Geochemical Significance of Biomarkers in the Methane Hydrate-Bearing Sediments from the Shenhu Area, the South China Sea
Abstract
:1. Introduction
2. Geological Background
3. Results and Discussion
3.1. Results
3.2. Discussion
4. Methods
4.1. Extraction and Separation
4.2. GC-MS and GC×GC-TOFMS Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sloan, E.D. Clathrate Hydrates of Natural Gases; Marcel Dekker: New York, NY, USA, 1990; p. 641. [Google Scholar]
- Max, M.D.; Johnson, A.H.; Dillon, W.P. Economic Geology of Natural Gas Hydrate; Springer: Dordrecht, The Netherlands, 2006; pp. 1–341. [Google Scholar]
- Makogon, Y.F. Natural gas hydrates—A promising source of energy. J. Nat. Gas Sci. Eng. 2010, 2, 49–59. [Google Scholar] [CrossRef]
- Chong, Z.R.; Yang, S.H.B.; Babu, P.; Linga, P.; Li, X.S. Review of natural gas hydrates as an energy resource: Prospects and challenges. Appl. Energy 2016, 162, 1633–1652. [Google Scholar] [CrossRef]
- Cragg, B.; Parkes, R.; Fry, J.; Weightman, A.; Rochelle, P.; Maxwell, J. Bacterial populations and processes in sediments containing gas hydrates (ODP Leg 146: Cascadia Margin). Earth Planet. Sci. Lett. 1996, 139, 497–507. [Google Scholar] [CrossRef]
- Inagaki, F.; Nunoura, T.; Nakagawa, S.; Teske, A.; Lever, M.; Lauer, A.; Suzuki, M.; Takai, K.; Delwiche, M.; Colwell, F.S.; et al. Biogeographical distribution and diversity of microbes in methane hydrate-bearing deep marine sediments on the Pacific Ocean Margin. Proc. Natl. Acad. Sci. USA 2006, 103, 2815–2820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanoil, B.D.; Sassen, R.; La Duc, M.T.; Sweet, S.T.; Nealson, K.H. Bacteria and Archaea physically associated with Gulf of Mexico gas hydrates. Appl. Environ. Microbiol. 2001, 67, 5143–5153. [Google Scholar] [CrossRef]
- Yang, J.Y.; Chung, K.H.; Jin, Y.K.; Shin, K.H. Characterizing lipid biomarkers in methanotrophic communities of gas hydrate-bearing sediments in the Sea of Okhotsk. Mar. Pet. Geol. 2011, 28, 1884–1898. [Google Scholar] [CrossRef]
- Jiao, L.; Su, X.; Wang, Y.; Jiang, H.; Zhang, Y.; Chen, F. Microbial diversity in the hydrate-containing and-free surface sediments in the Shenhu area, South China Sea. Geosci. Front. 2015, 6, 627–633. [Google Scholar] [CrossRef]
- Gong, J.; Sun, X.; Xu, L.; Lu, H. Contribution of thermogenic organic matter to the formation of biogenic gas hydrate: Evidence from geochemical and microbial characteristics of hydrate-containing sediments in the Taixinan Basin, South China Sea. Mar. Pet. Geol. 2017, 80, 432–449. [Google Scholar] [CrossRef]
- Bidle, K.A.; Kastner, M.; Bartlett, D.H. A phylogenetic analysis of microbial communities associated with methane hydrate containing marine fluids and sediments in the Cascadia margin (ODP site 892B). FEMS Microbiol. Lett. 1999, 177, 101–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Yang, S.; Liang, J.; Su, X.; Fu, S.; Sha, Z.; Yang, T. Variations of pore water sulfate gradients in sediments as indicator for underlying gas hydrate in Shenhu Area, the South China Sea. Sci. China Earth Sci. 2013, 56, 530–540. [Google Scholar] [CrossRef]
