Liposomal Taro Lectin Nanocapsules Control Human Glioblastoma and Mammary Adenocarcinoma Cell Proliferation
Abstract
:1. Introduction
2. Results
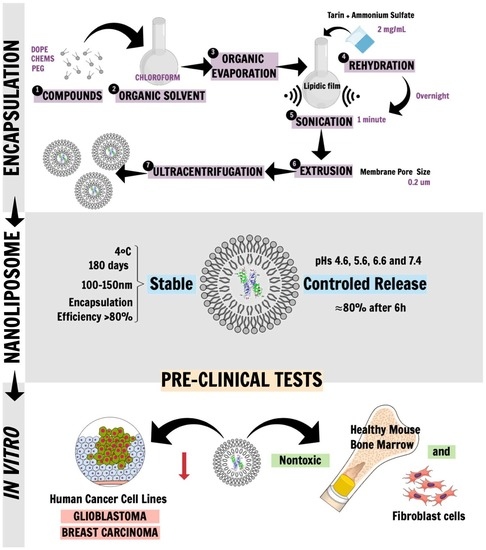
2.1. Liposomal Tarin Encapsulation and Characterization
2.2. Release Control under Physiological Conditions
2.3. In Vitro Pre-Clinical Tests
2.3.1. Toxicological Screening and Morphological Modifications of Healthy Mice Cells Treated with Free or Encapsulated Tarin
2.3.2. In Vitro Antitumoral Activity of Free and Encapsulated Tarin
3. Discussion
4. Material and Methods
4.1. Tarin Purification
4.2. Liposomal Nanocapsules Preparation
4.3. Morphological Liposome Characterization
4.4. Encapsulated Tarin Stability Determination
4.5. Kinetic Release of Encapsulated Tarin
4.6. Pre-Clinical In Vitro Tests
4.6.1. Animals
4.6.2. Tarin Cytotoxicity on Healthy Bone Marrow and Fibroblasts Cells
4.6.3. Morphology of Bone Marrow Cells Treated with Free and Encapsulated Tarin
4.6.4. Evaluation of Antitumoral Tarin Activity
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World health statistics 2018: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- American Cancer Society. About Breast Cancer. Available online: https://www.cancer.org/content/dam/CRC/PDF/Public/8577.00.pdf (accessed on 3 September 2018).
- American Cancer Society. Chemotherapy for Breast Cancer. Available online: https://www.cancer.org/cancer/breast-cancer/treatment/chemotherapy-for-breast-cancer.html (accessed on 3 September 2018).
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Rouse, C.; Chen, Y.; Dowling, J.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014, 16, iv1–iv63. [Google Scholar] [CrossRef]
- Agarwala, S.S.; Kirkwood, J.M. Temozolomide, a novel alkylating agent with activity in the central nervous system, may improve the treatment of advanced metastatic melanoma. Oncologist 2000, 5, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Tang, X.L. Natural products against cancer: A comprehensive bibliometric study of the research projects, publications, patents and drugs. J. Cancer Res. Ther. 2014, 10, 27. [Google Scholar] [PubMed]
- Wang, X.; Wang, Y.; Chen, Z.G.; Shin, D.M. Advances of cancer therapy by nanotechnology. Cancer Res. Treat 2009, 41. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.R.; Del Aguila, E.M.; Vericimo, M.A.; Zingali, R.B.; Paschoalin, V.M.F.; Silva, J.T. Purification and characterization of the lectin from taro (Colocasia esculenta) and its effect on mouse splenocyte proliferation in vitro and in vivo. Prot. J. 2014, 33, 92–99. [Google Scholar] [CrossRef]
- Pereira, P.R.; Winter, H.C.; Verícimo, M.A.; Meagher, J.L.; Stuckey, J.A.; Goldstein, I.J.; Paschoalin, V.M.; Silva, J.T. Structural analysis and binding properties of isoforms of tarin, the GNA-related lectin from Colocasia esculenta. BBA Proteins Proteom. 2015, 1854, 20–30. [Google Scholar] [CrossRef]
- Pereira, P.R.; Silva, J.T.; Verícimo, M.A.; Paschoalin, V.M.; Teixeira, G.A. Crude extract from taro (Colocasia esculenta) as a natural source of bioactive proteins able to stimulate haematopoietic cells in two murine models. J. Funct. Foods 2015, 18, 333–343. [Google Scholar] [CrossRef]
