Potent in Vitro α-Glucosidase Inhibition of Secondary Metabolites Derived from Dryopteris cycadina
Abstract
:1. Introduction
2. Results
2.1. Effect of In Vitro α-Glucosidase Activity
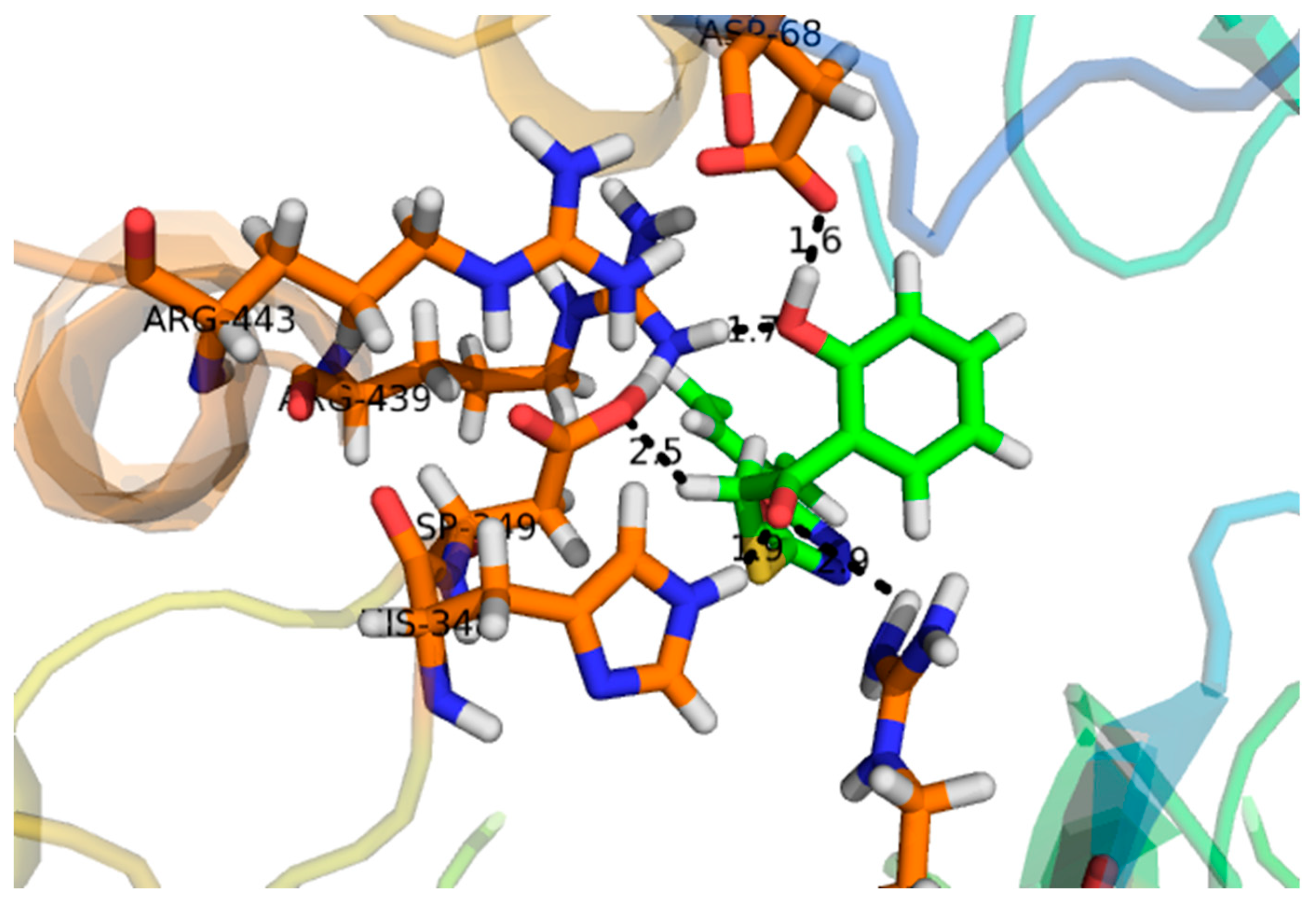
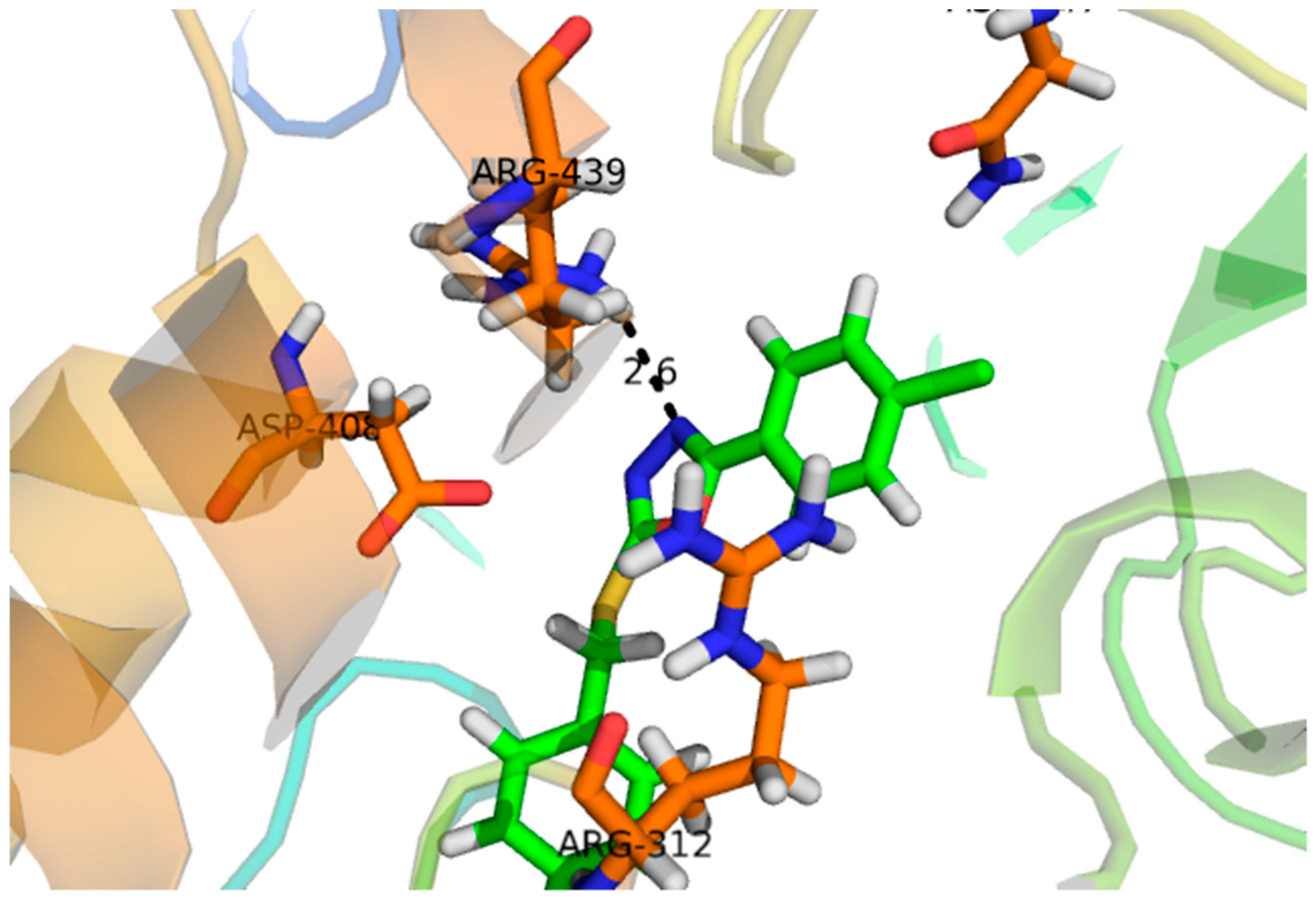
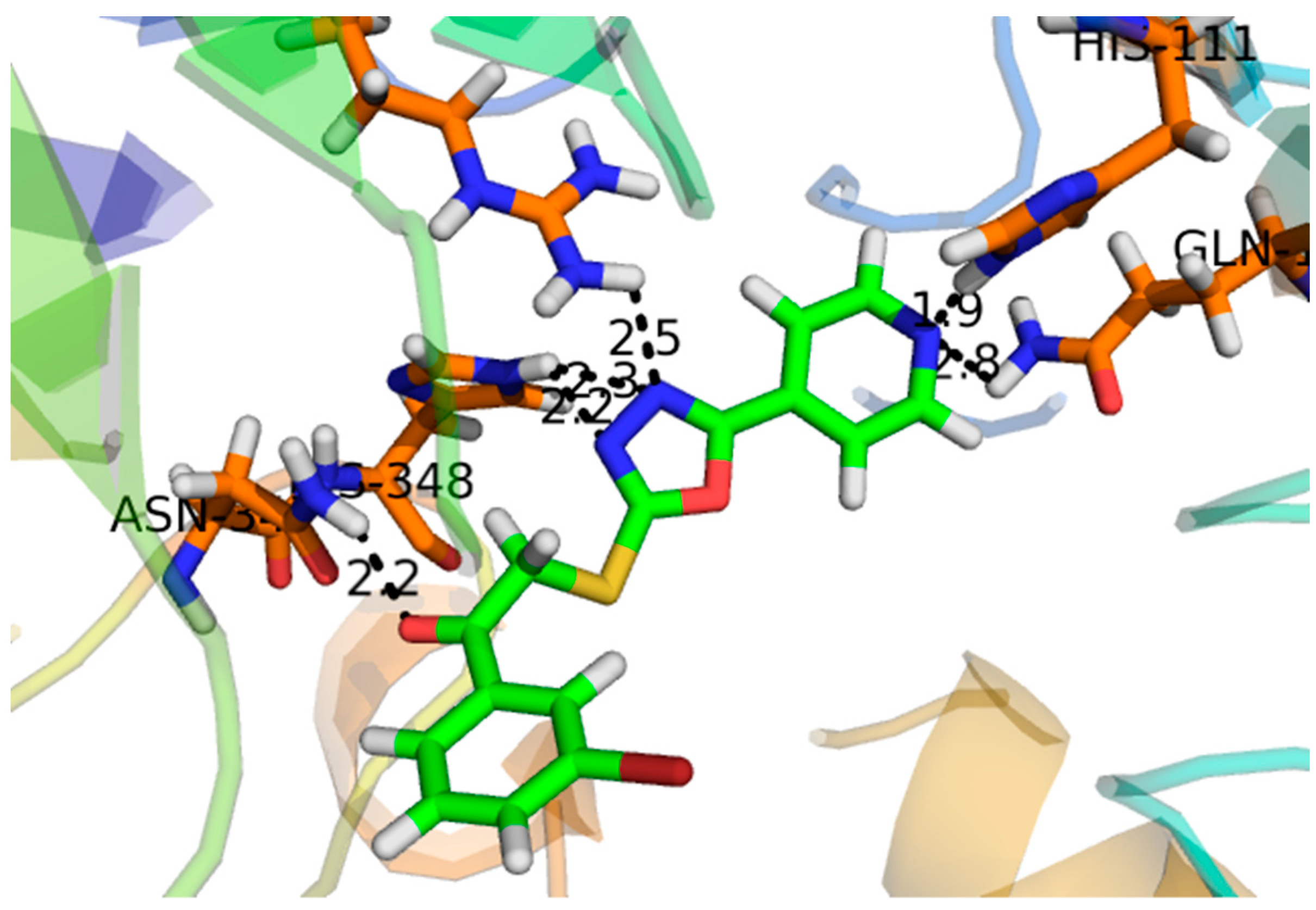
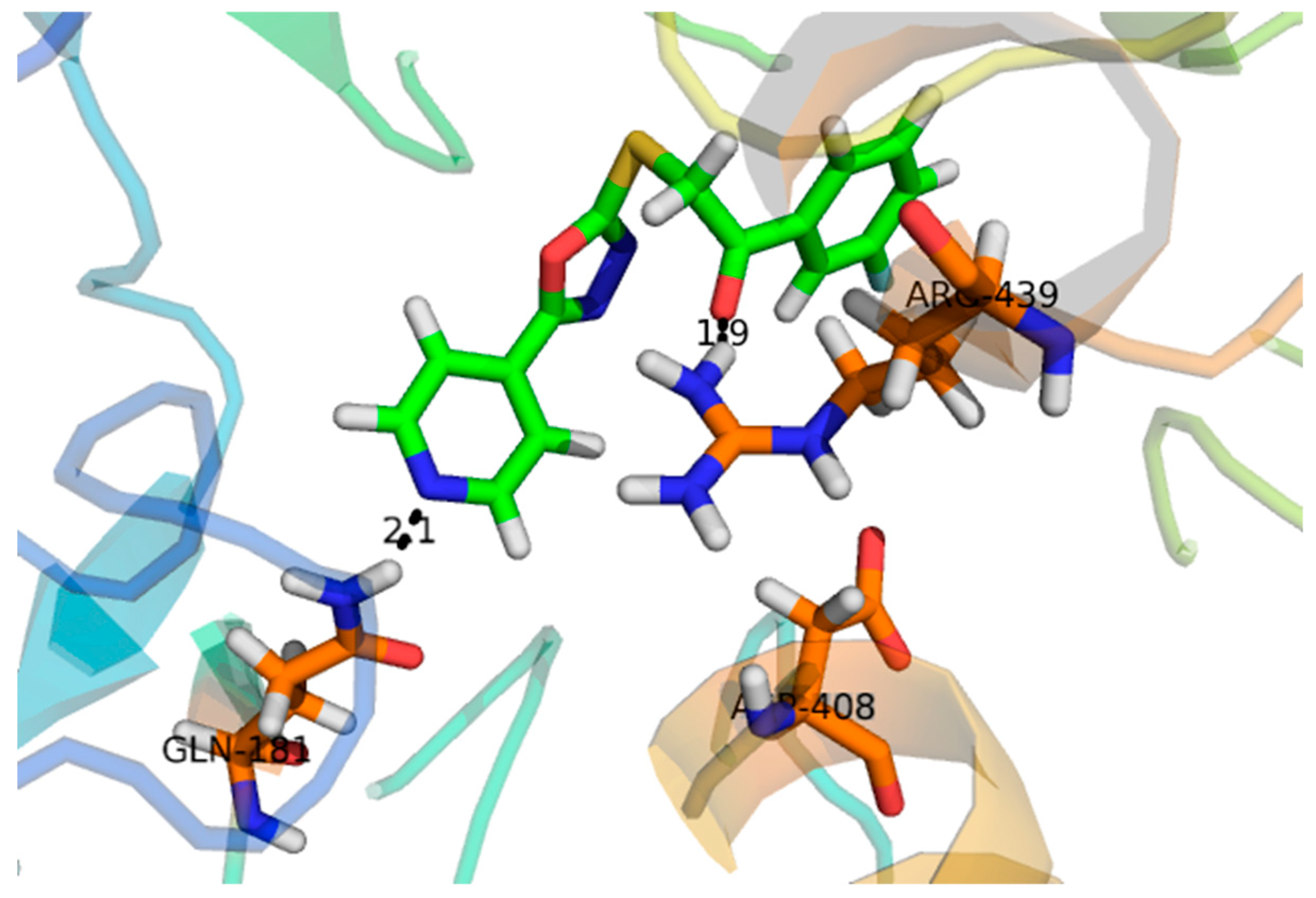
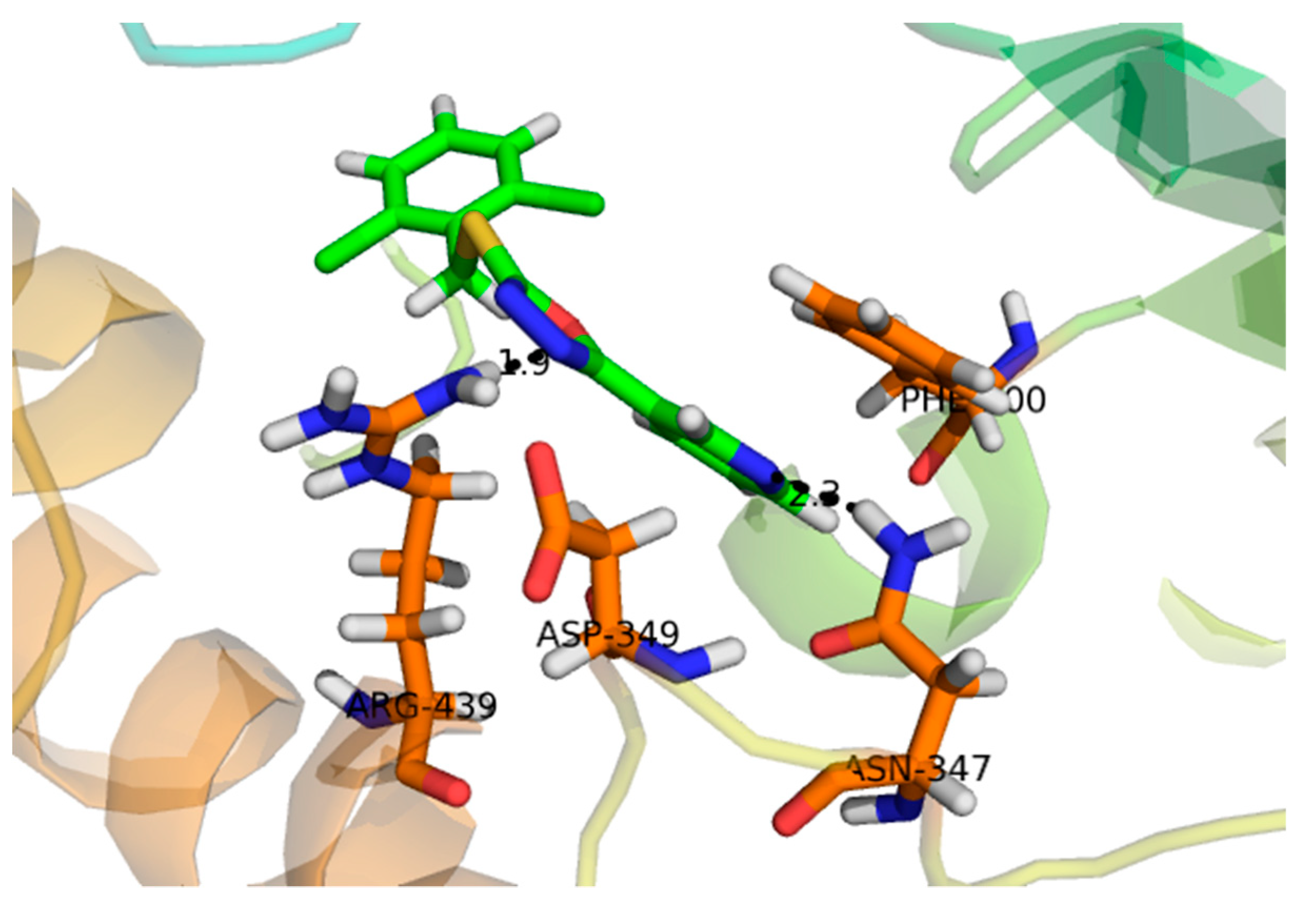
2.2. Effect of Molecular Docking
3. Materials and Methods
3.1. Materials
3.2. Assay Protocol
3.3. Half-Maximal Inhibitory Concentration of Compounds (IC50)
3.4. Computational Study
3.5. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qaisar, M.N.; Chaudhary, B.A.; Sajid, M.U.; Hussain, N. Evaluation of α-Glucosidase Inhibitory Activity of Dichloromethane and Methanol Extracts of Croton bonplandianum Baill. Trop. J. Pharm. Res. 2014, 13, 1833–1836. [Google Scholar] [CrossRef]
- Benalla, W.; Bellahcen, S.; Bnouham, M. Antidiabetic Medicinal Plants as a Source of alpha-Glucosidase Inhibitors. Curr. Diabetes Rev. 2010, 6, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Ravi, K.; Rajasekaran, S.; Subramanian, S. Antihyperlipidemic Effect of Eugenia jambolana Seed Kernel on Streptozotocin-induced Diabetes In Rats. Food Chem. Toxicol. 2005, 43, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- De Matos, A.M.; de Macedo, M.P.; Rauter, A.P. Bridging type 2 diabetes and alzheimer’s disease: assembling the puzzle pieces in the quest for the molecules with therapeutic and preventive potential. Med. Res. Rev. 2017, 38, 261–324. