Involvement of Hepatic SHIP2 and PI3K/Akt Signalling in the Regulation of Plasma Insulin by Xiaoyaosan in Chronic Immobilization-Stressed Rats
Abstract
1. Introduction
2. Result
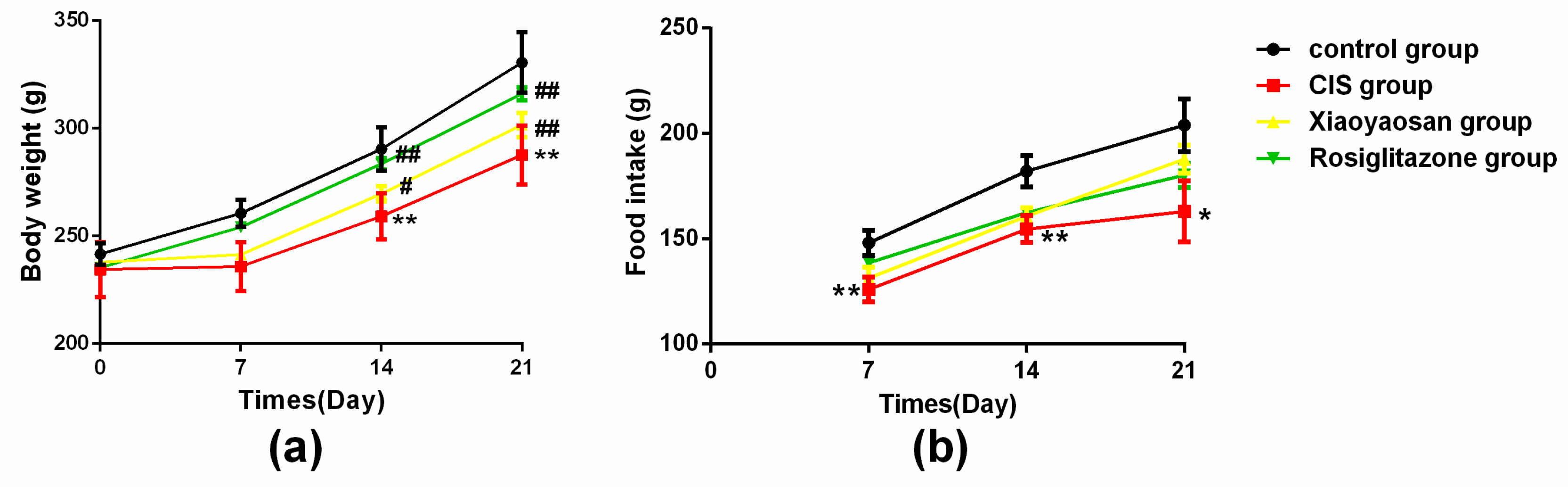
2.1. Effects of Xiaoyaosan on Weight and Food Intake in Rats Exposed to CIS
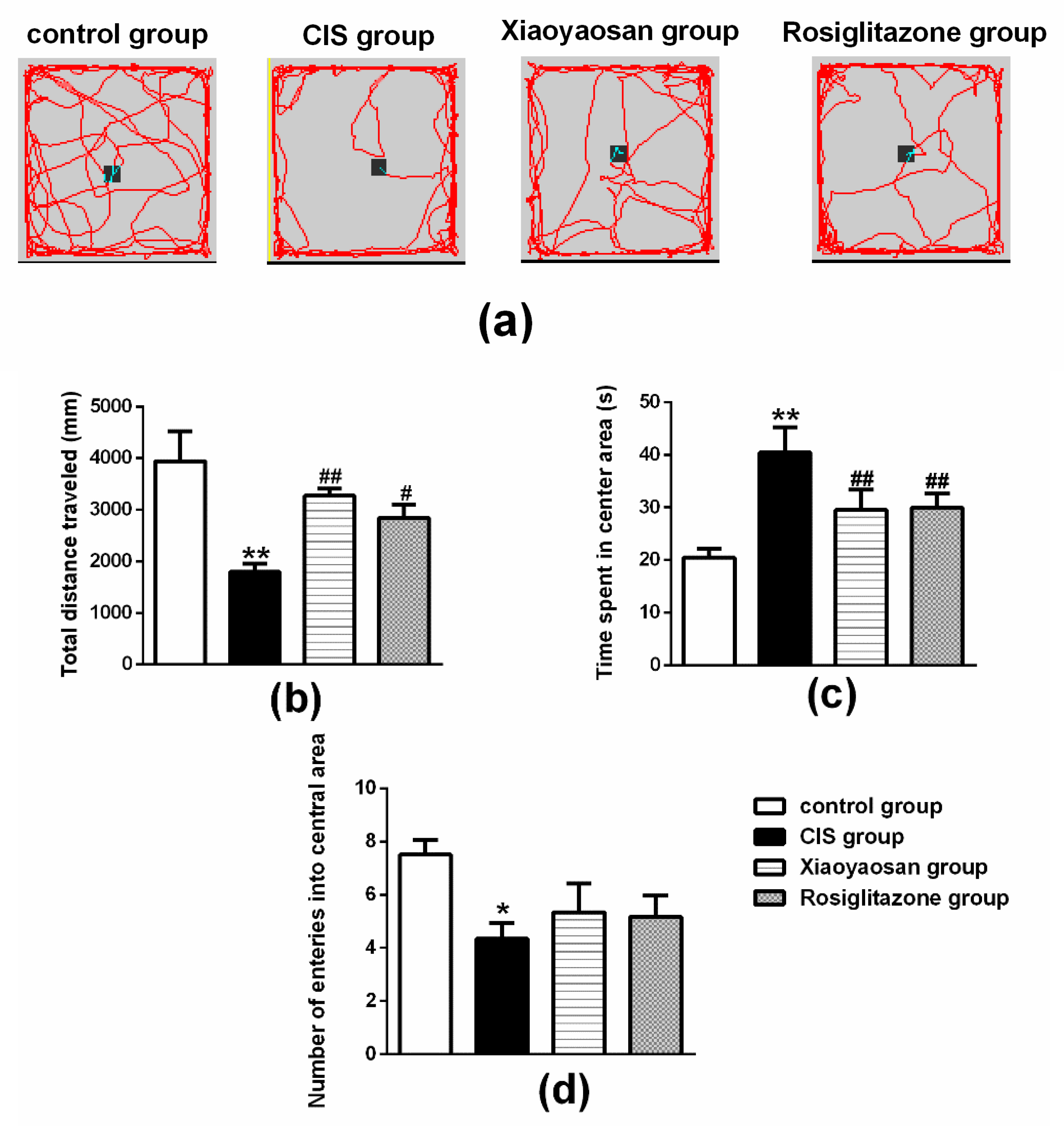
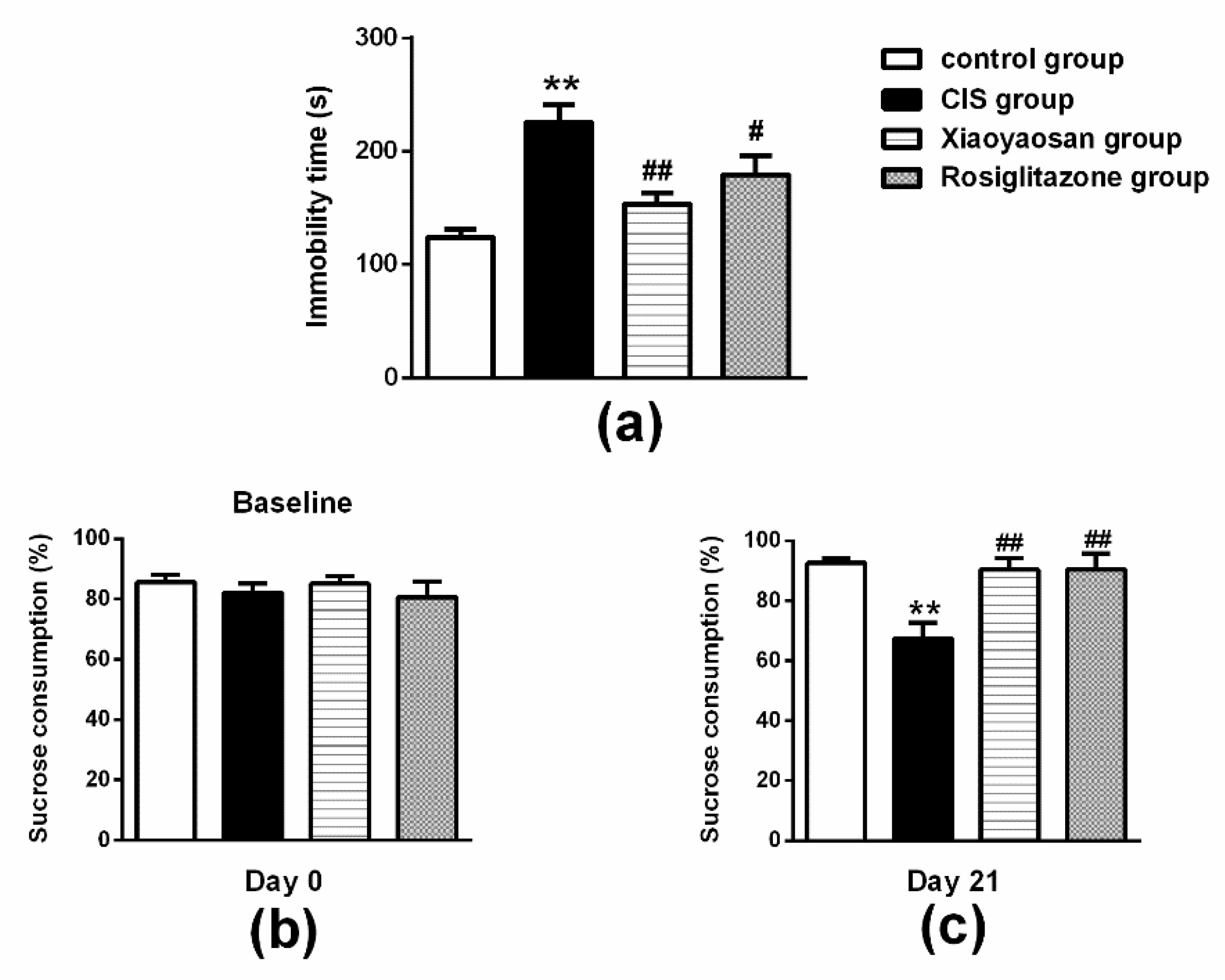
2.2. Effects of Xiaoyaosan on Behavioural Changes in Rats Exposed to CIS
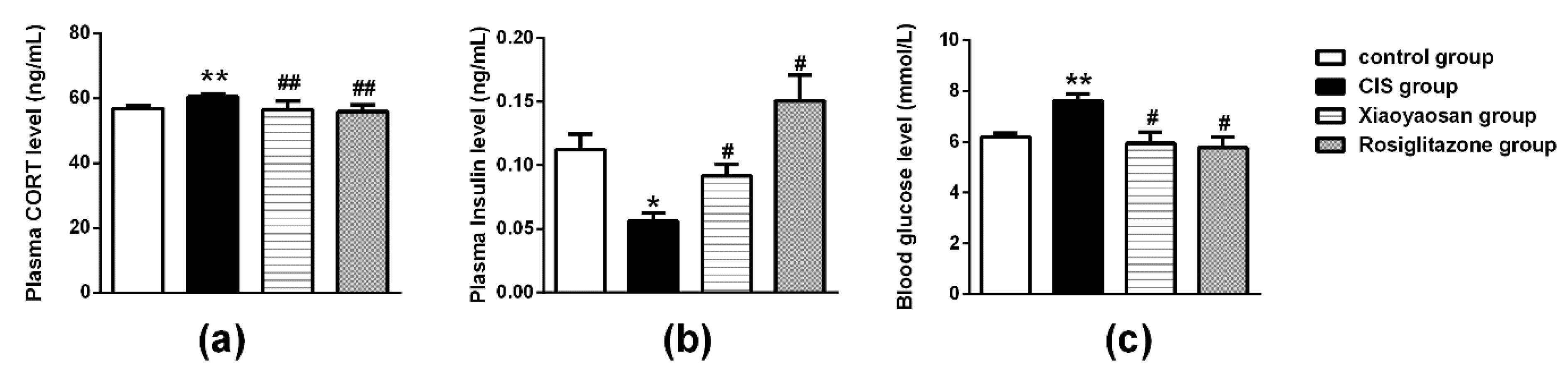
2.3. Effects of Xiaoyaosan on Plasma Cholesterol (CHOL), Triglyceride (TG), Low-Density Lipoprotein (LDL-C), High-Density Lipoprotein (HDL-C), Cortisol (CORT) and Insulin Levels in Rats Exposed to CIS
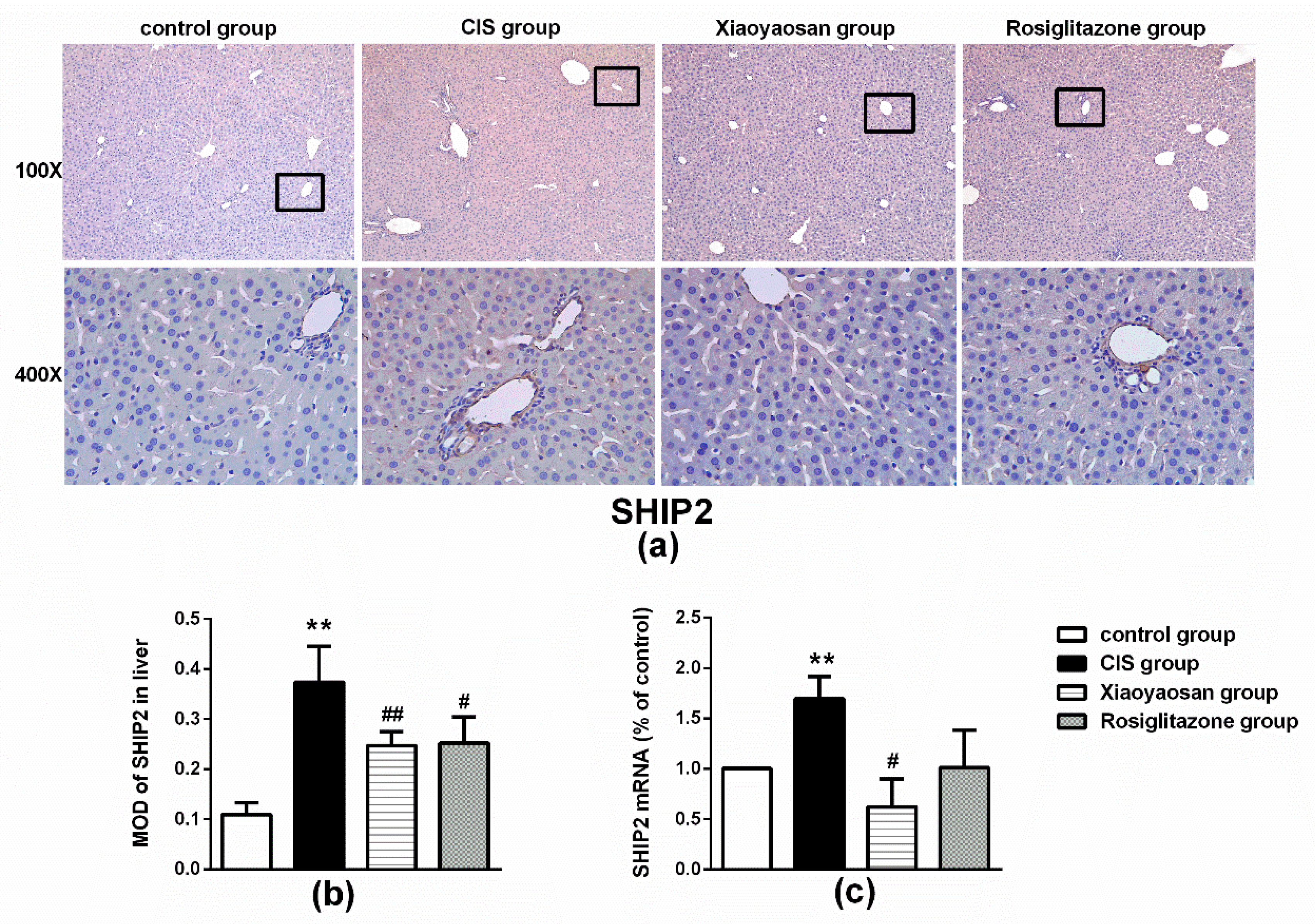
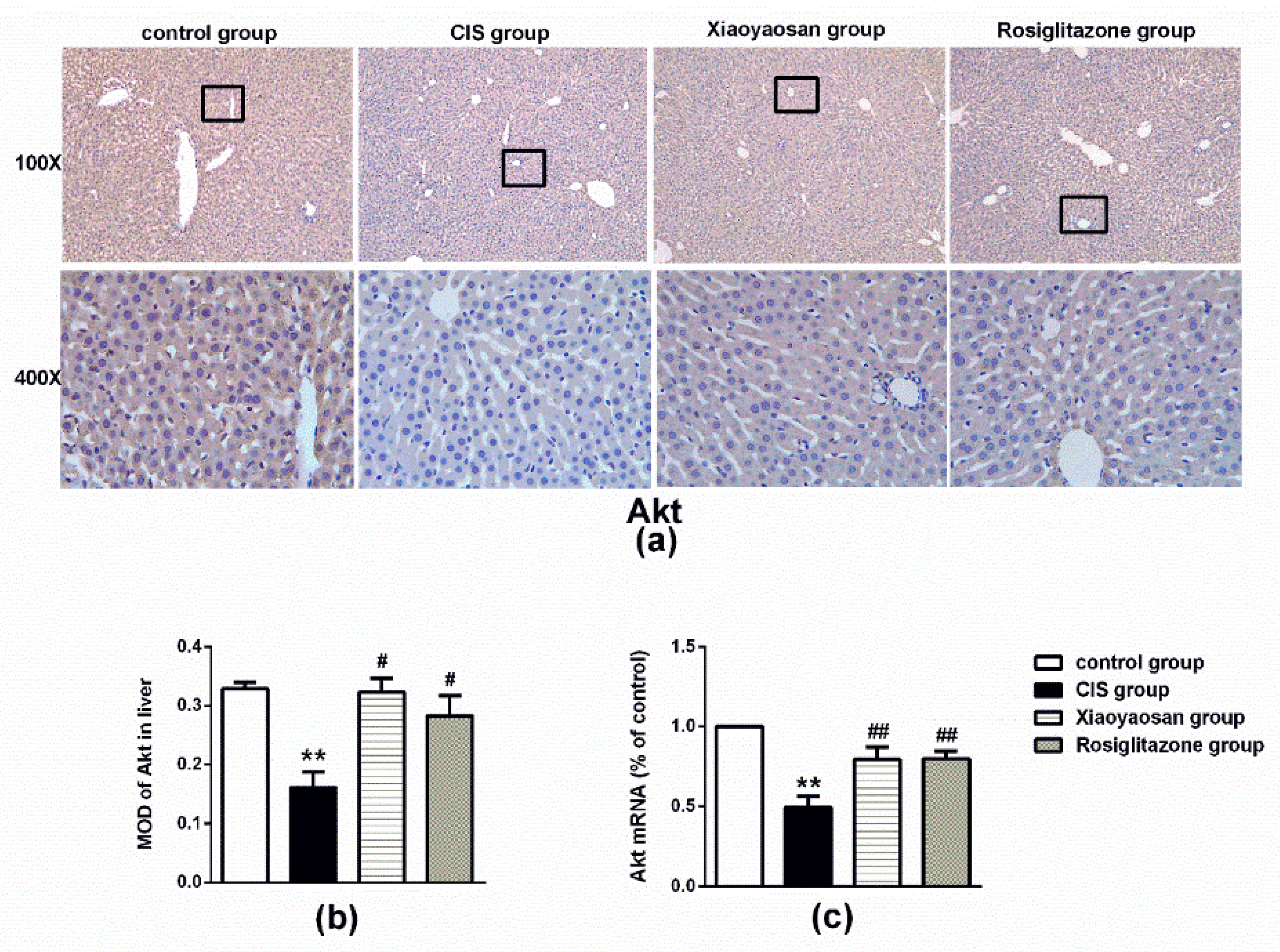
2.4. Effects of Xiaoyaosan on the Expression of SHIP2, p85 and Akt Immunolabelling and mRNAs in the Rat Liver
3. Discussion
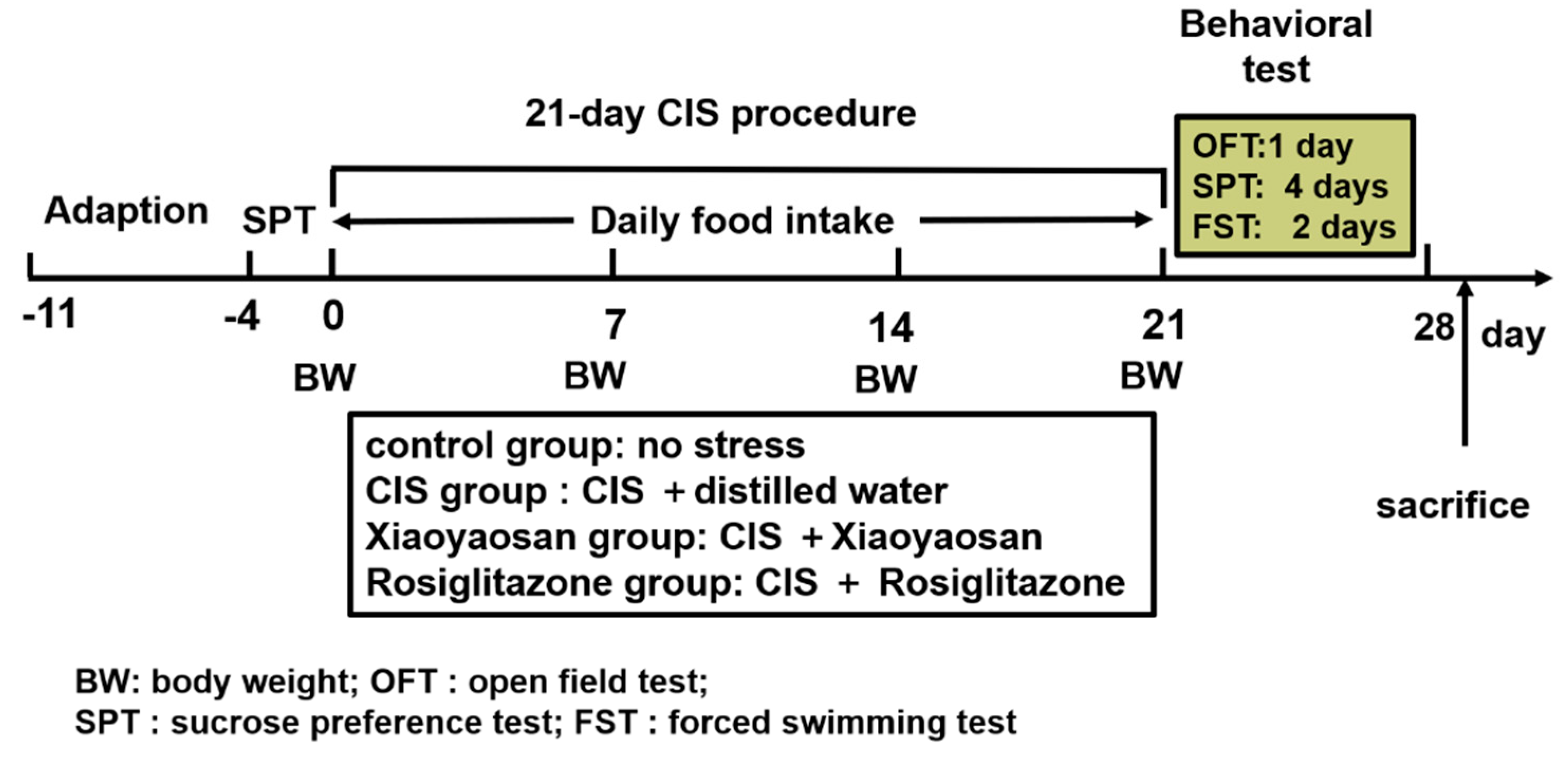
4. Materials and Methods
4.1. Animals
4.2. Chronic Immobilization Stress (CIS) Procedure
4.3. Drugs and Drug Administration
4.4. Body Weight and Food Intake
4.5. Behavioural Testing
4.6. Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.8. Liver Tissue Immunohistochemical Staining
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tamashiro, K.L.; Sakai, R.R.; Shively, C.A.; Karatsoreos, I.N.; Reagan, L.P. Chronic stress, metabolism, and metabolic syndrome. Stress Int. J. Biol. Stress 2011, 14, 468–474. [Google Scholar] [CrossRef]
- Zeugmann, S.; Quante, A.; Popovazeugmann, L.; Kössler, W.; Heuser, I.; Anghelescu, I. Pathways linking early life stress, metabolic syndrome, and the inflammatory marker fibrinogen in depressed inpatients. Psychiatr. Danub. 2012, 24, 57. [Google Scholar] [PubMed]
- Trombini, M.; Hulshof, H.J.; Graiani, G.; Carnevali, L.; Meerlo, P.; Quaini, F.; Sgoifo, A. Early maternal separation has mild effects on cardiac autonomic balance and heart structure in adult male rats. Stress Int. J. Biol. Stress 2012, 15, 457–470. [Google Scholar] [CrossRef]
- Sadeghimahalli, F.; Karbaschi, R.; Zardooz, H.; Khodagholi, F.; Rostamkhani, F. Effect of early life stress on pancreatic isolated islets’ insulin secretion in young adult male rats subjected to chronic stress. Endocrine 2015, 48, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.Y.; Kim, K.S.; Yeom, Y.I.; Lee, J.K.; Han, P.L. Chronic restraint stress massively alters the expression of genes important for lipid metabolism and detoxification in liver. Toxicol. Lett. 2003, 146, 49–63. [Google Scholar] [CrossRef]