- Dai, J.X.; Ni, Y.Y.; Huang, S.P.; Peng, W.L.; Han, W.X.; Gong, D.Y.; Wei, W. Genetic types of gas hydrates in China. Pet. Explor. Dev. 2017, 44, 887–898. [Google Scholar] [CrossRef]
- Fu, S.Y.; Lu, J.A. The characteristics and origin of gas hydrate in Shenhu area, South China Sea. Mar. Geol. Lett. 2010, 26, 6–10. (In Chinese) [Google Scholar]
- Liu, C.; Meng, Q.; He, X.; Li, C.; Ye, Y.; Lu, Z.; Zhu, Y.; Li, Y.; Liang, J. Comparison of the characteristics for natural gas hydrate recovered from marine and terrestrial areas in China. J. Geochem. Explor. 2015, 152, 67–74. [Google Scholar] [CrossRef]
- Biddle, J.F.; Lipp, J.S.; Lever, M.A.; Lloyd, K.G.; Sørensen, K.B.; Anderson, R.; Fredricks, H.F.; Elvert, M.; Kelly, T.J.; Schrag, D.P.; et al. Heterotrophic Archaea dominate sedimentary subsurface ecosystems off Peru. Proc. Natl. Acad. Sci. USA 2006, 103, 3846–3851. [Google Scholar] [CrossRef] [PubMed]
- Lipp, J.S.; Hinrichs, K.U. Structural diversity and fate of intact polar lipids in marine sediments. Geochim. Cosmochim. Acta 2009, 73, 6816–6833. [Google Scholar] [CrossRef]
- Oba, M.; Sakata, S.; Fujii, T. Archaeal polar lipids in subseafloor sediments from the Nankai Trough: Implications for the distribution of methanogens in the deep marine subsurface. Org. Geochem. 2015, 78, 153–160. [Google Scholar] [CrossRef]
- Orphan, V.J.; Jahnke, L.L.; Embaye, T.; Turk, K.A.; Pernthaler, A.; Summons, R.E.; Marais, D.J. Characterization and spatial distribution of methanogens and methanogenic biosignatures in hypersaline microbial mats of Baja California. Geobiology 2008, 6, 376–393. [Google Scholar] [CrossRef]
- Peters, K.E.; Walters, C.C.; Moldowan, J.M. The Biomarker Guide; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Li, J.B. (Ed.) Formation and Evolution of Marginal Seas in China and Their Effect on Resources; Geological Publishing House: Beijing, China, 2008; pp. 377–384. (In Chinese) [Google Scholar]
- Wu, N.Y.; Yang, S.X.; Wang, H.B.; Liang, J.Q.; Gong, Y.H.; Lu, Z.Q.; Wu, D.D.; Guan, H.X. Gas-bearing fluid influx subsystem for gas hydrate geological system in Shenhu Area, northern South China Sea. Chin. J. Geophys. 2009, 52, 1641–1650. (In Chinese) [Google Scholar]
- Pang, X.; Chen, C.M.; Peng, D.J.; Zhou, D.; Chen, H.H. The Pearl River Deep-Water Fan System and Petroleum in South China Sea; Science Press: Beijing, China, 2007. (In Chinese) [Google Scholar]
- Chen, F.; Su, X.; Zhou, Y.; Lu, H.F.; Liu, G.H.; Zhen, Z.X.; Chen, C.Y. Variations in biogenic components of late Miocene-Holocene sediments from Shenhu area in the northern South China Sea and their geological implication. Mar. Geol. Quat. Geol. 2009, 29, 1–8. (In Chinese) [Google Scholar]
- McDonnell, S.L.; Max, M.D.; Cherkis, N.Z.; Czarnecki, M.F. Tectono-sedimentary controls on the likelihood of gas hydrate occurrence near Taiwan. Mar. Pet. Geol. 2000, 17, 929–936. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Yang, S.X.; Wu, N.Y.; Su, X.; Holland, M. Successful and Surprising Results for China’s First Gas Hydrate Drilling Expedition; Fire in the Ice: Methane Hydrate Newsletter; National Energy Technology Laboratory, U.S. Department of Energy: Washington, DC, USA, 2007.
