Phytochemical Analysis and Potential Biological Activities of Essential Oil from Rice Leaf
Abstract
:1. Introduction
2. Results
2.1. Antioxidant and Xanthine Oxidase Inhibitory (XOI) Activities of Rice Leaf EO
2.2. Growth Inhibitory Activities of Rice Leaf Essential Oil
2.3. Identification of Phytochemicals in Rice Leaves EO
2.4. Identification and Quantification of MA and MB from Rice Leaf EO by UPLC/ESI–MS Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Materials
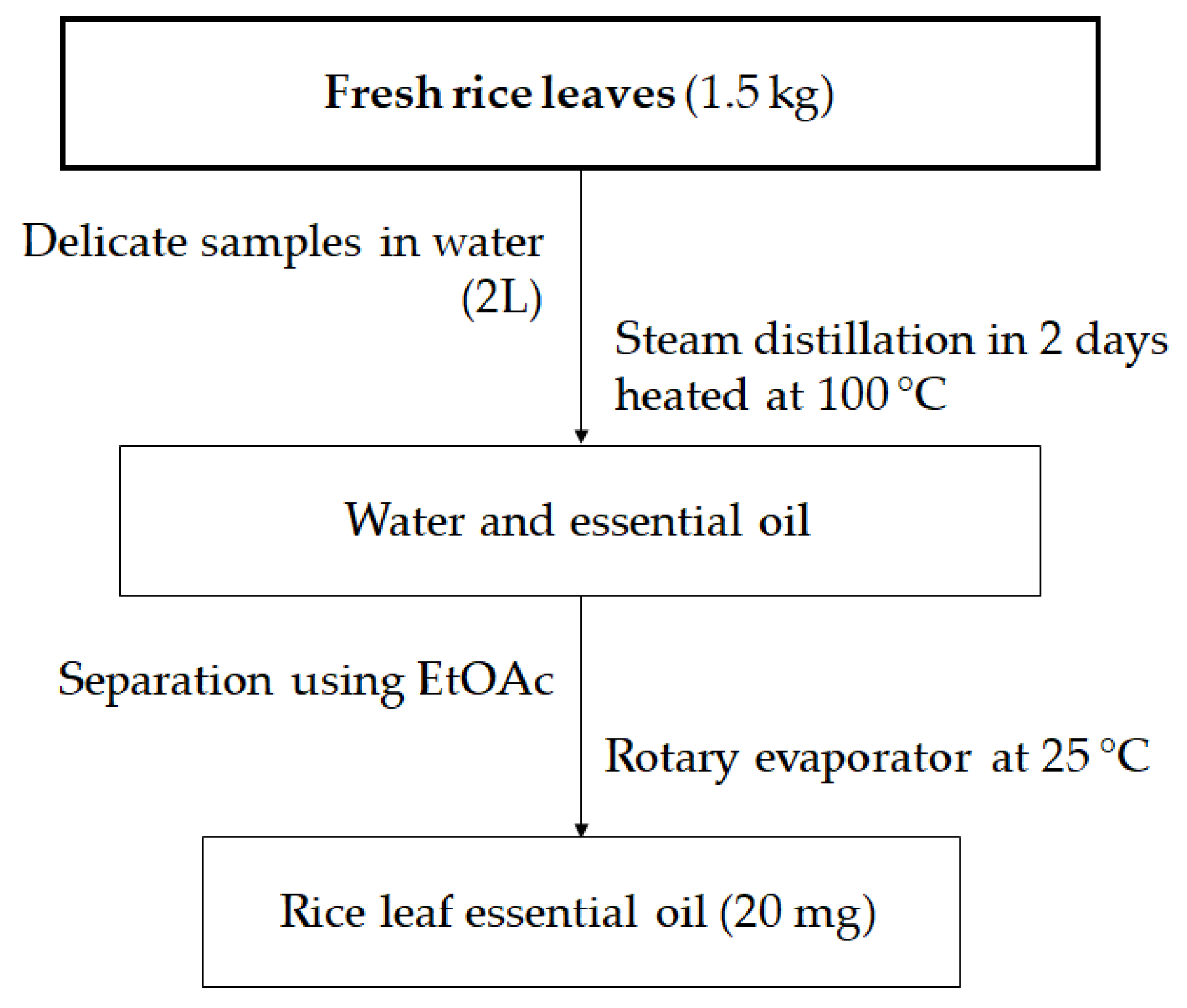
4.2. Preparation of Essential Oil
4.3. Antioxidant Assays
4.3.1. DPPH Radical Scavenging Assay
4.3.2. Reducing Power
4.3.3. ABTS Radical Scavenging Assay
4.3.4. Antioxidant Activity using the β-Carotene Bleaching Test
4.4. Growth Inhibitory Activity Bioassays
4.5. Xanthine Oxidase Inhibition (XOI) Activity
4.6. Identification of Volatile Compounds from Rice Leaf Essential Oil
4.7. Identification and Quantification of Momilactones A and B from EO of Rice Leaf by UPLC/ESI-MS
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Farmer, E.E. Surface-to-air signals. Nature 2001, 411, 854. [Google Scholar] [CrossRef] [PubMed]
- Kask, K.; Kännaste, A.; Niinemets, Ü. Emission of volatile organic compounds as a signal of plant stress. Sci. Bull. ESCORENA 2013, 8, 79–92. [Google Scholar]
- Villamar-Torres, R.; Mehdi, S.; Liuba-Delfini, G.; García, L.; Viot, C.R. Volatile organic compounds: Plant natural defense mechanisms against herbivorous arthropods and an opportunity for plant breeding of cotton. Sci. Agric. 2018, 9, 287–297. [Google Scholar] [CrossRef]
- Djilani, A.; Dicko, A. The therapeutic benefits of essential oils. In Nutrition, well-being and Health; Bouayed, J., Ed.; InTech: Beijing, China, 2012; 224p. [Google Scholar]
- Pickett, J.A.; Khan, Z.R. Plant volatile-mediated signaling and its application in agriculture: Successes and challenges. New Phytol. 2016, 212, 856–870. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.M.; Han, G.; Chung, H.J. A study on the trend analysis regarding the rice consumption of Korean adults using Korean National Health and Nutrition Examination Survey data from 1998, 2001 and 2005. Nutr. Res. Pract. 2012, 6, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Champagne, E.T. Rice aroma and flavor: A literature review. Cereal Chem. 2008, 85, 445–454. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Dang, P.; Zhao, S.; Lai, D.; Zhou, L. Rice secondary metabolites: Structures, roles, biosynthesis, and metabolic regulation. Molecules 2018, 23, 3098. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Ise, K.; Li, C.; Honda, I.; Iwai, Y.; Matsukura, U. Volatile components in stored rice [Oryza sativa (L.)] of varieties with and without lipoxygenase-3 in seeds. J. Agric. Food Chem. 1999, 47, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Fan, W.; Gao, Y.N.; Wu, S.F.; Wang, S.X. Study on volatile compounds in rice by HS-SPME and GC-MS. Julius-Kühn-Archiv. 2010, 425, 125. [Google Scholar]