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.K.; El-Sammad, N.M.; Mousa, A.M.; Mohammed, M.H.; Farrag, A.E.R.H.; Hashim, A.N.E.; Werner, V.; Lindequist, U.; Nawwar, M.A.E.-M. Hypoglycemic and antioxidant activities of Caesalpinia ferrea Martius leaf extract in streptozotocin-induced diabetic rats. Asian Pac. J. Trop. Biomed. 2015, 5, 462–471. [Google Scholar] [CrossRef] [Green Version]
- Rosas-Ramírez, D.; Escandón-Rivera, S.; Pereda-Miranda, R. Morning glory resin glycosides as α-glucosidase inhibitors: In vitro and in silico analysis. Phytochemistry 2018, 148, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, A.K. Management of Type 1 Diabetes. Nurs. Clin. N. Am. 2017, 52, 499–511. [Google Scholar] [CrossRef]
- Faggioni, M.; Baber, U.; Sartori, S.; Giustino, G.; Cohen, D.J.; Henry, T.D.; Farhan, S.; Ariti, C.; Dangas, G.; Gibson, M.; et al. Incidence, patterns, and associations between dual-antiplatelet therapy cessation and risk for adverse events among patients with and without diabetes mellitus receiving drug-eluting stents: Results from the PARIS registry. JACC Cardiovasc. Interv. 2017, 10, 645–654. [Google Scholar] [CrossRef]
- Chen, Y.-G.; Li, P.; Li, P.; Yan, R.; Zhang, X.-Q.; Wang, Y.; Zhang, X.-T.; Ye, W.-C.; Zhang, Q.-W. α-Glucosidase inhibitory effect and simultaneous quantification of three major flavonoid glycosides in Microctis folium. Molecules 2013, 18, 4221–4232. [Google Scholar] [CrossRef]