- Knittel, K.; Boetius, A. Anaerobic oxidation of methane: Progress with an unknown process. Annu. Rev. Microbiol. 2009, 63, 311–334. [Google Scholar] [CrossRef] [PubMed]
- Whiticar, M.J.; Faber, E.; Schoell, M. Biogenic methane formation in marine and freshwater environments: CO2 reduction vs. acetate fermentation-isotope evidence. Geochim. Cosmochim. Acta 1986, 50, 693–709. [Google Scholar] [CrossRef]
- Papendick, S.L.; Downs, K.R.; Vo, K.D.; Hamilton, S.K.; Dawson, G.K.; Golding, S.D.; Gilcrease, P.C. Biogenic methane potential for Surat Basin, Queensland coal seams. Int. J. Coal Geol. 2011, 88, 123–134. [Google Scholar] [CrossRef]
- Meslé, M.; Dromart, G.; Oger, P. Microbial methanogenesis in subsurface oil and coal. Res. Microbiol. 2013, 164, 959–972. [Google Scholar] [CrossRef] [PubMed]
- Brocks, J.J.; Pearson, A. Building the biomarker tree of life. In Reviews in Mineralogy and Geochemistry; Banfield, J., Nealson, K., Cervini-Silva, J., Eds.; The Mineralogical Society of America: Chantilly, VA, USA, 2005; Volume 59, pp. 233–258. [Google Scholar]
- Tran, T.C.; Logan, G.A.; Grosjean, E.; Ryan, D.; Marriott, P.J. Use of comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry for the characterization of biodegradation and unresolved complex mixtures in petroleum. Geochim. Cosmochim. Acta 2010, 74, 6468–6484. [Google Scholar] [CrossRef]
- Ventura, G.T.; Kenig, F.; Reddy, C.M.; Frysinger, G.S.; Nelson, R.K.; Mooy, B.V.; Gaines, R.B. Analysis of unresolved complex mixtures of hydrocarbons extracted from Late Archean sediments by comprehensive two-dimensional gas chromatography (GC × GC). Org. Geochem. 2008, 39, 846–867. [Google Scholar] [CrossRef]