- Yang, D.S.; Lee, K.S.; Jeong, O.Y.; Kim, K.J.; Kays, S.J. Characterization of volatile aroma compounds in cooked black rice. J. Agric. Food Chem. 2007, 56, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.S.; Shewfelt, R.L.; Lee, K.S.; Kays, S.J. Comparison of odor-active compounds from six distinctly different rice flavor types. J. Agric. Food Chem. 2008, 56, 2780–2787. [Google Scholar] [CrossRef] [PubMed]
- Sukhonthara, S.; Theerakulkait, C.; Miyazawa, M. Characterization of volatile aroma compounds from red and black rice bran. J. Oleo Sci. 2009, 58, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Liyanaarachchi, G.D.; Kottearachchi, N.S.; Samarasekera, R. Volatile profiles of traditional aromatic rice varieties in Sri Lanka. J. Natl. Sci. Found. Sri Lanka 2014, 42, 87–93. [Google Scholar] [CrossRef]
- Yajima, I.; Yanai, T.; Nakamura, M.; Sakakibara, H.; Habu, T. Volatile flavor components of cooked rice. Agric. Biol. Chem. 1978, 42, 1229–1233. [Google Scholar]
- Kongkiattikajorn, J. Effect of storage time and temperature on volatile aroma compounds and physicochemical properties of rice. Kasetsart J. Nat. Sci. 2008, 42, 111–117. [Google Scholar]
- Kim, H.R.; Kim, K.M.; Woo, K.; Jeong, H.S.; Kim, K.O. Changes in volatile compounds of waxy rice and gangjeong (a traditional Korean oil-puffed snack) under different steeping conditions. Food Sci. Biotechnol. 2015, 24, 1565–1572. [Google Scholar] [CrossRef]
- Sirisantimethakom, L.; Laopaiboon, L.; Danvirutai, P.; Laopaiboon, P. Volatile compounds of a traditional Thai rice wine. Biotechnology 2008, 7, 505–513. [Google Scholar]
- Lee, S.M.; Han, H.Y.; Lee, S.J. Volatile compounds in takju (rice wine) using different types of fermentation starters. Food Eng. Prog. 2014, 18, 348–354. [Google Scholar] [CrossRef]
- Minh, T.N.; Xuan, T.D.; Ahmad, A.; Elzaawely, A.A.; Teschke, R.; Van, T.M. Momilactones A and B: Optimization of yields from isolation and purification. Separations 2018, 5, 28. [Google Scholar] [CrossRef]
- Minh, T.N.; Xuan, T.D.; Ahmad, A.; Elzaawely, A.A.; Teschke, R.; Van, T.M. Efficacy from different extractions for chemical profile and biological activities of rice husk. Sustainability 2018, 10, 1356. [Google Scholar] [CrossRef]
- Laosinwattana, C.; Wichittrakarn, P.; Teerarak, M. Chemical composition and herbicidal action of essential oil from Tagetes erecta L. leaves. Ind. Crops Prod. 2018, 126, 129–134. [Google Scholar] [CrossRef]
- Tsasi, G.; Mailis, T.; Daskalaki, A.; Sakadani, E.; Razis, P.; Samaras, Y.; Skaltsa, H. The effect of harvesting on the composition of essential oils from five varieties of Ocimum basilicum L. cultivated in the island of Kefalonia, Greece. Plants 2017, 6, 18. [Google Scholar] [CrossRef]
- Ahmad, A.; Xuan, T.D.; Minh, T.N.; Siddiqui, N.A.; Quan, N.V. Comparative extraction and simple isolation improvement techniques of active constituents’ momilactone A and B from rice husks of Oryza sativa by HPLC analysis and column chromatography. Saudi Pharm. J. 2019, 27, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Sefidkon, F.; Abbasi, K.; Khaniki, G.B. Influence of drying and extraction methods on yield and chemical composition of the essential oil of Satureja hortensis. Food Chem. 2006, 99, 19–23. [Google Scholar] [CrossRef]