- Haque, Z.; Akbar, N.; Yasmin, F.; Haleem, M.A.; Haleem, D.J. Inhibition of immobilization stress-induced anorexia, behavioral deficits, and plasma corticosterone secretion by injected leptin in rats. Stress Int. J. Biol. Stress 2013, 16, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Zareian, P.; Karimi, M.V.; Dorneyani, G. The comparison of the effects of acute swimming stress on plasma corticosterone and leptin concentration in male and female rats. Acta Med. Iran. 2011, 49, 284–287. [Google Scholar] [PubMed]
- Norman, K.J.; Seiden, J.A.; Klickstein, J.A.; Han, X.; Hwa, L.S.; Debold, J.F.; Miczek, K.A. Social stress and escalated drug self-administration in mice I. Alcohol and corticosterone. Psychopharmacology 2015, 232, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Radahmadi, M.; Alaei, H.; Sharifi, M.R.; Hosseini, N. Effects of different timing of stress on corticosterone, BDNF and memory in male rats. Physiol. Behav. 2015, 139, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Ghalami, J.; Zardooz, H.; Rostamkhani, F.; Farrokhi, B.; Hedayati, M. High-fat diet did not change metabolic response to acute stress in rats. EXCLI J. 2011, 10, 205–217. [Google Scholar] [PubMed]
- Jia, H.M.; Qi, L.; Chao, Z.; Meng, Y.; Yong, Y.; Zhang, H.W.; Gang, D.; Hai, S.; Zou, Z.M. Chronic unpredictive mild stress leads to altered hepatic metabolic profile and gene expression. Sci. Rep. 2016, 6, 23441. [Google Scholar] [CrossRef] [PubMed]
- Elsharkawy, E.E.; Yahia, D.; El-Nisr, N.A. Sub-chronic exposure to chlorpyrifos induces hematological, metabolic disorders and oxidative stress in rat: Attenuation by glutathione. Environ. Toxicol. Pharmacol. 2013, 35, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.L.; Luo, Z.; Pan, Y.X.; Zheng, J.L.; Zhu, Q.L.; Sun, L.D.; Zhuo, M.Q.; Hu, W. Differential induction of enzymes and genes involved in lipid metabolism in liver and visceral adipose tissue of juvenile yellow catfish Pelteobagrus fulvidraco exposed to copper. Aquat. Toxicol. 2013, 136–137, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Liem, M.; Ang, C.S.; Mathivanan, S. Insulin mediated activation of PI3K/Akt signalling pathway modifies the proteomic cargo of extracellular vesicles. Proteomics 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, P.; Stephens, L. PI3K signalling in inflammation. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2015, 1851, 882–897. [Google Scholar] [CrossRef]
