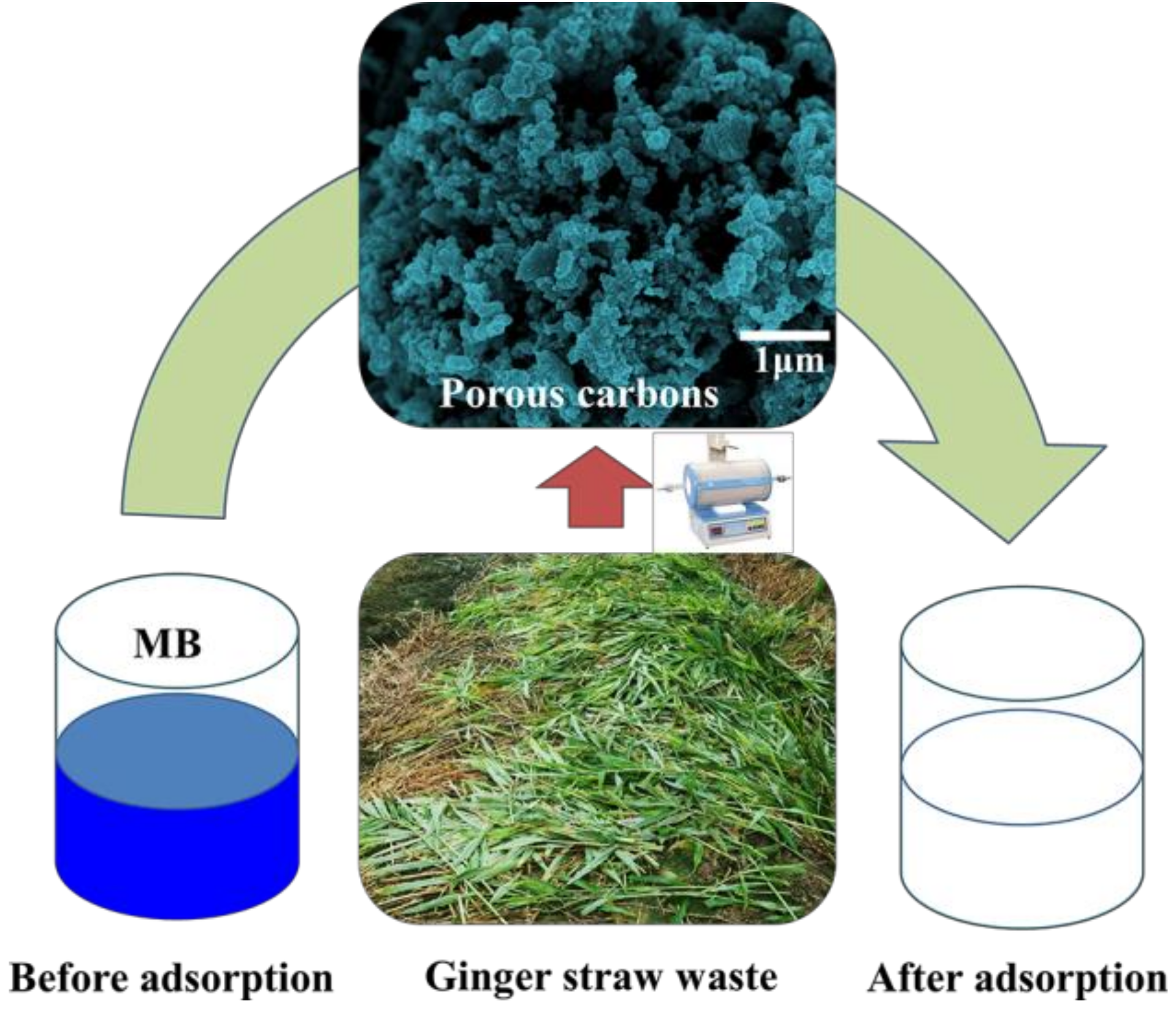
Ginger Straw Waste-Derived Porous Carbons as Effective Adsorbents toward Methylene Blue
Abstract
:1. Introduction
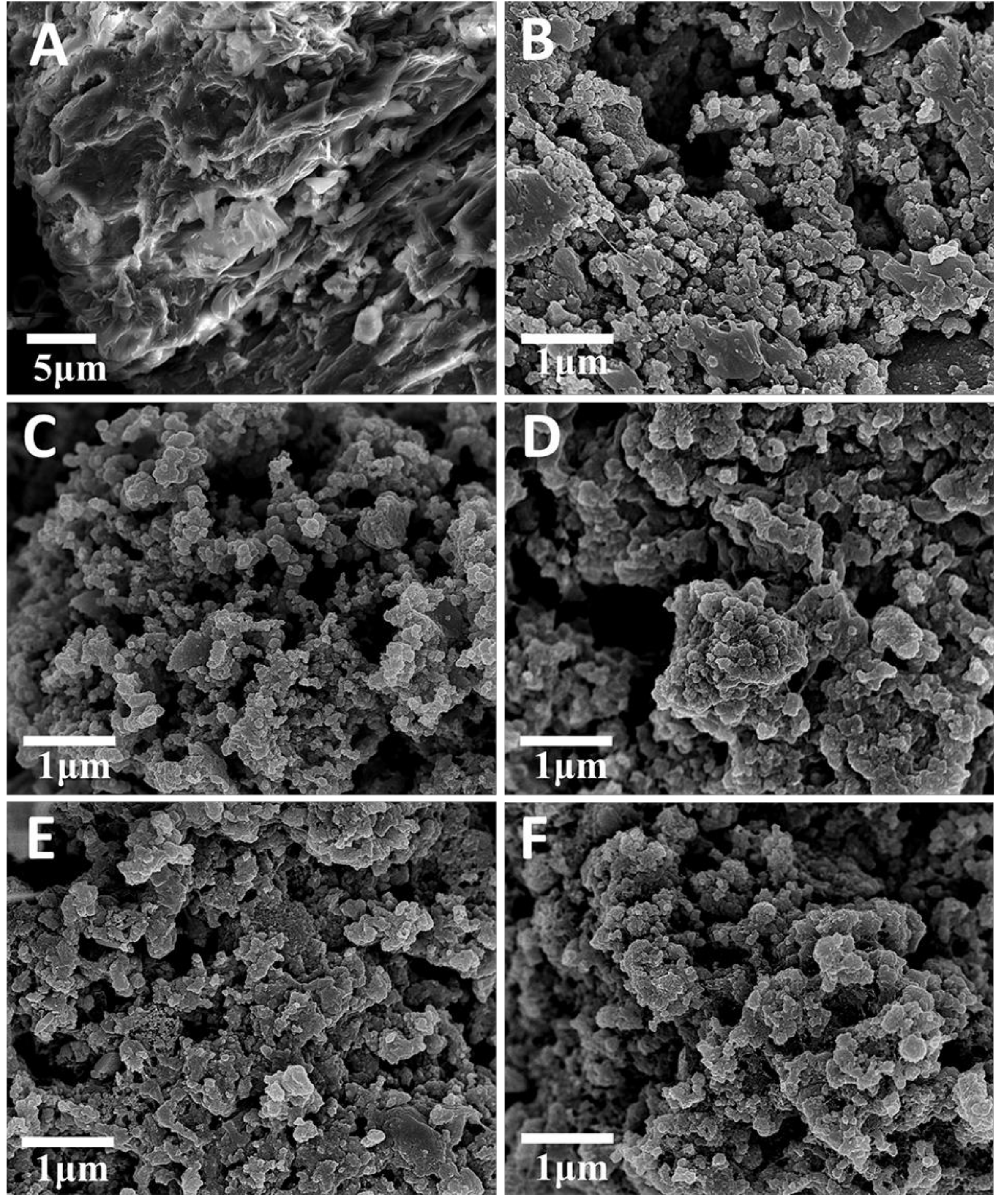
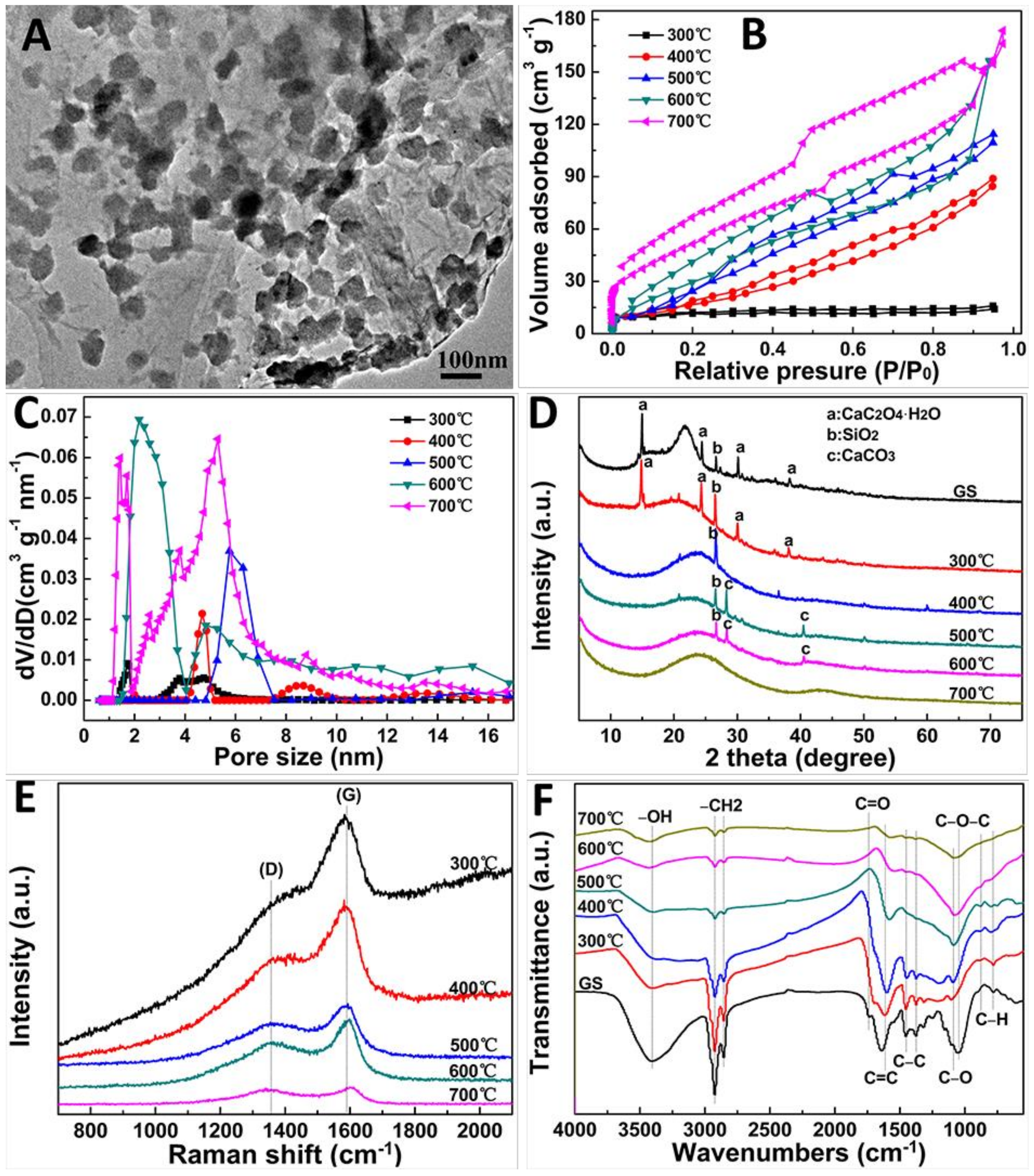
2. Results and Discussion
3. Material and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mor, S.; Chhavi, M.K.; Sushil, K.K.; Ravindra, K. Assessment of hydrothermally modified fly ash for the treatment of methylene blue dye in the textile industry wastewater. Environ. Dev. Sustain. 2018, 20, 625–639. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Naushad, M.; Kumar, A.; Al-Muhtaseb, A.a.H.; Dhiman, P.; Ghfar, A.A.; Stadler, F.J.; Khan, M.R. Photoremediation of toxic dye from aqueous environment using monometallic and bimetallic quantum dots based nanocomposites. J. Clean Prod. 2018, 172, 2919–2930. [Google Scholar] [CrossRef]
- Bhatia, D.; Datta, D.; Joshi, A.; Gupta, S.; Gote, Y. Adsorption study for the separation of isonicotinic acid from aqueous solution using activated carbon/Fe3O4 composites. J. Chem. Eng. Data. 2018, 63, 436–445. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, Z. Citrus pectin-derived carbon microspheres with superior adsorption ability for methylene blue. Nanomaterials 2017, 7, 161. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Wan, Y.; Hu, X.; Wang, S.; Zimmerman, A.R.; Gao, B. Sorption of lead and methylene blue onto hickory biochars from different pyrolysis temperatures: Importance of physicochemical properties. J. Ind. Eng. Chem. 2016, 37, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Inyang, M.I.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.; Mosa, A.; Pullammanappallil, P.; Ok, Y.S.; Cao, X. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit. Rev. Environ. Sci. Technol. 