- Božović, M.; Navarra, A.; Garzoli, S.; Pepi, F.; Ragno, R. Esential oils extraction: A 24-h steam distillation systematic methodology. Nat. Prod. Res. 2017, 31, 2387–2396. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, J.; Kovačević, D.B.; Putnik, P.; Gabrić, D.; Bilušić, T.; Krešić, G.; Stulić, V.; Barba, F.J.; Chemat, F.; Barbosa-Cánovas, G.; et al. Extraction of bioactive compounds and essential oils from mediterranean herbs by conventional and green innovative techniques: A review. Food Res. Int. 2018, 113, 245–262. [Google Scholar] [CrossRef]
- Amic, D.; Davidovic-Amic, D.; Beslo, D.; Rastija, V.; Lucic, B.; Trinajstic, N. SAR and QSAR of the antioxidant activity of flavonoids. Curr. Med. Chem. 2007, 14, 827–845. [Google Scholar] [CrossRef]
- Toscano-Garibay, J.D.; Arriaga-Alba, M.; Sánchez-Navarrete, J.; Mendoza-García, M.; Flores-Estrada, J.J.; Moreno-Eutimio, M.A.; Espinosa-Aguirre, J.J.; González-Ávila, M.; Ruiz-Pérez, N.J. Antimutagenic and antioxidant activity of the essential oils of Citrus sinensis and Citrus latifolia. Sci. Rep. 2017, 7, 11479. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Juliani, H.R. Essential oils in combination and their antimicrobial properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef]
- Miyazawa, M.; Nagai, S.; Oshima, T. Volatile components of the straw of Oryza sativa L. J. Oleo Sci. 2008, 57, 139–143. [Google Scholar] [CrossRef]
- Oloyede, G.K. Antioxidant activities of methyl ricinoleate and ricinoleic acid dominated Ricinus communis seeds extract using lipid peroxidation and free radical scavenging methods. Res. J. Med. Plant 2012, 6, 511–520. [Google Scholar] [CrossRef]
- Karimi, E.; Jaafar, H.Z.; Ghasemzadeh, A.; Ebrahimi, M. Fatty acid composition, antioxidant and antibacterial properties of the microwave aqueous extract of three varieties of Labisia pumila Benth. Biol. Res. 2015, 48, 9. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H. Convergent or parallel molecular evolution of momilactone A and B: Potent allelochemicals, momilactones have been found only in rice and the moss Hypnum plumaeforme. J. Plant Physiol. 2011, 168, 1511–1516. [Google Scholar] [CrossRef] [PubMed]
- Xuan, T.D.; Minh, T.N.; Anh, L.H.; Khanh, T.D. Allelopathic momilactones A and B are implied in rice drought and salinity tolerance, not weed resistance. Agron. Sustain. Dev. 2016, 36, 52. [Google Scholar] [CrossRef]
- Charles, D.J.; Simon, J.E. Comparison of extraction methods for the rapid determination of essential oil content and composition of basil. J. Am. Soc. Hortic. Sci. 1990, 115, 458–462. [Google Scholar] [CrossRef]