- Okkenhaug, K. Signaling by the Phosphoinositide 3-Kinase Family in Immune Cells. Annu. Rev. Immunol. 2013, 31, 675–704. [Google Scholar] [CrossRef] [PubMed]
- Steve, J.; Kiger, A.A. Classes of phosphoinositide 3-kinases at a glance. J. Cell Sci. 2014, 127, 923–928. [Google Scholar]
- Cesar, O.F.; Amira, K. Dissecting signalling by individual Akt/PKB isoforms, three steps at once. Biochem. J. 2015, 470, 13–16. [Google Scholar]
- Dummler, B.; Hemmings, B.A. Physiological roles of PKB/Akt isoforms in development and disease. Biochem. Soc. Trans. 2007, 35, 231–235. [Google Scholar] [CrossRef]
- Schultze, S.M.; Jensen, J.; Hemmings, B.A.; Tschopp, O.; Niessen, M. Promiscuous affairs of PKB/AKT isoforms in metabolism. Arch. Physiol. Biochem. 2011, 117, 70–77. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Z.; Rasschaert, J.; Blero, D.L.; Schurmans, S.; Erneux, C.; Pesesse, X. SHIP2 controls PtdIns(3,4,5)P-3 levels and PKB activity in response to oxidative stress. Cell Signal. 2007, 19, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Lazar, D.F.; Saltiel, A.R. Lipid phosphatases as drug discovery targets for type 2 diabetes. Nat. Rev. Drug Discov. 2006, 5, 333–342. [Google Scholar] [CrossRef]
- Watt, N.T.; Gage, M.C.; Patel, P.A.; Viswambharan, H.; Sukumar, P.; Galloway, S.; Yuldasheva, N.Y.; Imrie, H.; Amn, W.; Griffin, K.J. Endothelial SHIP2 Suppresses Nox2 NADPH Oxidase-Dependent Vascular Oxidative Stress, Endothelial Dysfunction, and Systemic Insulin Resistance. Diabetes 2017, 66, 2808–2821. [Google Scholar] [CrossRef] [PubMed]
- Sleeman, M.W.; Wortley, K.E.; Lai, K.M.V.; Gowen, L.C.; Kintner, J.; Kline, W.O.; Garcia, K.; Stitt, T.N.; Yancopoulos, G.D.; Wiegand, S.J.; et al. Absence of the lipid phosphatase SHIP2 confers resistance to dietary obesity. Nat. Med. 2005, 11, 199–205. [Google Scholar] [CrossRef]
- Clément, S.; Krause, U.; Desmedt, F.; Tanti, J.F.; Behrends, J.; Pesesse, X.; Sasaki, T.; Penninger, J.; Doherty, M.; Malaisse, W.; et al. The lipid phosphatase SHIP2 controls insulin sensitivity. Nature 2001, 409, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Cheng-Lai, A.; Levine, A. Rosiglitazone: An agent from the thiazolidinedione class for the treatment of type 2 diabetes. Heart Dis. 2000, 2, 326–333. [Google Scholar]