- Ventura, G.T.; Raghuraman, B.; Nelson, R.K.; Mullins, O.C.; Reddy, C.M. Compound class oil fingerprinting techniques using comprehensive two-dimensional gas chromatography (GC × GC). Org. Geochem. 2010, 41, 1026–1035. [Google Scholar] [CrossRef]
- Eiserbeck, C.; Nelson, R.K.; Grice, K.; Curiale, J.; Reddy, C.M. Comparison of GC–MS, GC–MRM-MS, and GC × GC to characterise higher plant biomarkers in Tertiary oils and rock extracts. Geochim. Cosmochim. Acta 2012, 87, 299–322. [Google Scholar] [CrossRef]
- Li, S.; Cao, J.; Hu, S.; Zhang, D.; Fan, R. Analysis of terpanes in biodegraded oils from China using comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry. Fuel 2014, 133, 153–162. [Google Scholar] [CrossRef]
- Li, S.; Cao, J.; Hu, S. Analyzing hydrocarbon fractions in crude oils by two-dimensional gas chromatography/time-of-flight mass spectrometry under reversed-phase column system. Fuel 2015, 158, 191–199. [Google Scholar] [CrossRef]
- Li, S.; Cao, J.; Hu, S.; Luo, G. Characterization of compounds in unresolved complex mixtures (UCM) of a Mesoproterzoic shale by using GC× GC-TOFMS. Mar. Pet. Geol. 2015, 66, 791–800. [Google Scholar] [CrossRef]
- Hu, S.Z.; Li, S.F.; Wang, J.H.; Cao, J. Origin of unresolved complex mixtures (UCMs) in biodegraded oils: Insights from artificial biodegradation experiments. Fuel 2018, 231, 53–60. [Google Scholar] [CrossRef]
