 104 | 1.9 × 104 |
| DT7 | 726 | 0.609 | 13 | 4.7 × 104 | 3.0 × 104 |
| DT8 | 733 | 0.625 | 12 | 5.2 × 104 | 3.1 × 104 |
| Sample | Au–Au Distance [Å] | Au–π Distance [Å] | θ [deg] a) |
|---|---|---|---|
| DT6 | 3.40 | 3.58 | 15 |
| DT7 | 3.29 | 3.38 | 2.4 |
| DT8 | 3.25 | 3.39 | 7.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsutsumi, O.; Tamaru, M.; Nakasato, H.; Shimai, S.; Panthai, S.; Kuroda, Y.; Yamaguchi, K.; Fujisawa, K.; Hisano, K. Highly Efficient Aggregation-Induced Room-Temperature Phosphorescence with Extremely Large Stokes Shift Emitted from Trinuclear Gold(I) Complex Crystals. Molecules 2019, 24, 4606. https://doi.org/10.3390/molecules24244606
Tsutsumi O, Tamaru M, Nakasato H, Shimai S, Panthai S, Kuroda Y, Yamaguchi K, Fujisawa K, Hisano K. Highly Efficient Aggregation-Induced Room-Temperature Phosphorescence with Extremely Large Stokes Shift Emitted from Trinuclear Gold(I) Complex Crystals. Molecules. 2019; 24(24):4606. https://doi.org/10.3390/molecules24244606
Chicago/Turabian StyleTsutsumi, Osamu, Masakazu Tamaru, Hitoya Nakasato, Shingo Shimai, Supattra Panthai, Yuki Kuroda, Kenta Yamaguchi, Kaori Fujisawa, and Kyohei Hisano. 2019. "Highly Efficient Aggregation-Induced Room-Temperature Phosphorescence with Extremely Large Stokes Shift Emitted from Trinuclear Gold(I) Complex Crystals" Molecules 24, no. 24: 4606. https://doi.org/10.3390/molecules24244606
APA StyleTsutsumi, O., Tamaru, M., Nakasato, H., Shimai, S., Panthai, S., Kuroda, Y., Yamaguchi, K., Fujisawa, K., & Hisano, K. (2019). Highly Efficient Aggregation-Induced Room-Temperature Phosphorescence with Extremely Large Stokes Shift Emitted from Trinuclear Gold(I) Complex Crystals. Molecules, 24(24), 4606. https://doi.org/10.3390/molecules24244606