- Ichiki, H.; Miura, T.; Kubo, M.; Ishihara, E.; Komatsu, Y.; Tanigawa, K.; Okada, M. New antidiabetic compounds, mangiferin and its glucoside. Biolog. Pharm. Bull. 1998, 21, 1389–1390. [Google Scholar] [CrossRef]
- Ikeda, K.; Takahashi, M.; Nishida, M.; Miyauchi, M.; Kizu, H.; Kameda, Y.; Arisawa, M.; Watson, A.A.; Nash, R.J.; Fleet, G.W. Homonojirimycin analogues and their glucosides from Lobelia sessilifolia and Adenophora spp. (Campanulaceae). Carbohyd. Res. 1999, 323, 73–80. [Google Scholar] [CrossRef]
- Abbas, G.; Al-Harrasi, A.S.; Hussain, H. Discovery and Development of Antidiabetic Agents from Natural Products; Elsevier: Amsterdam, The Netherlands, 2017; pp. 251–269. [Google Scholar]
- Kim, S.D. α-Glucosidase Inhibitor Isolated from Coffee. J. Microbiol. Biotechnol. 2015, 25, 174–177. [Google Scholar] [CrossRef]
- Hakamata, W.; Kurihara, M.; Okuda, H.; Nishio, T.; Oku, T. Design and screening strategies for α-glucosidase inhibitors based on enzymological information. Curr. Top. Med. Chem. 2009, 9, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Samoshin, A.V.; Dotsenko, I.A.; Samoshina, N.M.; Franz, A.H.; Samoshin, V.V. Thio-beta-D-glucosides: Synthesis and evaluation as glycosidase inhibitors and activators. Int. J. Carbohyd. Chem. 2014, 2014, 941059. [Google Scholar] [CrossRef]