- Sutton, P.A.; Lewis, C.A.; Rowland, S.J. Isolated of individual hydrocarbons from the unresolved complex hydrocarbon mixture of a biodegraded crude oil using preparative capillary gas chromatography. Org. Geochem. 2005, 36, 963–970. [Google Scholar] [CrossRef]
- Vinson, D.S.; Blair, N.E.; Martini, A.M.; Larter, S.; Orem, W.H.; McIntosh, J.C. Microbial methane from in situ biodegradation of coal and shale: A review and reevaluation of hydrogen and carbon isotope signatures. Chem. Geol. 2017, 453, 128–145. [Google Scholar] [CrossRef]
- Chen, F.; Su, X.; Lu, H.F.; Zhou, Y.; Zhuang, C. Relations between biogenic component (foraminifera) and highly saturated gas hydrates distribution from Shenhu area, northern South China Sea. Earth Sci. J. China Univ. Geosci. 2013, 38, 907–915. (In Chinese) [Google Scholar]
- Stefanova, M. Head-to-head linked isoprenoids in Miocene coal lithotypes. Fuel 2000, 79, 755–758. [Google Scholar] [CrossRef]
- Greenwood, P.F.; Summons, R.E. GC–MS detection and significance of crocetane and pentamethylicosane in sediments and crude oils. Org. Geochem. 2003, 34, 1211–1222. [Google Scholar] [CrossRef]
- Hayes, J.M.; Freeman, K.H.; Popp, B.N.; Hoham, C.H. Compound-specific isotopic analyses: A novel tool for reconstruction of ancient biogeochemical processes. Org. Geochem. 1990, 16, 1115–1128. [Google Scholar] [CrossRef]
- Damsté, J.S.S.; Schouten, S. Is there evidence for a substantial contribution of prokaryotic biomass to organic carbon in Phanerozoic carbonaceous sediments? Org. Geochem. 1997, 26, 517–530. [Google Scholar] [CrossRef]