2016, 46, 406–433. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Zhang, W.; Zhou, Z.; Li, C.M. γ-Fe2O3 nanocrystals-anchored macro/meso-porous graphene as a highly efficient adsorbent toward removal of methylene blue. J. Colloid Interface Sci. 2016, 476, 200–205. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, L.Y.; Zhao, X.J.; Zhou, Z. Citrus pectin derived porous carbons as a superior adsorbent toward removal of methylene blue. J. Solid State Chem. 2016, 243, 101–105. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.; Li, J.; Ma, D.; Han, R. Characterization of bio-char from pyrolysis of wheat straw and its evaluation on methylene blue adsorption. Desalin. Water Treat. 2012, 46, 115–123. [Google Scholar] [CrossRef]
- Mashhadi, S.; Javadian, H.; Ghasemi, M.; Saleh, T.A.; Gupta, V.K. Microwave-induced H2SO4 activation of activated carbon derived from rice agricultural wastes for sorption of methylene blue from aqueous solution. Desalin. Water Treat. 2016, 57, 21091–21104. [Google Scholar]
- Vadivelan, V.; Kumar, K.V. Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J. Colloid Interface Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Huff, M.D.; Kumar, S.; Lee, J.W. Comparative analysis of pinewood, peanut shell, and bamboo biomass derived biochars produced via hydrothermal conversion and pyrolysis. J. Environ. Manage. 2014, 146, 303–308. [Google Scholar] [CrossRef] [PubMed]
- An, K.; Zhao, D.; Wang, Z.; Wu, J.; Xu, Y.; Xiao, G. Comparison of different drying methods on Chinese ginger (Zingiber officinale Roscoe): Changes in volatiles, chemical profile, antioxidant properties, and microstructure. Food Chem. 2016, 197, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Munroe, P.; Joseph, S.; Kimber, S.; Van Zwieten, L. Nanoscale organo-mineral reactions of biochars in ferrosol: an investigation using microscopy. Plant Soil 2012, 357, 369–380. [Google Scholar] [CrossRef]