- Pereira, P.R.; Meagher, J.L.; Winter, H.C.; Goldstein, I.J.; Paschoalin, V.M.; Silva, J.T.; Stuckey, J.A. High-resolution crystal structures of Colocasia esculenta tarin lectin. Glycobiology 2016, 27, 50–56. [Google Scholar] [CrossRef]
- Sang Chan, Y.; Ho Wong, J.; Bun Ng, T. A cytokine-inducing hemagglutinin from small taros. Protein Peptide Lett. 2010, 17, 823–830. [Google Scholar] [CrossRef]
- Kundu, N.; Campbell, P.; Hampton, B.; Lin, C.-Y.; Ma, X.; Ambulos, N.; Zhao, X.F.; Goloubeva, O.; Holt, D.; Fulton, A.M. Antimetastatic activity isolated from Colocasia esculenta (taro). Anticancer Drugs 2012, 23, 200. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.R.; Corrêa, A.C.N.T.F.; Vericimo, M.A.; Paschoalin, V.M.F. Tarin, a Potential Immunomodulator and COX-Inhibitor Lectin Found in Taro (Colocasia esculenta). Compr. Rev. Food Sci. Food Saf. 2018. [Google Scholar] [CrossRef]
- Merida, L.A.; Mattos, E.B.; Corrêa, A.C.; Pereira, P.R.; Paschoalin, V.M.; Pinho, M.F.; Vericimo, M.A. Tarin stimulates granulocyte growth in bone marrow cell cultures and minimizes immunosuppression by cyclo-phosphamide in mice. PLoS ONE 2018, 13, e0206240. [Google Scholar] [CrossRef]
- Santos, N.C.; Castanho, M.A. Liposomes: Has the magic bullet hit the target? Quim. Nova 2002, 25, 1181–1185. [Google Scholar] [CrossRef]
- Papachristos, A.; Pippa, N.; Ioannidis, K.; Sivolapenko, G.; Demetzos, C. Liposomal forms of anticancer agents beyond anthracyclines: Present and future perspectives. J. Liposome Res. 2015, 25, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C.A.; Correia, M.T.; Coelho, L.C.; Nascimento, S.C.; Santos-Magalhães, N.S. Antitumor activity of Cratylia mollis lectin encapsulated into liposomes. Int. J. Pharm. 2004, 278, 435–445. [Google Scholar] [CrossRef]
- dos Santos Ferreira, D.; Faria, S.D.; de Araújo Lopes, S.C.; Teixeira, C.S.; Malachias, A.; Magalhães-Paniago, R.; de Souza Filho, J.D.; Oliveira, B.L.d.J.P.; Guimarães, A.R.; Caravan, P. Development of a bone-targeted pH-sensitive liposomal formulation containing doxorubicin: Physicochemical characterization, cytotoxicity, and biodistribution evaluation in a mouse model of bone metastasis. Int. J. Nanomed. 2016, 11, 3737. [Google Scholar]
- Mukthavaram, R.; Jiang, P.; Saklecha, R.; Simberg, D.; Bharati, I.S.; Nomura, N.; Chao, Y.; Pastorino, S.; Pingle, S.C.; Fogal, V. High-efficiency liposomal encapsulation of a tyrosine kinase inhibitor leads to improved in vivo toxicity and tumor response profile. Int. J. Nanomed. 2013, 8, 3991. [Google Scholar] [Green Version]
- Peterson, G.L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal. Biochem. 1977, 83, 346–356. [Google Scholar] [CrossRef]
- Douglas, S.; Tuluc, F. Morphology of Monocytes and Macrophages. In Williams Hematology, 8e; The McGraw-Hill Companies: New York, NY, USA, 2010; Chapter 67. [Google Scholar]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal formulations in clinical use: An updated review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho, J.F.; Ferreira, P.C.; Alves, P.; Cordeiro, R.; Fonseca, A.C.; Góis, J.R.; Gil, M.H. Drug delivery systems: Advanced technologies potentially applicable in personalized treatments. EPMA J. 2010, 1, 164–209. [Google Scholar] [CrossRef] [Green Version]