- Raskin, P.; Rappaport, E.B.; Cole, S.T.; Yan, Y.; Patwardhan, R.; Freed, M.I. Rosiglitazone short-term monotherapy lowers fasting and post-prandial glucose in patients with Type II diabetes. Diabetologia 2000, 43, 278–284. [Google Scholar] [CrossRef]
- Phillips, L.S.; Grunberger, G.; Miller, E.; Patwardhan, R.; Rappaport, E.B.; Salzman, A. Once- and Twice-Daily Dosing With Rosiglitazone Improves Glycemic Control in Patients With Type 2 Diabetes. Diabetes Care 2001, 24, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Min-Xiang, L.; Xiao-Yun, X.; Lan, L.; Yan-Mei, S.; Juan, M.; Shan, W. Rosiglitazone inhibits high glucose-induced apoptosis in human umbilical vein endothelial cells through the PI3K/Akt/eNOS pathway. Can. J. Physiol. Pharm. 2009, 87, 549–555. [Google Scholar]
- Meng, Z.; Li, P.; Qiao, Z.; Hongtao, H.E.; Ye, Z.; Zhuo, X.U. Rosiglitazone inhibits human HepG2 cell proliferation via PI3K/PTEN/Akt signaling pathway. Chin. J. Cell. Mol. Immunol. 2014, 30, 147–150. [Google Scholar]
- Tiziana, S.; Alessandra, T.; Vincenzo, U.; Ilias, S.; Filippo, D.; Claudio, B.; Salvatore, S. Reversible inhibition of vasoconstriction by thiazolidinediones related to PI3K/Akt inhibition in vascular smooth muscle cells. Biochem. Pharmacol. 2013, 85, 551–559. [Google Scholar]
- Damme, M.; Suntio, T.; Saftig, P.; Eskelinen, E.L. Autophagy in neuronal cells: General principles and physiological and pathological functions. Acta Neuropathol. 2015, 129, 337–362. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, W.X.; Yang, J. Effects of the Chinese traditional prescription Xiaoyaosan decoction on chronic immobilization stress-induced changes in behavior and brain BDNF, TrkB, and NT-3 in rats. Cell. Mol. Neurobiol. 2008, 28, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.F.; Zhao, X.H.; Tao, Y.; Zhong, W.C.; Fan, Q.; Diao, J.X.; Liu, Y.L.; Chen, Y.Y.; Chen, J.X.; Lv, Z.P. Xiao Yao San Improves Depressive-Like Behaviors in Rats with Chronic Immobilization Stress through Modulation of Locus Coeruleus-Norepinephrine System. Evid.-Based Complement. Altern. Med. 2014, 2014, 902516. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Ma, Q.Y.; Jiang, Y.M.; Bai, X.H.; Yan, Z.Y.; Liu, Q.; Pan, Q.X.; Liu, Y.Y.; Chen, J.X. Xiaoyaosan exerts anxiolytic-like effects by down-regulating the TNF-α/JAK2-STAT3 pathway in the rat hippocampus. Sci. Rep. 2017, 7, 353. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Lee, D.H.; Kang, S.S. Effects of chronic restraint stress on body weight, food intake, and hypothalamic gene expressions in mice. Endocrinol. Metab. 2013, 28, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Calvez, J.; Fromentin, G.; Nadkarni, N.; Darcel, N.; Tomé, D.; Ballet, N.; Chaumontet, C. Effect of chronic variable stress on central regulation of food intake and neurogenesis. Appetite 2010, 54, 637. [Google Scholar] [CrossRef]
- Rahmati, R.; Semnani, S.; Sepehry, H.; Veghari, G.; Hoseiny, S.; Mashadi, Z.H.; Mohammadi, R. Effect of peppermint extract on food intake and body weight following immobilization stress in mice. Koomesh 2013, 14, 367–372. [Google Scholar]
- Rabasa, C.; Dickson, S.L. Impact of stress on metabolism and energy balance. Curr. Opin. Behav. Sci. 2016, 9, 71–77. [Google Scholar] [CrossRef]
- Ottenweller, J.E.; Servatius, R.J.; Tapp, W.N.; Drastal, S.D.; Bergen, M.T.; Natelson, B.H. A chronic stress state in rats: Effects of repeated stress on basal corticosterone and behavior. Physiol. Behav. 1992, 51, 689–698. [Google Scholar] [CrossRef]
- Pardon, M.-C.; Gould, G.G.; Garcia, A.; Phillips, L.; Cook, M.C.; Miller, S.A.; Mason, P.A.; Morilak, D.A. Stress reactivity of the brain noradrenergic system in three rat strains differing in their neuroendocrine and behavioral responses to stress: Implications for susceptibility to stress-related neuropsychiatric disorders. Neuroscience 2002, 115, 229–242. [Google Scholar] [CrossRef]
- Strekalova, T.; Spanagel, R.; Dolgov, O.; Bartsch, D. Stress-induced hyperlocomotion as a confounding factor in anxiety and depression models in mice. Behav. Pharmacol. 2005, 16, 171–180. [Google Scholar] [CrossRef]
- Nagasawa, K.; Matsuura, N.; Takeshita, Y.; Ito, S.; Sano, Y.; Yamada, Y.; Uchinaka, A.; Murohara, T.; Nagata, K. Attenuation of cold stress-induced exacerbation of cardiac and adipose tissue pathology and metabolic disorders in a rat model of metabolic syndrome by the glucocorticoid receptor antagonist RU486. Nutr. Diabetes 2016, 6, e207. [Google Scholar] [CrossRef]
- Mathews, E.H.; Liebenberg, L. A practical quantification of blood glucose production due to high-level chronic stress. Stress Health J. Int. Soc. Investig. Stress 2012, 28, 327–332. [Google Scholar] [CrossRef]