- Mink, J.N.; Singhurst, J.R.; Holmes, W.C. Dryopteris marginalis (Dryopteridaceae): New to Texas. Phytoneuron 2010, 53, 1–6. [Google Scholar]
- Vasudeva, S. Economic importance of pteridophytes. Indian Fern J. 1999, 16, 130–152. [Google Scholar]
- Joshi, K.; Joshi, A.R. Ethnobotanical studies on some lower plants of the central development region, Nepal. Ethnobot. Leaflets 2008, 12, 832–840. [Google Scholar]
- Gao, Z.; Ali, Z.; Zhao, J.; Qiao, L.; Lei, H.; Lu, Y.; Khan, I.A. Phytochemical investigation of the rhizomes of Dryopteris crassirhizoma. Phytochem. Lett. 2008, 1, 188–190. [Google Scholar] [CrossRef]
- Singh, I.P.; Bharate, S.B. Phloroglucinol compounds of natural origin. Nat. Prod. Rep. 2006, 23, 558–591. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-S.; Lee, S.-S. Flavonol glycosides with α-glucosidase inhibitory activities and new flavone C-Diosides from the leaves of Machilus konishii. Helv. Chim. Acta 2014, 97, 1672–1682. [Google Scholar] [CrossRef]
- Han, X.; Li, Z.; Li, C.Y.; Jia, W.N.; Wang, H.T.; Wang, C.H. Phytochemical Constituents and Biological Activities of Plants from the Genus Dryopteris. Chem. Biodives. 2015, 12, 1131–1162. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hu, H.-J.; Li, P.-F.; Yang, Y.-B.; Wu, L.-H.; Chou, G.-X.; Wang, Z.