- Mahmud, M.; Piwoni, A.; Filiczak, N.; Janicka, M.; Gubernator, J. Long-circulating curcumin-loaded liposome formulations with high incorporation efficiency, stability and anticancer activity towards pancreatic adenocarcinoma cell lines in vitro. PLoS ONE 2016, 11, e0167787. [Google Scholar] [CrossRef] [PubMed]
- Woodle, M.; Collins, L.; Sponsler, E.; Kossovsky, N.; Papahadjopoulos, D.; Martin, F. Sterically stabilized liposomes. Reduction in electrophoretic mobility but not electrostatic surface potential. Biophys. J. 1992, 61, 902–910. [Google Scholar] [CrossRef] [Green Version]
- Sharma, U.S.; Sharma, A.; Chau, R.I.; Straubinger, R.M. Liposome-mediated therapy of intracranial brain tumors in a rat model. Pharm. Res. 1997, 14, 992–998. [Google Scholar] [CrossRef]
- Nakhla, T.; Marek, M.; Kovalcik, T. Issues associated with large-scale production of liposomal formulations. Drug Deliv. Technol. 2002, 2. [Google Scholar]
- Comiskey, S.J.; Heath, T.D. Leakage and delivery of liposome-encapsulated methotrexate-γ-aspartate in a chemically defined medium. Biochim. Biophys Acta Biomembr. 1990, 1024, 307–317. [Google Scholar] [CrossRef]
- Tabata, Y.; Ikada, Y. Phagocytosis of polymer microspheres by macrophages. In New Polymer Materials; Springer: Berlin, Germany, 1990; pp. 107–141. [Google Scholar]
- Stolnik, S.; Illum, L.; Davis, S. Long circulating microparticulate drug carriers. Adv. Drug Deliv. Rev. 1995, 16, 195–214. [Google Scholar] [CrossRef]
- Caldorera-Moore, M.; Guimard, N.; Shi, L.; Roy, K. Designer nanoparticles: Incorporating size, shape and triggered release into nanoscale drug carriers. Expert Opin. Drug Deliv. 2010, 7, 479–495. [Google Scholar] [CrossRef]
- Ferreira, D.d.S.; Lopes, S.C.d.A.; Franco, M.S.; Oliveira, M.C. pH-sensitive liposomes for drug delivery in cancer treatment. Ther. Deliv. 2013, 4, 1099–1123. [Google Scholar] [CrossRef] [PubMed]
- Thakor, F.K.; Wan, K.-W.; Welsby, P.J.; Welsby, G. Pharmacological effects of asiatic acid in glioblastoma cells under hypoxia. Mol. Cell. Biochem. 2017, 430, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Baer, J.; Freeman, A.; Newlands, E.; Watson, A.; Rafferty, J.; Margison, G. Depletion of O 6-alkylguanine-DNA alkyltransferase correlates with potentiation of temozolomide and CCNU toxicity in human tumour cells. Br. J. Cancer 1993, 67, 1299. [Google Scholar] [CrossRef]
- Yin, L.L.; Wen, X.M.; Lai, Q.H.; Li, J.; Wang, X.W. Lenalidomide improvement of cisplatin antitumor efficacy on triple-negative breast cancer cells in vitro. Oncol. Lett. 2018, 15, 6469–6474. [Google Scholar] [CrossRef] [PubMed]
- Pilco-Ferreto, N.; Calaf, G.M. Influence of doxorubicin on apoptosis and oxidative stress in breast cancer cell lines. Int. J. Oncol. 2016, 49, 753–762. [Google Scholar] [CrossRef]
- Roy, A.; Banerjee, S.; Majumder, P.; Das, S. Efficiency of mannose-binding plant lectins in controlling a homopteran insect, the red cotton bug. J. Agric. Food Chem. 2002, 50, 6775–6779. [Google Scholar] [CrossRef]