- M Loredana, M.; Francesco, C. The effects of acute and chronic stress on diabetes control. Sci. Signal. 2012, 5, pt10. [Google Scholar]
- Flak, J.N.; Ryan, J.; Solomon, M.B.; Krause, E.G.; Herman, J.P. Opposing effects of chronic stress and weight restriction on cardiovascular, neuroendocrine and metabolic function. Physiol. Behav. 2011, 104, 228–234. [Google Scholar] [CrossRef]
- Lin, Y.H.; Liu, A.H.; Xu, Y.; Lu, T.; Yu, H.M.; Li, X.J. Effect of chronic unpredictable mild stress on brain–pancreas relative protein in rat brain and pancreas. Behav. Brain Res. 2005, 165, 63–71. [Google Scholar] [CrossRef]
- Han, B.; Yu, L.; Geng, Y.; Shen, L.; Wang, H.; Wang, Y.; Wang, J.; Wang, M. Chronic Stress Aggravates Cognitive Impairment and Suppresses Insulin Associated Signaling Pathway in APP/PS1 Mice. J. Alzheimers Dis. JAD 2016, 53, 1539–1552. [Google Scholar] [CrossRef]
- Fu, J.H.; Xie, S.R.; Kong, S.J.; Wang, Y.; Wei, W.; Shan, Y.; Luo, Y.M. The combination of a high-fat diet and chronic stress aggravates insulin resistance in Wistar male rats. Exp. Clin. Endocrinol. Diabetes 2009, 117, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Holden, R.J. The role of brain insulin in the neurophysiology of serious mental disorders: Review. Med. Hypotheses 1999, 52, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Jen-Chieh, C.; Huxing, C.; Mason, B.L.; Melissa, M.; Bookout, A.L.; Yu, H.G.; Mario, P.; Elmquist, J.K.; Repa, J.J.; Zigman, J.M. Chronic social defeat stress disrupts regulation of lipid synthesis. J. Lipid Res. 2010, 51, 1344–1353. [Google Scholar]
- Rosmond, R.; Dallman, M.F.; Björntorp, P. Stress-related cortisol secretion in men: Relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J. Clin. Endocrinol. Metab. 1998, 83, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Gallo, L.C.; Roesch, S.C.; Fortmann, A.L.; Carnethon, M.R.; Penedo, F.J.; Perreira, K.; Birnbaumweitzman, O.; Wassertheilsmoller, S.; Talavera, G.A.; Sotresalvarez, D. Associations of chronic stress burden, perceived stress, and traumatic stress with cardiovascular disease prevalence and risk factors in the Hispanic Community Health Study/Study of Latinos Sociocultural Ancillary Study. Psychosom. Med. 2016, 76, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Zhou, H.; Jing, L.; Chen, S.; Liu, Y. The model of rat lipid metabolism disorder induced by chronic stress accompanying high-fat-diet. Lipids Health Dis. 2011, 10, 153. [Google Scholar]