- Minh, T.N.; Khang, D.T.; Tuyen, P.T.; Minh, L.T.; Anh, L.H.; Quan, N.V.; Ha, P.T.T.; Quan, N.T.; Toan, N.P.; Elzaawely, A.A.; et al. Phenolic compounds and antioxidant activity of Phalaenopsis orchid hybrids. Antioxidants 2016, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Minh, T.N.; Tuyen, P.T.; Khang, D.T.; Quan, N.V.; Ha, P.T.T.; Quan, N.T.; Yusuf, A.; Fan, X.; Van, T.M.; Khanh, T.D.; et al. Potential use of plant wastes of moth orchid (Phalaenopsis Sogo Yukidian ‘V3′) as an antioxidant source. Foods 2017, 6, 85. [Google Scholar] [CrossRef]
- Tuyen, P.T.; Xuan, T.D.; Khang, D.T.; Ahmad, A.; Quan, N.V.; Anh, T.T.T.; Anh, L.H.; Minh, T.N. Phenolic compositions and antioxidant properties in bark, flower, inner skin, kernel and leaf extracts of Castanea crenata Sieb. et Zucc. Antioxidants 2017, 6, 31. [Google Scholar] [CrossRef]
- Xuan, T.D.; Tsuzuki, E.; Terao, H.; Matstuo, M.; Khanh, T.D.; Murayama, S.; Hong, N.H. Alfalfa, rice by-products and their incorporation for weed control in rice. Weed Biol. Manag. 2003, 3, 137–144. [Google Scholar] [CrossRef]
- Nguyen, M.T.T.; Awale, S.; Tezuka, Y.; Tran, Q.; Le Watanabe, H.; Kadota, S. Xanthine oxidase inhibitory activity of Vietnamese medicinal plants. Biol. Pharm. Bull. 2004, 27, 1414–1421. [Google Scholar] [CrossRef]
- Van, T.M.; Xuan, T.D.; Minh, T.N.; Quan, N.V. Isolation and purification of potent growth inhibitors from Piper methysticum root. Molecules 2018, 23, 1907. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Mazumdar, S. Electrospray ionization mass spectrometry: A technique to access the information beyond the molecular weight of the analyte. Int. J. Anal. Chem. 2012, 2012, 282574. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, S. Improved abundance sensitivity of molecular ions in positive-ion APCI MS analysis of petroleum in toluene. J. Am. Soc. Mass Spectr. 2010, 21, 386–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prokudina, E.A.; Havlíček, L.; Al-Maharik, N.; Lapčík, O.; Strnad, M.; Gruz, J. Rapid UPLC-ESI-MS/MS method for the analysis of isoflavonoids and other phenylpropanoids. J. Food Compos. Anal. 2012, 26, 36–42. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

| Sample | IC50 (µg/mL) | LPI (%) | IC50 (µg/mL) XOI | ||
|---|---|---|---|---|---|
| DPPH | ABTS | FRAP | β-carotene | ||
| Rice leaf EO | 73.1 ± 1.4 | 198.3 ± 2.2 | 700.8 ± 5.7 | 79.0% | 526.0 ± 2.3 |
| BHT * | 9.3 ± 1.1 | 45.7 ± 1.4 | 426.7 ± 3.8 | 90.0% | - |
| Allopurinol * | - | - | - | - | 21.5 ± 0.2 |
| Plant Species | Inhibition Percentage (%) | IC50 (µg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| Root | Shoot | |||||||
| 100 * | 500 * | 1000 * | 100 * | 500 * | 1000 * | Root | Shoot | |
| Oryza sativa L. | −10.0 | −12.0 | −3.0 | −5.0 | −7.0 | −11.0 | St ** | St ** |
| Echinochloa crus-galli | 38.0 | 51.0 | 63.0 | 18.0 | 25.0 | 51.0 | 455.6 ± 11.5 b | 964.3 ± 12.1 a |
| Bidens pilosa L. | 12.0 | 20.0 | 52.0 | 16.0 | 19.0 | 56.0 | 912.5 ± 10.3 a | 869.2 ± 6.1 bc |
| Raphanus sativus L. | 4.0 | 21.0 | 60.0 | 7.0 | 20.0 | 60.0 | 916.3 ± 10.0 a | 866.2 ± 26.1 c |
| Lactuca sativa L. | 6.0 | 34.0 | 55.0 | 12.0 | 24.0 | 55.0 | 926.7 ± 11.6 a | 908.0 ± 11.1 b |
| Chemical Formula | Compounds | Molecular Weight | Retention Time (min) | