Synthesis and Biological Evaluation of Some Novel Thiazole-Based Heterocycles as Potential Anticancer and Antimicrobial Agents
Abstract
1. Introduction
2. Results and Discussion
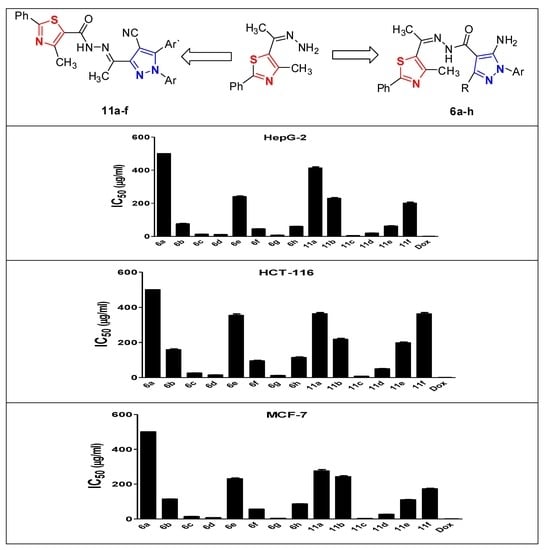
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. Cytotoxic Activity
- (1)
- Attachment of chlorine (6d) or methoxy group (6c) at position 4 in the aryl moiety of the pyrazole ring is important for cytotoxic activity with chlorine having the higher impact in compound (6d).
- (2)
- Addition of another chlorine atom in position 2 in the aryl moiety of compound (6g) increases the activity which reaches the double against MCF-7 cells.
- (1)
- Substitution on only one of the aryl moieties of the pyrazole ring in compounds (11c,d) induces cytotoxic activity, most prominently by chlorine in compound (11c).
- (2)
- Substitution on the second aryl moiety of the pyrazole ring by methyl group as in compounds (11b,f) induces great reduction (nearly abolishes) the cytotoxic activity.
2.2.2. Evaluation of the Antimicrobial Activity
2.2.3. In Vitro Hepatoprotective Activity
3. Materials and Methods
3.1. Chemistry
General Information
3.2. Biological Assays
3.2.1. In Vitro Cytotoxic Activity
3.2.2. Antimicrobial Evaluation
3.2.3. Hepatoprotective Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Satoh, A.; Nagatomi, Y.; Hirata, Y.; Ito, S.; Suzuki, G.; Kimura, T.; Maehara, S.; Hikichi, H.; Satow, A.; Hata, M.; et al. Discovery and in vitro and in vivo profiles of 4-fluoro-N-[4-[6-(isopropylamino)pyrimidin-4-yl]-1,3-thiazol-2-yl]-N-methylbenzamide as novel class of an orally active metabotropic glutamate receptor 1 (mGluR1) antagonist. Bioorg. Med. Chem. Lett. 2009, 19, 5464–5468. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.; Ahsan, W. Synthesis, anticonvulsant and toxicity screening of thiazolyl–thiadiazole derivatives. Med. Chem. Res. 2011, 20, 261–268. [Google Scholar] [CrossRef]
- Dawane, B.S.; Konda, S.G.; Mandawad, G.G.; Shaikh, B.M. Poly(ethylene glycol) (PEG-400) as an alternative reaction solvent for the synthesis of some new 1-(4-(4′-chlorophenyl)-2-thiazolyl)-3-aryl-5-(2-butyl-4-chloro-1H-imidazol-5yl)-2-pyrazolines and their in vitro antimicrobial evaluation. Eur. J. Med. Chem. 2010, 45, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Adibpour, N.; Khalaj, A.; Rajabalian, S. Synthesis and antibacterial activity of isothiazolyloxazolidinones and analogous 3(2H)-isothiazolones. Eur. J. Med. Chem. 2010, 45, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Sondhi, S.M.; Singh, N.; Lahoti, A.M.; Bajaj, K.; Kumar, A.; Lozach, O.; Meijer, L. Synthesis of acridinyl-thiazolino derivatives and their evaluation for anti-inflammatory, analgesic and kinase inhibition activities. Bioorg. Med. Chem. 2005, 13, 4291–4299. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Khalil, K.D. A convenient ultrasound-promoted synthesis and cytotoxic activity of some new thiazole derivatives bearing a coumarin nucleus. Molecules 2012, 17, 9335–9347. [Google Scholar] [CrossRef]
- Luzina, E.L.; Popov, A.V. Synthesis and anticancer activity of N-bis(trifluoromethyl)alkyl-N′-thiazolyl and N-bis(trifluoromethyl)alkyl-N’-benzothiazolylureas. Eur. J. Med. Chem. 2009, 44, 4944–4953. [Google Scholar] [CrossRef]
- Iino, T.; Hashimoto, N.; Sasaki, K.; Ohyama, S.; Yoshimoto, R.; Hosaka, H.; Hasegawa, T.; Chiba, M.; Nagata, Y.; Nishimura, J.E.T. Structure activity relationships of 3,5-disubstituted benzamides as glucokinase activators with potent in vivo efficacy. Bioorg. Med. Chem. 2009, 17, 3800–3809. [Google Scholar] [CrossRef]
- Rawal, R.K.; Tripathi, R.; Katti, S.B.; Pannecouque, C.; Clercq, E.D. Design and synthesis of 2-(2,6-dibromophenyl)-3-heteroaryl-1,3-thiazolidin-4-ones as anti-HIV agents. Eur. J. Med. Chem. 2008, 43, 2800–2806. [Google Scholar] [CrossRef]
- Shiradkar, M.R.; Akula, K.C.; Dasari, V.; Baru, V.; Chiningiri, B.; Gandhi, S.; Kaur, R. Clubbed thiazoles by MAOS: A novel approach to cyclin-dependent kinase 5/p25 inhibitors as a potential treatment for Alzheimer’s disease. Bioorg. Med. Chem. 2007, 15, 2601–2610. [Google Scholar] [CrossRef]
- Gomha, S.M.; Edrees, M.M.; Altalbawy, F.M.A. Synthesis and characterization of some new bis-pyrazolyl-thiazoles incorporating the thiophene moiety as potent anti-tumor agents. Inter. J. Mol. Sci. 2016, 17, 1499. [Google Scholar] [CrossRef] [PubMed]
- Turan-Zitouni, G.; Chevallet, P.; Kiliç, F.S.; Erol, K. Synthesis of some thiazolyl-pyrazoline derivatives and preliminary investigation of their hypotensive activity. Eur. J. Med. Chem. 2000, 35, 635–641. [Google Scholar] [CrossRef]
- Shih, M.H.; Ke, F.Y. Syntheses and evaluation of antioxidant activity of sydnonyl substituted thiazolidinone and thiazoline derivatives. Bioorg. Med. Chem. 2004, 12, 4633–4643. [Google Scholar] [CrossRef]
- Kheder, N.A.; Riyadh, S.M.; Asiry, A.M. Azoles and bis-Azoles: Synthesis and Biological Evaluation as Antimicrobial and Anti-Cancer Agents. Chem. Pharm. Bull. 2013, 61, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Salah, T.A.; Abdelhamid, A.O. Synthesis, characterization and pharmacological evaluation of some novel thiadiazoles and thiazoles incorporating pyrazole moiety as potent anticancer agents. Monatsh. Chem. 2015, 146, 149–158. [Google Scholar] [CrossRef]
- Chen, Z.Z.; Wang, Z.L.; Deng, C.Y.; Zheng, H.; Wang, X.H.; Ma, L.; Ye, X.; Ma, Y.H.; Xie, C.F.; Chen, L.J.; et al. (Z)-5-(4-methoxybenzylidene) thiazolidine-2,4-dione protects rats from carbon tetrachloride-induced liver injury and fibrogenesis. World J. Gastroenterol. 2012, 18, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, E.; Zamora, P.; Feliu, J.; González Barón, M. Classification of anticancer drugs—A new system based on therapeutic. Cancer Treat Rev. 2003, 29, 515–523. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Marcellin, P.; Kutala, B.K. Liver diseases: A major, neglected global public health problem requiring urgent actions and large-scale screening. Liver Int. 2018, 38, 2–6. [Google Scholar] [CrossRef]
- Gomha, S.M.; Kheder, N.A.; Abdelaziz, M.R.; Mabkhot, Y.N.; Alhajoj, A.M. A facile synthesis and anticancer activity of some novel thiazoles carrying 1,3,4-thiadiazole moiety. Chem. Central J. 2017, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Riyadh, S.M.; Abbas, I.M.; Bauomi, M.A. Synthetic utility of ethylidenethiosemi- carbazide: Synthesis and anti-cancer activity of 1,3-thiazines and thiazoles with imidazole moiety. Heterocycles 2013, 87, 341–356. [Google Scholar]
- Gomha, S.M.; Riyadh, S.M.; Abdalla, M.M. Solvent-drop grinding method: Efficient synthesis, DPPH radical scavenging and anti-diabetic activities of chalcones, bis-chalcones, azolines, and bis-azolines. Curr. Org. Synth. 2015, 12, 220–228. [Google Scholar] [CrossRef]
- Gomha, S.M.; Abdelaziz, M.R.; Kheder, N.A.; Abdel-aziz, H.M.; Alterary, S.; Mabkhot, Y.N. A facile access and evaluation of some novel thiazole and 1,3,4-thiadiazole derivatives incorporating thiazole moiety as potent anticancer agents. Chem. Central J. 2017, 11, 105. [Google Scholar] [CrossRef]
- Gomha, S.M.; Salah, T.A.; Hassaneen, H.M.E.; Abdel-aziz, H.; Khedr, M.A. Synthesis, characterization and molecular docking of novel bioactive thiazolyl-thiazole derivatives as promising cytotoxic antitumor drug. Molecules 2016, 21, 3. [Google Scholar] [CrossRef]
- Gomha, S.M.; Eldebss, T.M.A.; Badrey, M.G.; Abdulla, M.M.; Mayhoub, A.S. Novel 4-heteroaryl-antipyrines as DPP-IV Inhibitors. Chem. Biol. Drug Des. 2015, 86, 1292–1303. [Google Scholar] [CrossRef] [PubMed]
- Tazoo, D.; Oniga, O.; Bohle, D.S.; Chua, Z.; Dongo, E. General two-step preparation of chalcones containing thiazole. J. Heterocycl. Chem. 2012, 49, 768–773. [Google Scholar] [CrossRef]
- Gorolets, N.Y.; Yousefi, B.H.; Belaj, F.; Kappe, C.O. Rapid microwave-assisted solution phase synthesis of substituted 2-pyridone libraries. Tetrahedron 2004, 60, 8633–8644. [Google Scholar] [CrossRef]
- Dieckmann, W.; Platz, L. Ueber eine neue bildungsweise von osotetrazonen. Ber. Dtsch. Chem. Ges. 1905, 38, 2986–2990. [Google Scholar] [CrossRef]
- Abushamleh, A.S.; Al-Aqarbeh, M.M.; Day, V. Transition metal complexes of derivatized chiral dihydro- 1,2,4-triazin-6-ones. Template synthesis of nickel (II) tetraaza-(4N-M) complexes incorporating the triazinone moiety. Am. J. Appl. Sci. 2008, 5, 750–754. [Google Scholar] [CrossRef]
- Eweiss, N.F.; Abdelhamid, A.O. Synthesis of heterocycles. Part II. New routes to acetyl thiadiazolines and alkylazothiazoles. J. Heterocycl. Chem. 1980, 17, 1713–1717. [Google Scholar] [CrossRef]
- Shawali, A.S.; Eweiss, N.F.; Hassaneen, H.M.; Al-gharib, M.S. Synthesis and rearrangement of ethyl aryloxyglyoxalate arylhydrazones. Bull. Chem. Soc. Jpn. 1975, 48, 365–366. [Google Scholar] [CrossRef]
- Shawali, A.S.; Abdelhamid, A.O. Synthesis and reactions of phenylcarbamoylarylhydrazidic chlorides. Tetrahedron 1971, 27, 2517–2528. [Google Scholar] [CrossRef]
- Rao, P.S.; Venkataratnam, R.V. Zinc Chloride as a New Catalyst for Knoevenagel Condensation. Tetrahedron Lett. 1991, 32, 5821–5822. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement; CLSI Document M100-S22; CLSI: Wayne, PA, USA, 2012. [Google Scholar]
- Ghorab, M.M.; Alsaid, M.S.; El-Gaby, M.S.A.; Safwat, N.A.; Elaasser, M.M.; Soliman, A.M. Biological evaluation of some new N-(2,6-dimethoxypyrimidinyl) thioureido benzenesulfonamide derivatives as potential antimicrobial and anticancer agents. Eur. J. Med. Chem. 2016, 124, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Thirunavukkarasu, P.; Asha, S.; Ramanathan, T.; Balasubramanian, T.; Shanmogapriya, R.; Renugadevi, G. In vitro Hepatoprotective activity of isolated fractions of Cressa cretica. Pharm. Chem. J. 2014, 48, 121–126. [Google Scholar] [CrossRef]
- Reese, J.A.; Byard, J.L. Isolation and culture of adult hepatocytes from liver biopsies. In Vitro 1981, 17, 935–940. [Google Scholar] [CrossRef]
- Wilson, A.P. Cytotoxicity and viability assays. In Animal Cell Culture: A Practical Approach, 3rd ed.; Oxford University Press: Oxford, UK, 2000; pp. 175–219. [Google Scholar]
Sample Availability: Samples of the compounds 6a–h and 11a–f are available from the authors. |





| No. | Time (min) | Yield % | |
|---|---|---|---|
| TEA | g-Chitosan | ||
| 6a | 4 | 67 | 80 |
| 6b | 6 | 69 | 82 |
| 6c | 9 | 68 | 84 |
| 6d | 5 | 73 | 85 |
| 6e | 10 | 73 | 84 |
| 6f | 8 | 68 | 83 |
| 6g | 7 | 76 | 88 |
| 6h | 7 | 73 | 81 |
| No. | Time (min) | Yield % | |
|---|---|---|---|
| Piperidine | g-Chitosan | ||
| 11a | 5 | 69 | 83 |
| 11b | 7 | 71 | 81 |
| 11c | 5 | 74 | 86 |
| 11d | 8 | 72 | 81 |
| 11e | 3 | 71 | 84 |
| 11f | 7 | 73 | 85 |
| Tested Compounds | IC50 (μg/mL) | ||
|---|---|---|---|
| HepG-2 | HCT-116 | MCF-7 | |
| 6a | >500 | >500 | >500 |
| 6b | 75.5 ± 2.7 | 159 ± 4.7 | 114 ± 1.2 |
| 6c | 13.1 ± 0.4 | 25.4 ± 1.3 | 13.9 ± 0.9 |
| 6d | 11.4 ± 0.2 | 14.8 ± 0.6 | 7.36 ± 0.4 |
| 6e | 240 ± 4.3 | 354 ± 8.9 | 231 ± 4.5 |
| 6f | 44.8 ± 1.3 | 95 ± 3.8 | 56.1 ± 0.7 |
| 6g | 7.4 ± 0.2 | 11.8 ± 0.5 | 3.77 ± 0.2 |
| 6h | 60 ± 1.1 | 114 ± 4.1 | 86.2 ± 1.1 |
| 11a | 413 ± 6.9 | 364 ± 6.9 | 276 ± 7.8 |
| 11b | 230 ± 4.6 | 218 ± 5.3 | 243 ± 4.9 |
| 11c | 4.24 ± 0.3 | 7.35 ± 0.4 | 2.99 ± 0.2 |
| 11d | 19.3 ± 0.8 | 49.6 ± 1.7 | 26.8 ± 0.8 |
| 11e | 62.1 ± 2.6 | 198 ± 4.2 | 110 ± 1.9 |
| 11f | 201 ± 5.9 | 363 ± 7.8 | 173 ± 3.5 |
| Doxorubicin | 0.36 ± 0.04 | 0.49 ± 0.07 | 0.35 ± 0.03 |
| Sample | Microorganisms | |||||
|---|---|---|---|---|---|---|
| Fungi | Gram Positive Bacteria | Gram Negative Bacteria | ||||
| AF | CA | SA | BS | EC | PV | |
| 6a | NA | NA | 12 ± 0.6 | 11 ± 0.5 | 10 ± 0.3 | NA |
| 6b | NA | NA | 13 ± 0.8 | 16 ± 0.7 | 12 ± 0.7 | NA |
| 6c | NA | NA | 14 ± 0.6 | 15 ± 0.4 | 14 ± 0.4 | NA |
| 6d | NA | NA | 12 ± 0.7 | 16 ± 0.9 | 13 ± 0.6 | NA |
| 6e | NA | NA | 11 ± 0.4 | 17 ± 0.8 | 12 ± 0.8 | NA |