- Gao, S.; Han, X.; Fu, J.; Yuan, X.; Sun, X.; Li, Q. Influence of chronic stress on the compositions of hepatic cholesterol and triglyceride in male Wistar rats fed a high fat diet. Hepatol. Res. 2012, 42, 686–695. [Google Scholar] [CrossRef]
- Ricart-Jané, D.; Rodríguez-Sureda, V.; Benavides, A.; Peinado-Onsurbe, J.; López-Tejero, M.D.; Llobera, M. Immobilization stress alters intermediate metabolism and circulating lipoproteins in the rat. Metab. Clin. Exp. 2002, 51, 925–931. [Google Scholar] [CrossRef]
- Macht, M. How emotions affect eating: A five-way model. Appetite 2008, 50, 1–11. [Google Scholar] [CrossRef]
- Awad, A.; Gassama-Diagne, A. PI3K/SHIP2/PTEN pathway in cell polarity and hepatitis C virus pathogenesis. World J. Hepatol. 2017, 9, 18–29. [Google Scholar] [CrossRef]
- Garofalo, N.A.; Neto, F.J.T.; Pereira, C.D.N.; Pignaton, W.; Vicente, F.; Alvaides, R.K. Cardiorespiratory and neuroendocrine changes induced by methadone in conscious and in isoflurane anaesthetised dogs. Vet. J. 2012, 194, 398–404. [Google Scholar] [CrossRef]
- Syota, K.; Yoshiyuki, S.; Hajime, I.; Takeshi, O.; Masakiyo, S.; Saori, Y.; Ryo, O.; Tsutomu, W.; Hiroshi, T.; Toshiyasu, S. Impact of transgenic overexpression of SH2-containing inositol 5’-phosphatase 2 on glucose metabolism and insulin signaling in mice. Endocrinology 2008, 149, 642–650. [Google Scholar]
- Yu, X.; Shen, N.; Zhang, M.L.; Pan, F.Y.; Wang, C.; Jia, W.P.; Liu, C.; Gao, Q.; Gao, X.; Xue, B.; et al. Egr-1 decreases adipocyte insulin sensitivity by tilting PI3K/Akt and MAPK signal balance in mice. EMBO J. 2014, 30, 3754–3765. [Google Scholar] [CrossRef]
- Kazuhito, F.; Tsutomu, W.; Syota, K.; Kiyofumi, N.; Mariko, I.; Hajime, I.; Masashi, K.; Toshiyasu, S. Impact of the liver-specific expression of SHIP2 (SH2-containing inositol 5’-phosphatase 2) on insulin signaling and glucose metabolism in mice. Diabetes 2005, 54, 1958–1967. [Google Scholar]
- National Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China 2015 Edition; China Medicine Science Press: Beijing, China, 2015; p. 1356. [Google Scholar]
- Ding, X.F.; Li, Y.H.; Chen, J.X.; Sun, L.J.; Jiao, H.Y.; Wang, X.X.; Zhou, Y. Involvement of the glutamate/glutamine cycle and glutamate transporter GLT-1 in antidepressant-like effects of Xiao Yao san on chronically stressed mice. BMC Complement. Altern. Med. 2017, 17, 326. [Google Scholar] [CrossRef]