-T. Diterpenoids and phenylethanoid glycosides from the roots of Clerodendrum bungei and their inhibitory effects against angiotensin converting enzyme and α-glucosidase. Phytochemistry 2014, 103, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Khan, S.A.; Rauf, A.; Khan, H.; Shah, M.R.; Ahmad, M.; Mubarak, M.S.; Hadda, T.B. Characterization and antinociceptive activity (in vivo) of kempferol-3, 4′-di-O-α-L-rhamnopyranoside isolated from Dryopteris cycadina. Med. Chem. Res. 2015, 24, 3218–3229. [Google Scholar] [CrossRef]
- Ali, M.; Rauf, A.; Ben Hadda, T.; Bawazeer, S.; Abu-Izneid, T.; Khan, H.; Raza, M.; Khan, A.; Shah, S.; Pervez, S. Mechanisms underlying anti-hyperalgesic properties of Kaempferol-3, 7-di-O-α-L-rhamnopyranoside isolated from Dryopteris cycadina. Curr. Top. Med. Chem. 2017, 17, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Taukoorah, U.; Mahomoodally, M.F. Crude Aloe vera Gel Shows Antioxidant Propensities and Inhibits Pancreatic Lipase and Glucose Movement In Vitro. Adv. Pharmacol. Sci. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, B.; Riaz, N.; Saleem, M.; Naveed, M.A.; Ashraf, M.; Alam, U.; Rafiq, H.M.; Tareen, R.B.; Jabbar, A. Isolation of natural compounds from Phlomis stewartii showing α-glucosidase inhibitory activity. Phytochemistry 2013, 96, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Ismail, N.H.; Imran, S.; Wadood, A.; Rahim, F.; Ali, M.; Rehman, A.U. Novel quinoline derivatives as potent In vitro α-glucosidase inhibitors: In silico studies and SAR predictions. Med. Chem. Comm. 2015, 2015. [Google Scholar] [CrossRef]