- Ourisson, G.; Albrecht, P.; Rohmer, M. Predictive microbial biochemistry-from molecular fossils to procaryotic membranes. Trends Biochem. Sci. 1982, 7, 236–239. [Google Scholar] [CrossRef]
- Zhang, S.C.; Gong, Z.S.; Liang, D.G.; Wu, K.Q.; Wang, J.R.; Song, F.Q.; Wang, P.R.; Wang, H.T.; Zhong, H. Geochemistry of petroleum systems in the Eastern Pearl River Mouth Basin-1: Oil family classification, oil-source correlation and mixed oil analysis. Acta Sedimentol. Sin. 2004, 22, 15–26. (In Chinese) [Google Scholar]
- Jin, X.; Pan, C.; Yu, S.; Li, E.; Wang, J.; Fu, X.; Qin, J.; Xie, Z.; Zheng, P.; Wang, L.; et al. Organic geochemistry of marine source rocks and pyrobitumen-containing reservoir rocks of the Sichuan Basin and neighbouring areas, SW China. Mar. Pet. Geol. 2014, 56, 147–165. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Pang, X.; Xiao, S.; Peng, H.; Li, Q.; Song, S.; Wu, L.; Chen, D.; Hu, T. Secondary migration of hydrocarbons in the zhujiang formation in the huixi half-graben, Pearl River Mouth Basin, South China Sea. Can. J. Earth Sci. 2016, 53, 189–201. [Google Scholar] [CrossRef]
- Wu, S.; Dong, D.; Yang, S.; Zhang, G.; Wang, Z.; Li, Q.; Liang, J.; Gong, Y.; Sun, Y. Genetic model of the hydrate system in the fine grain sediments in the northern continental slope of South China Sea. Chin. J. Geophys. 2009, 52, 1849–1857. [Google Scholar]