| 6f | NA | NA | 20 ± 0.9 | 22 ± 1.3 | 17 ± 0.5 | 12 ± 0.9 |
| 6g | NA | NA | 14 ± 0.6 | 16 ± 0.4 | 13 ± 0.7 | NA |
| 6h | NA | NA | 12 ± 0.8 | 11 ± 0.6 | 16 ± 0.5 | 15 ± 0.7 |
| 11a | NA | NA | 10 ± 0.7 | 12 ± 0.8 | 11 ± 0.4 | 10 ± 0.3 |
| 11b | NA | NA | NA | 13 ± 0.5 | 9 ± 0.2 | 11 ± 0.4 |
| 11c | NA | NA | 16 ± 0.4 | 12 ± 0.7 | 15 ± 0.9 | 13 ± 0.5 |
| 11d | NA | NA | 14 ± 0.7 | 12 ± 0.4 | 13 ± 0.6 | 14 ± 0.7 |
| 11e | NA | NA | 15 ± 0.9 | 11 ± 0.6 | 12 ± 0.7 | 10 ± 0.2 |
| 11f | NA | NA | 9 ± 0.4 | NA | 10 ± 0.3 | NA |
| Ketoconazole | 17 ± 0.4 | 20 ± 0.8 | - | - | - | - |
| Gentamycin | - | - | 24 ± 1.2 | 26 ± 0.7 | 30 ± 0.9 | 25 ± 0.8 |
| Sample | Microorganisms | |||||
|---|---|---|---|---|---|---|
| Fungi | Gram Positive Bacteria | Gram Negative Bacteria | ||||
| AF | CA | SA | BS | EC | PV | |
| 6a | NA | NA | 625 | 5000 | 5000 | NA |
| 6b | NA | NA | 2500 | 312.5 | 625 | NA |
| 6c | NA | NA | 312.5 | 1250 | 625 | NA |
| 6d | NA | NA | 156.25 | 625 | 312.5 | NA |
| 6e | NA | NA | 5000 | 625 | 1250 | NA |
| 6f | NA | NA | 78.13 | 396 | 156.25 | 315 |
| 6g | NA | NA | 312.5 | 78.13 | 625 | NA |
| 6h | NA | NA | 1250 | 5000 | 156.25 | 312.5 |
| 11a | NA | NA | 5000 | 2500 | 2500 | 5000 |
| 11b | NA | NA | NA | 1250 | 10,000 | 5000 |
| 11c | NA | NA | 312.5 | 1250 | 625 | 1250 |
| 11d | NA | NA | 625 | 1250 | 1250 | 312.5 |
| 11e | NA | NA | 625 | 2500 | 2500 | 5000 |
| 11f | NA | NA | 10,000 | NA | 5000 | NA |
| Tested Compounds | Hepatoprotective Activity (EC50 µg/mL) |
|---|---|
| 6a | NA |
| 6b | NA |
| 6c | 368 ± 14.6 |
| 6d | 972 ± 96.2 |
| 6e | NA |
| 6f | 1350 ± 87 |
| 6g | 456 ± 32 |
| 6h | 1324 ± 64.6 |
| 11a | NA |
| 11b | NA |
| 11c | 724 ± 31.7 |
| 11d | 936 ± 64 |
| 11e | 1980 ± 213 |
| 11f | NA |
| Silymarin | 34.9 ± 0.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Melha, S.; Edrees, M.M.; Salem, H.H.; Kheder, N.A.; Gomha, S.M.; Abdelaziz, M.R. Synthesis and Biological Evaluation of Some Novel Thiazole-Based Heterocycles as Potential Anticancer and Antimicrobial Agents. Molecules 2019, 24, 539. https://doi.org/10.3390/molecules24030539
Abu-Melha S, Edrees MM, Salem HH, Kheder NA, Gomha SM, Abdelaziz MR. Synthesis and Biological Evaluation of Some Novel Thiazole-Based Heterocycles as Potential Anticancer and Antimicrobial Agents. Molecules. 2019; 24(3):539. https://doi.org/10.3390/molecules24030539
Chicago/Turabian StyleAbu-Melha, Sraa, Mastoura M. Edrees, Heba H. Salem, Nabila A. Kheder, Sobhi M. Gomha, and Mohamad R. Abdelaziz. 2019. "Synthesis and Biological Evaluation of Some Novel Thiazole-Based Heterocycles as Potential Anticancer and Antimicrobial Agents" Molecules 24, no. 3: 539. https://doi.org/10.3390/molecules24030539
APA StyleAbu-Melha, S., Edrees, M. M., Salem, H. H., Kheder, N. A., Gomha, S. M., & Abdelaziz, M. R. (2019). Synthesis and Biological Evaluation of Some Novel Thiazole-Based Heterocycles as Potential Anticancer and Antimicrobial Agents. Molecules, 24(3), 539. https://doi.org/10.3390/molecules24030539