- Azam, S.S.; Uddin, R.; Wadood, A. Structure and dynamics of alpha-glucosidase through molecular dynamics simulation studies. J. Mol. Liquids 2012, 174, 58–62. [Google Scholar] [CrossRef]
- Rauf, A.; Uddin, G.; Siddiqui, B.S.; Khan, A.; Khan, H.; Arfan, M.; Muhammad, N.; Wadood, A. In-vivo antinociceptive, anti-inflammatory and antipyretic activity of pistagremic acid isolated from Pistacia integerrima. Phytomedicine 2014, 21, 1509–1515. [Google Scholar] [CrossRef]
- Ayaz, M.; Junaid, M.; Ullah, F.; Subhan, F.; Sadiq, A.; Ali, G.; Ovais, M.; Shahid, M.; Ahmad, A.; Wadood, A.; et al. Anti-Alzheimer’s Studies on β-Sitosterol Isolated from Polygonum hydropiper L. Front. Pharmcol. 2017, 8, 697. [Google Scholar] [CrossRef]
- Atta ur, R.; Zareen, S.; Choudhary, M.I.; Akhtar, M.N.; Khan, S.N. α-Glucosidase inhibitory activity of triterpenoids from Cichorium intybus. J. Nat. Prod. 2008, 71, 910–913. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Ismail, N.H.; Imran, S.; Wadood, A.; Ali, M.; Rahim, F.; Khan, A.A.; Riaz, M. Novel thiosemicarbazide–oxadiazole hybrids as unprecedented inhibitors of yeast α-glucosidase and in silico binding analysis. RSC Adv. 2016, 6, 33733–33742. [Google Scholar] [CrossRef]
- Khan, H.; Saeed, M.; Gilani, A.H.; Muhammad, N.; ur Rehman, N.; Mehmood, M.H.; Ashraf, N. Antispasmodic and antidiarrheal activity of rhizomes of Polygonatum verticillatum maneuvered predominately through activation of K+ channels: Components identification through TLC. Toxicol. Ind. Health 2016, 32, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Marya; Khan, H. Anti-inflammatory potential of alkaloids as a promising therapeutic modality. Lett. Drug Des. Discov. 2017, 14, 240–249. [Google Scholar] [CrossRef]
- Marya; Khan, H.; Mushtaq, G.; Amjad Kamal, M.; Ahmad, I. Glycosides as possible lead antimalarial in new drug discovery: Future perspectives. Curr. Drug Metab. 2017, 18, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Marya; Khan, H.; Nabvi, S.M.; Habtemariam, S. Anti-diabetic potential of peptides: Future prospects as therapeutic agents. Life Sci. 2018, 193, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Khan, H.; D’Onofrio, G.; Šamec, D.; Shirooie, S.; Dehpour, A.R.; Argüelles, S.; Habtemariam, S.; Sobarzo-Sanchez, E. Apigenin as neuroprotective agent: Of mice and men. Pharmacol. Res. 2018, 128, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Rauf, A.; Khan, H.; Khalid, S.; Mubarak, M.S. Potential health benefits of natural products derived from truffles: A review. Trends Food Sci. Technol. 2017, 70, 1–8. [Google Scholar] [CrossRef]
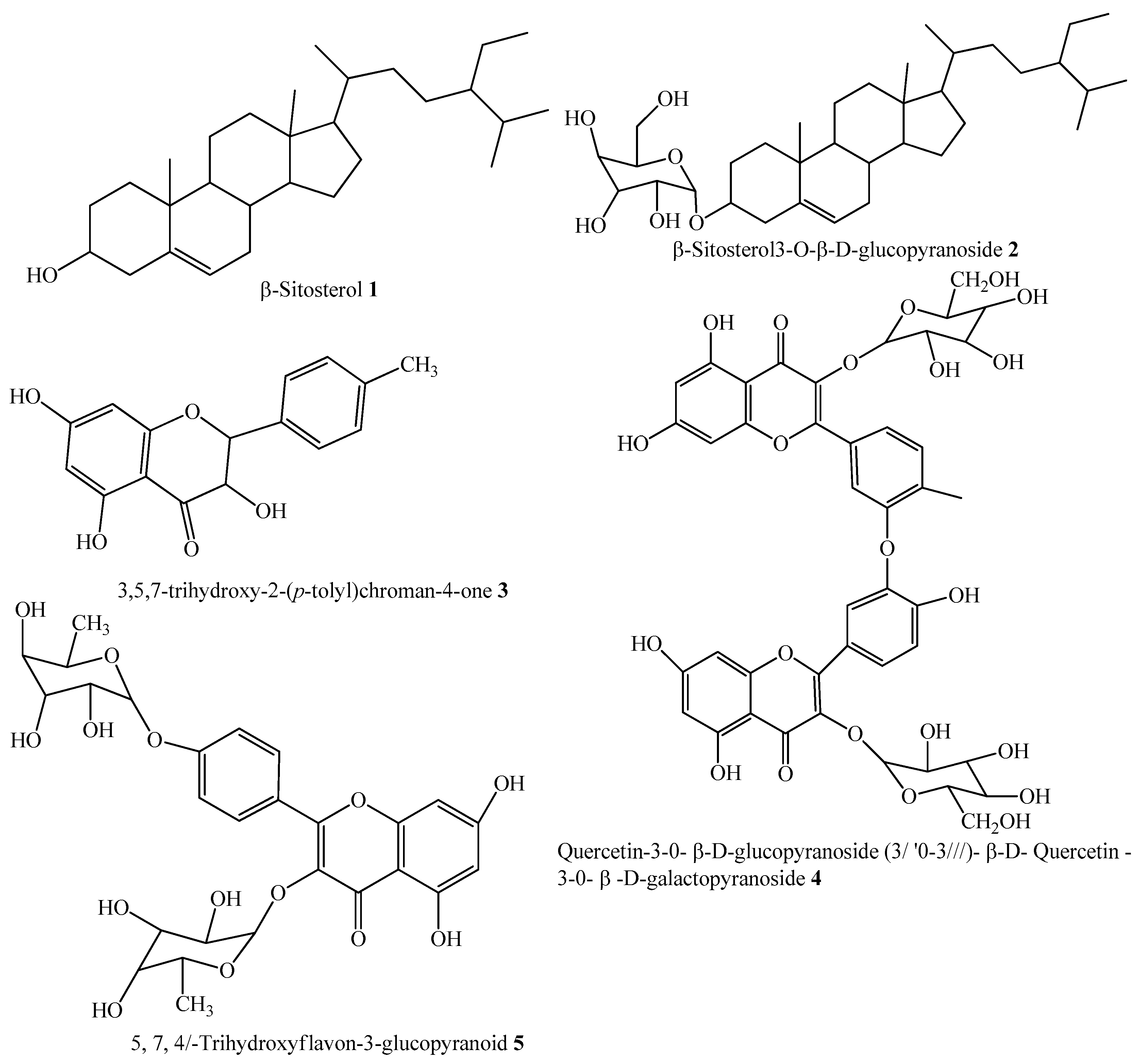
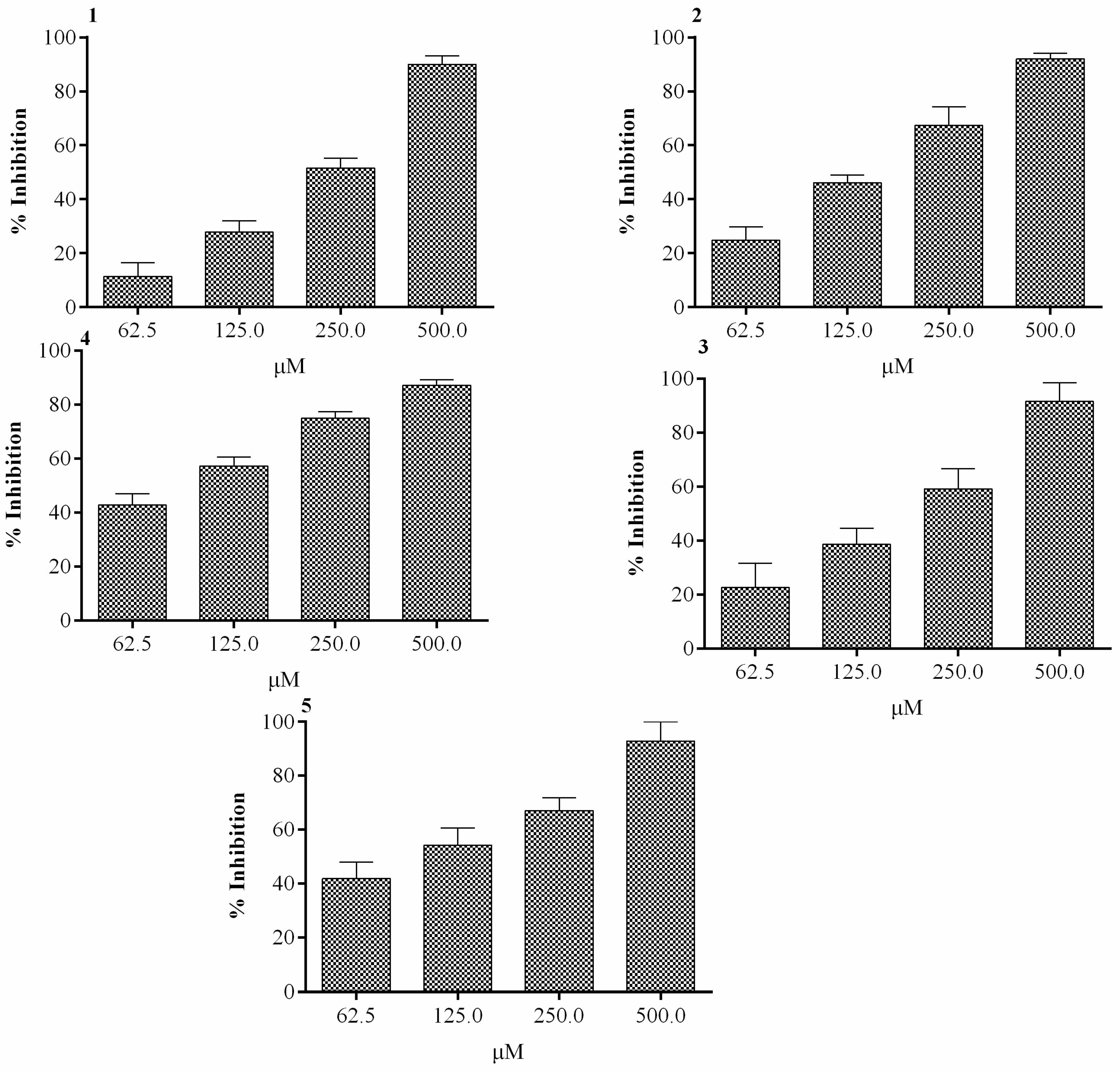
| α- Glucosidase | Compounds | IC50 ± SEM (µM) |
| 1 | 143 ± 0.47 | |
| 2 | 314 ± 4.58 | |
| 3 | 133 ± 6.90 | |
| 4 | 298 ± 0.67 | |
| 5 | 146 ± 1.93 | |
| Acarbose | 290 ± 0.54 |