- Jiang, H.; Pang, X.; Shi, H.; Yu, Q.; Cao, Z.; Yu, R.; Chen, D.; Long, Z.; Jiang, F. Source rock characteristics and hydrocarbon expulsion potential of the Middle Eocene Wenchang formation in the Huizhou depression, Pearl River Mouth basin, South China sea. Mar. Pet. Geol. 2015, 67, 635–652. [Google Scholar] [CrossRef]
- He, D.; Hou, D.; Zhang, P.; Harris, M.; Mi, J.; Chen, T.; Li, J. Reservoir characteristics in the LW3-1 structure in the deepwater area of the Baiyun sag, South China Sea. Arab. J. Geosci. 2016, 9, 1–12. [Google Scholar] [CrossRef]
- Li, L.L.; Zhang, X.H.; Sha, Z.B. Gas hydrate and associated free gas in the Dongsha Area of northern South China Sea. Mar. Petrol. Geol. 2013, 39, 92–101. [Google Scholar] [CrossRef]
- Yang, R.; Su, M.; Qiao, S.H.; Cong, X.R.; Su, Z.; Liang, J.Q.; Wu, N.Y. Migration of methane associated with gas hydrates of the Shenhu Area, northern slope of South China Sea. Mar. Geophys. Res. 2015, 36, 253–261. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |

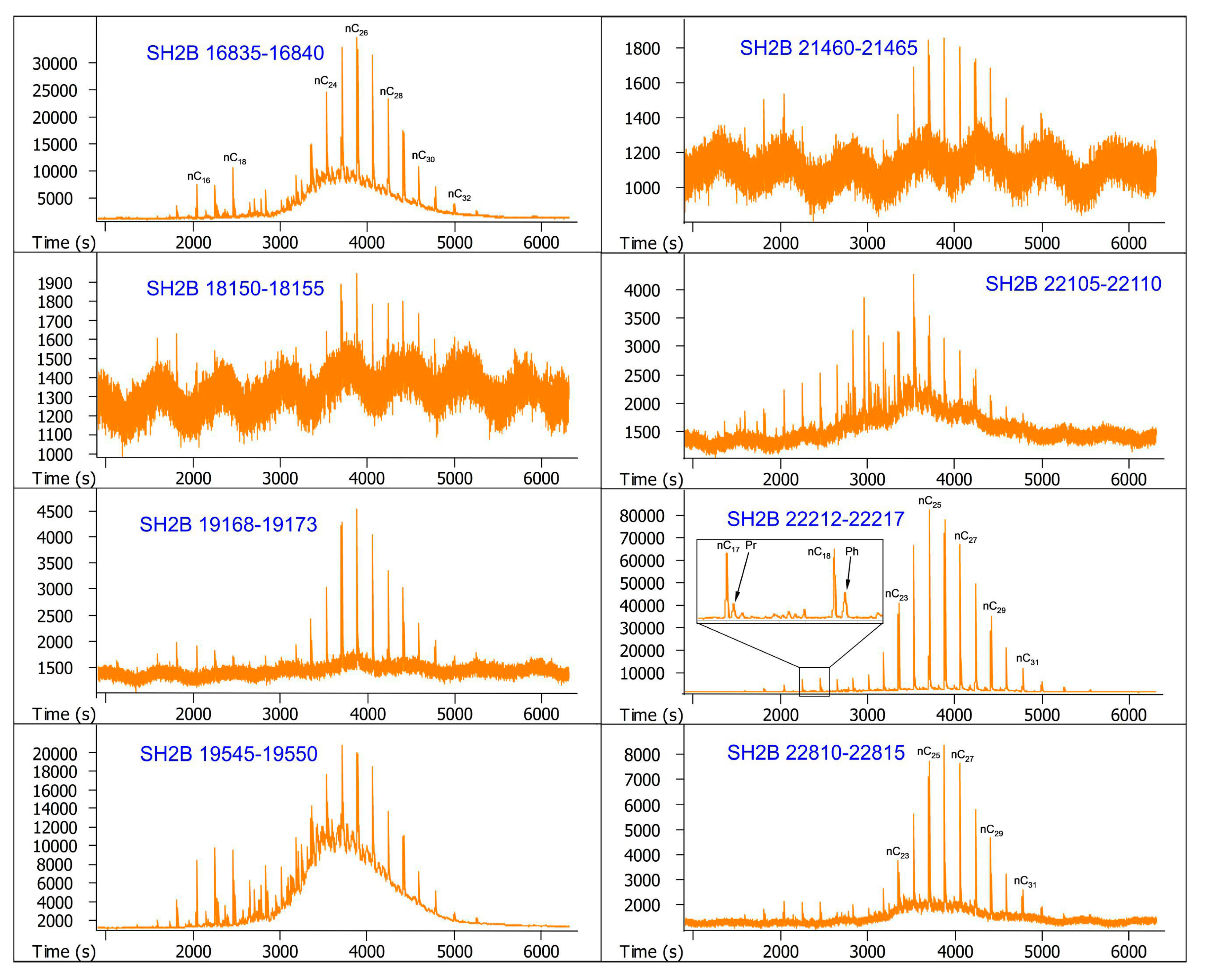
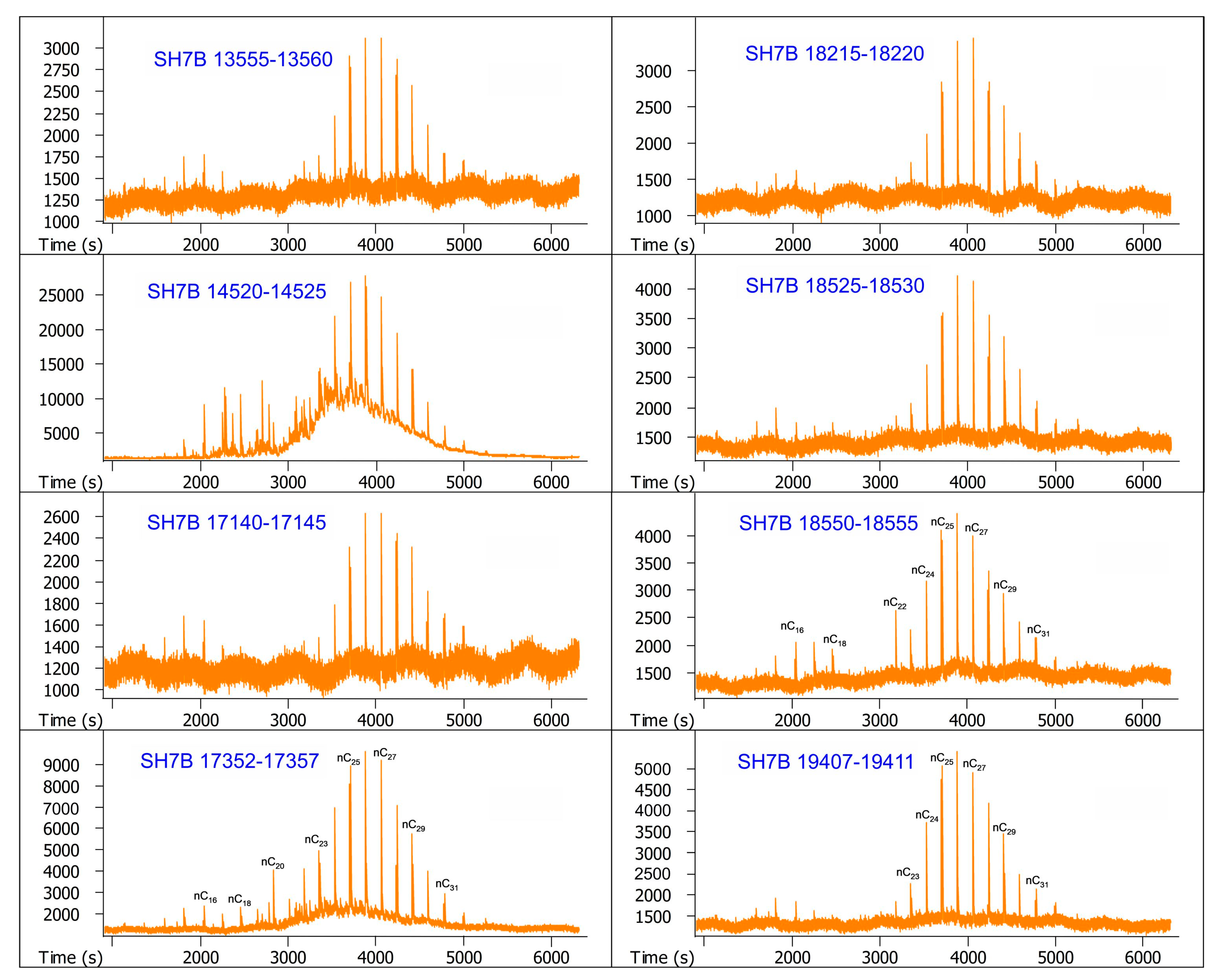
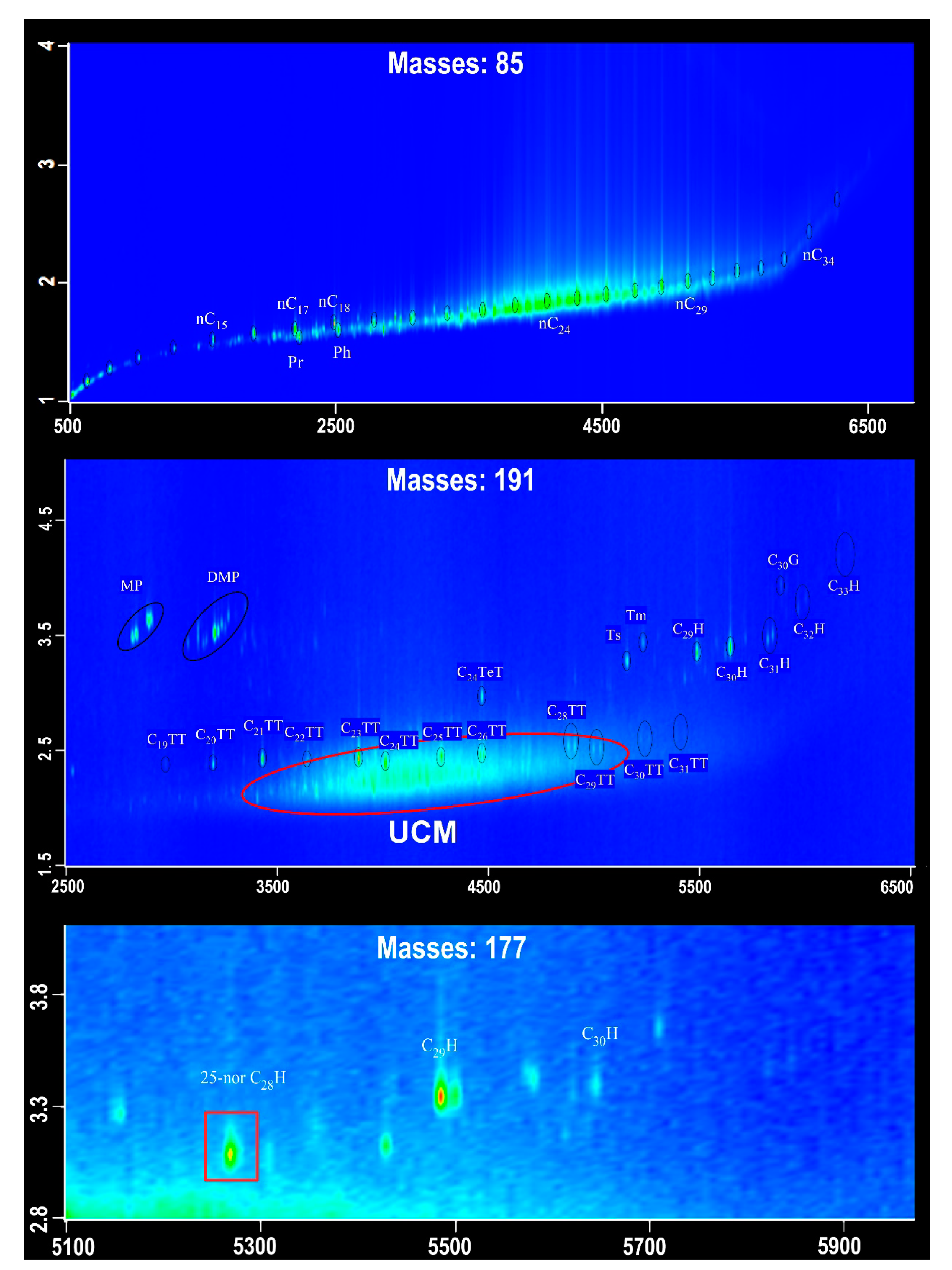
| Sample Number | Core Number | Depth Interval (cm) | Lithology | Layers |
|---|---|---|---|---|
| 1 | SH2B | 16,835–16,840 | Silty clay | Overlying layer |
| 2 | SH2B | 18,150–18,155 | Silty clay | Overlying layer |
| 3 | SH2B | 19,168–19,173 | Silty clay | Methane hydrate-bearing layer |
| 4 | SH2B | 19,545–19,550 | Silty clay | Methane hydrate-bearing layer |
| 5 | SH2B | 21,460–21,465 | Silty clay | Methane hydrate-bearing layer |
| 6 | SH2B | 22,105–22,110 | Silty clay | Methane hydrate-bearing layer |