- Archanjo, B.S.; Mendoza, M.E.; Albu, M.; Mitchell, D.R.; Hagemann, N.; Mayrhofer, C.; Anh Mai T., L.; Weng, Z.; Kappler, A.; Behrens, S.; Munroe, P.; Achete C., A.; Donne, S.; Araujo J., R.; Zwieten, L.; Horvat, J.; Enders, A.; Joseph, S.; Munroe, P. Nanoscale analyses of the surface structure and composition of biochars extracted from field trials or after co-composting using advanced analytical electron microscopy. Geoderma 2017, 294, 70–79. [Google Scholar] [CrossRef]
- Li, H.; Sun, Z.; Zhang, L.; Tian, Y.; Cui, G.; Yan, S. A cost-effective porous carbon derived from pomelo peel for the removal of methyl orange from aqueous solution. Colloid Surf. A Physicochem. Eng. Asp. 2016, 489, 191–199. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Yi, H.T.; Chen, X.Y. Zinc citrate-based nanoporous carbon materials: Large capacitive enhancement using redox active electrolyte of p-phenylenediamine. J. Alloys Compd. 2015, 651, 414–422. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, X.; Wei, X.; Zhang, S.; Chen, J.; Ren, Z.J. CO2 activation promotes available carbonate and phosphorus of antibiotic mycelial fermentation residue-derived biochar support for increased lead immobilization. Chem. Eng. J. 2018, 334, 1101–1107. [Google Scholar] [CrossRef]
- Fan, S.; Tang, J.; Wang, Y.; Li, H.; Zhang, H.; Tang, J.; Wang, Z.; Li, X. Biochar prepared from co-pyrolysis of municipal sewage sludge and tea waste for the adsorption of methylene blue from aqueous solutions: Kinetics, isotherm, thermodynamic and mechanism. J. Mol. Liq. 2016, 220, 432–441. [Google Scholar] [CrossRef]