| Compound | Docking Score | Interacting Residues of the Receptor |
|---|---|---|
| 1 | −16.097 | ASP-215, ASP-352, ARG-442, GLN-182 |
| 2 | −7.756 | ASN-415 |
| 3 | −22.480 | ARG-315, ASP-307, HIS-280, LYS-156, SER-240, THR-310, TYR-158 |
| 4 | −12.931 | ARG-442, TYR-158 |
| 5 | −15.752 | ASP-242, LYS-156, PRO-312, TYR-158 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amin, S.; Ullah, B.; Ali, M.; Rauf, A.; Khan, H.; Uriarte, E.; Sobarzo-Sánchez, E. Potent in Vitro α-Glucosidase Inhibition of Secondary Metabolites Derived from Dryopteris cycadina. Molecules 2019, 24, 427. https://doi.org/10.3390/molecules24030427
Amin S, Ullah B, Ali M, Rauf A, Khan H, Uriarte E, Sobarzo-Sánchez E. Potent in Vitro α-Glucosidase Inhibition of Secondary Metabolites Derived from Dryopteris cycadina. Molecules. 2019; 24(3):427. https://doi.org/10.3390/molecules24030427
Chicago/Turabian StyleAmin, Surriya, Barkat Ullah, Mumtaz Ali, Abdur Rauf, Haroon Khan, Eugenio Uriarte, and Eduardo Sobarzo-Sánchez. 2019. "Potent in Vitro α-Glucosidase Inhibition of Secondary Metabolites Derived from Dryopteris cycadina" Molecules 24, no. 3: 427. https://doi.org/10.3390/molecules24030427
APA StyleAmin, S., Ullah, B., Ali, M., Rauf, A., Khan, H., Uriarte, E., & Sobarzo-Sánchez, E. (2019). Potent in Vitro α-Glucosidase Inhibition of Secondary Metabolites Derived from Dryopteris cycadina. Molecules, 24(3), 427. https://doi.org/10.3390/molecules24030427