- Murtey, M.D.; Ramasamy, P. Sample Preparations for Scanning Electron Microscopy–Life Sciences. In Modern Electron Microscopy in Physical and Life Sciences; InTech: London, UK, 2016. [Google Scholar]
- Deniz, A.; Sade, A.; Severcan, F.; Keskin, D.; Tezcaner, A.; Banerjee, S. Celecoxib-loaded liposomes: Effect of cholesterol on encapsulation and in vitro release characteristics. Biosci. Rep. 2010, 30, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Iwama, A.; Oguro, H.; Negishi, M.; Kato, Y.; Morita, Y.; Tsukui, H.; Ema, H.; Kamijo, T.; Katoh-Fukui, Y.; Koseki, H. Enhanced self-renewal of hematopoietic stem cells mediated by the polycomb gene product Bmi-1. Immunity 2004, 21, 843–851. [Google Scholar] [CrossRef]
- Schindelin, J.; Rueden, C.T.; Hiner, M.C.; Eliceiri, K.W. The ImageJ ecosystem: An open platform for biomedical image analysis. Mol. Reprod. Dev. 2015, 82, 518–529. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |






| Membrane Pore Size | Storage Time Intervals at 4 °C (days) | Size Distribution (nm) | Average Size (nm) | Polydispersity Index (PdI) | Peak (nm) | Entrapment Efficiency |
|---|---|---|---|---|---|---|
| 0.2 µm | 1 | 50.75–396.1 | 154.6 | 0.168 | 93.55 ± 38.75 | 0.83 |
| 10 | 43.82–396.1 | 155.0 | 0.191 | 78.74 ± 35.19 | ||
| 90 | 50.75–396.1 | 149.8 | 0.163 | 88.21 ± 35.69 | ||
| 120 | 43.82–396.1 | 149.2 | 0.192 | 84.65 ± 34.57 | ||
| 150 | 50.75–396.1 | 151.3 | 0.191 | 39.64 ± 22.36 | ||
| 180 | 58.77–396.1 | 149.9 | 0.135 | 99.12 ± 36.50 |
| Tumoral Cell Lines | Antitumoral Molecules | IC50 (µg/mL) | Reference |
|---|---|---|---|
| U-87 MG | Cisplatin | 29.00 | [35] |
| Temozolomide | 33.40 | [36] | |
| Tarin liposomal nanocapsules | 39.36 | Present study | |
| MDA-MB-231 | Cisplatin | 2.30 | [37] |
| Doxorubicin | 0.50 | [38] | |
| Tarin liposomal nanocapsules | 71.38 | Present study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrêa, A.C.N.T.F.; Vericimo, M.A.; Dashevskiy, A.; Pereira, P.R.; Paschoalin, V.M.F. Liposomal Taro Lectin Nanocapsules Control Human Glioblastoma and Mammary Adenocarcinoma Cell Proliferation. Molecules 2019, 24, 471. https://doi.org/10.3390/molecules24030471
Corrêa ACNTF, Vericimo MA, Dashevskiy A, Pereira PR, Paschoalin VMF. Liposomal Taro Lectin Nanocapsules Control Human Glioblastoma and Mammary Adenocarcinoma Cell Proliferation. Molecules. 2019; 24(3):471. https://doi.org/10.3390/molecules24030471
Chicago/Turabian StyleCorrêa, Anna C. N. T. F., Mauricio A. Vericimo, Andriy Dashevskiy, Patricia R. Pereira, and Vania M. F. Paschoalin. 2019. "Liposomal Taro Lectin Nanocapsules Control Human Glioblastoma and Mammary Adenocarcinoma Cell Proliferation" Molecules 24, no. 3: 471. https://doi.org/10.3390/molecules24030471
APA StyleCorrêa, A. C. N. T. F., Vericimo, M. A., Dashevskiy, A., Pereira, P. R., & Paschoalin, V. M. F. (2019). Liposomal Taro Lectin Nanocapsules Control Human Glioblastoma and Mammary Adenocarcinoma Cell Proliferation. Molecules, 24(3), 471. https://doi.org/10.3390/molecules24030471