| 7 | SH2B | 22,212–22,217 | Silty clay | Methane hydrate-bearing layer |
| 8 | SH2B | 22,810–22,815 | Silty clay | Underlying layer |
| 9 | SH7B | 13,555–13,560 | Sand | Overlying layer |
| 10 | SH7B | 14,520–14,525 | Sand | Overlying layer |
| 11 | SH7B | 17,140–17,145 | Silty clay | Methane hydrate-bearing layer |
| 12 | SH7B | 17,352–17,357 | Silty clay | Methane hydrate-bearing layer |
| 13 | SH7B | 18,215–18,220 | Silty clay | Underlying layer |
| 14 | SH7B | 18,525–18,530 | Silty clay | Underlying layer |
| 15 | SH7B | 18,550–18,555 | Silty clay | Underlying layer |
| 16 | SH7B | 19,407–19,411 | Silty clay | Underlying layer |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.-Z.; Li, Y.; Chen, F.; Li, S.-F.; Dong, S.-J.; Zhang, F.-L.; Xu, X.-M.; Wang, J.-H. Geochemical Significance of Biomarkers in the Methane Hydrate-Bearing Sediments from the Shenhu Area, the South China Sea. Molecules 2019, 24, 456. https://doi.org/10.3390/molecules24030456
Zhou Q-Z, Li Y, Chen F, Li S-F, Dong S-J, Zhang F-L, Xu X-M, Wang J-H. Geochemical Significance of Biomarkers in the Methane Hydrate-Bearing Sediments from the Shenhu Area, the South China Sea. Molecules. 2019; 24(3):456. https://doi.org/10.3390/molecules24030456
Chicago/Turabian StyleZhou, Qian-Zhi, Yan Li, Fang Chen, Shui-Fu Li, Shu-Jun Dong, Feng-Lin Zhang, Xiao-Ming Xu, and Jiang-Hai Wang. 2019. "Geochemical Significance of Biomarkers in the Methane Hydrate-Bearing Sediments from the Shenhu Area, the South China Sea" Molecules 24, no. 3: 456. https://doi.org/10.3390/molecules24030456