- Fan, S.; Wang, Y.; Wang, Z.; Tang, J.; Tang, J.; Li, X. Removal of methylene blue from aqueous solution by sewage sludge-derived biochar: Adsorption kinetics, equilibrium, thermodynamics and mechanism. J. Environ. Chem. Eng. 2017, 5, 601–611. [Google Scholar] [CrossRef]
- Leng, L.; Yuan, X.; Huang, H.; Shao, J.; Wang, H.; Chen, X.; Zeng, G. Bio-char derived from sewage sludge by liquefaction: Characterization and application for dye adsorption. Appl. Surf. Sci. 2015, 346, 223–231. [Google Scholar] [CrossRef]
- Sun, L.; Wan, S.; Luo, W. Biochars prepared from anaerobic digestion residue, palm bark, and eucalyptus for adsorption of cationic methylene blue dye: Characterization, equilibrium, and kinetic studies. Bioresour. Technol. 2013, 140, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Moon, J.H.; Ma, X.; Zhang, L.; Chen, Q.; Chen, L.; Peng, R.; Si, P.; Feng, J.; Li, Y.; et al. High performance graphene oxide nanofiltration membrane prepared by electrospraying for wastewater purification. Carbon 2018, 130, 487–494. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, L.Y.; Zhao, X.J.; Zhou, Z. Citrus pectin derived ultrasmall Fe3O4@C nanoparticles as a high-performance adsorbent toward removal of methylene blue. J. Mol. Liq. 2016, 222, 995–1002. [Google Scholar] [CrossRef]
- Güzel, F.; Sayğılı, H.; Akkaya Sayğılı, G.; Koyuncu, F.; Yılmaz, C. Optimal oxidation with nitric acid of biochar derived from pyrolysis of weeds and its application in removal of hazardous dye methylene blue from aqueous solution. J. Clean Prod. 2017, 144, 260–265. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, Y.; Ho, S.; Zhou, Y.; Ren, N. Highly efficient adsorption of dyes by biochar derived from pigments-extracted macroalgae pyrolyzed at different temperature. Bioresour. Technol. 2018, 259, 104–110. [Google Scholar] [CrossRef]
- Sun, L.; Chen, D.; Wan, S.; Yu, Z. Performance, kinetics, and equilibrium of methylene blue adsorption on biochar derived from eucalyptus saw dust modified with citric, tartaric, and acetic acids. Bioresour. Technol. 2015, 198, 300–308. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Gong, Y.; Wu, D.; Li, Z.; Li, Q.; Zheng, L.; Chen, W. Palladium-cobalt nanodots anchored on graphene: In-situ synthesis, and application as an anode catalyst for direct formic acid fuel cells. Appl. Surf. Sci. 2019, 469, 305–311. [Google Scholar] [CrossRef]
- Li, X.Y.; Han, D.; Xie, J.F.; Wang, Z.B.; Gong, Z.Q.; Li, B. Hierarchical porous activated biochar derived from marine macroalgae wastes (Enteromorpha prolifera): facile synthesis and its application on Methylene Blue removal. RSC Adv. 2018, 8, 29237–29247. [Google Scholar] [CrossRef]
Sample Availability: Samples are available from the authors. |

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Li, H.; Tang, J.; Lu, H.; Liu, Y. Ginger Straw Waste-Derived Porous Carbons as Effective Adsorbents toward Methylene Blue. Molecules 2019, 24, 469. https://doi.org/10.3390/molecules24030469
Zhang W, Li H, Tang J, Lu H, Liu Y. Ginger Straw Waste-Derived Porous Carbons as Effective Adsorbents toward Methylene Blue. Molecules. 2019; 24(3):469. https://doi.org/10.3390/molecules24030469
Chicago/Turabian StyleZhang, Wenlin, Huihe Li, Jianmin Tang, Hongjia Lu, and Yiqing Liu. 2019. "Ginger Straw Waste-Derived Porous Carbons as Effective Adsorbents toward Methylene Blue" Molecules 24, no. 3: 469. https://doi.org/10.3390/molecules24030469
APA StyleZhang, W., Li, H., Tang, J., Lu, H., & Liu, Y. (2019). Ginger Straw Waste-Derived Porous Carbons as Effective Adsorbents toward Methylene Blue. Molecules, 24(3), 469. https://doi.org/10.3390/molecules24030469