- Teegarden, S. Behavioral Phenotyping in Rats and Mice. Mater. Method 2012, 2. [Google Scholar] [CrossRef]
- Slattery, D.A.; Cryan, J.F. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat. Protoc. 2012, 7, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Belovicova, K.; Bogi, E.; Csatlosova, K.; Dubovicky, M. Animal tests for anxiety-like and depression-like behavior in rats. Interdiscip. Toxicol. 2017, 10, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.H.; Chen, W.C.; Lu, K.H.; Chen, P.J.; Hsieh, S.C.; Pan, T.M.; Chen, S.T.; Sheen, L.Y. Down-regulation of Slit-Robo pathway mediating neuronal cytoskeletal remodeling processes facilitates the antidepressive-like activity of Gastrodia elata Blume. J. Agric. Food Chem. 2014, 62, 10493–10503. [Google Scholar] [CrossRef]
Sample Availability: Samples of the Xiaoyaosan are available from the authors. |

| Group | CHOL (mmol/L) | HDL-C (mmol/L) | LDL-C (mmol/L) | TG (mmol/L) |
|---|---|---|---|---|
| Control group | 3.5510.508 | 1.6420.146 | 1.0920.085 | 0.5520.121 |
| CIS group | 2.2430.443 ** | 1.1150.053 ** | 0.6520.160 ** | 0.3520.065 ** |
| Xiaoyaosan group | 2.7550.410 ## | 1.3220.214 # | 0.5080.100 # | 0.3950.056 |
| Rosiglitazone group | 2.7100.466 # | 1.1900.186 | 0.6770.116 | 0.4130.076 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Q.; Wu, J.; Liu, Y.; Li, X.; Chen, J. Involvement of Hepatic SHIP2 and PI3K/Akt Signalling in the Regulation of Plasma Insulin by Xiaoyaosan in Chronic Immobilization-Stressed Rats. Molecules 2019, 24, 480. https://doi.org/10.3390/molecules24030480
Pan Q, Wu J, Liu Y, Li X, Chen J. Involvement of Hepatic SHIP2 and PI3K/Akt Signalling in the Regulation of Plasma Insulin by Xiaoyaosan in Chronic Immobilization-Stressed Rats. Molecules. 2019; 24(3):480. https://doi.org/10.3390/molecules24030480
Chicago/Turabian StylePan, Qiuxia, Jiajia Wu, Yueyun Liu, Xiaojuan Li, and Jiaxu Chen. 2019. "Involvement of Hepatic SHIP2 and PI3K/Akt Signalling in the Regulation of Plasma Insulin by Xiaoyaosan in Chronic Immobilization-Stressed Rats" Molecules 24, no. 3: 480. https://doi.org/10.3390/molecules24030480
APA StylePan, Q., Wu, J., Liu, Y., Li, X., & Chen, J. (2019). Involvement of Hepatic SHIP2 and PI3K/Akt Signalling in the Regulation of Plasma Insulin by Xiaoyaosan in Chronic Immobilization-Stressed Rats. Molecules, 24(3), 480. https://doi.org/10.3390/molecules24030480




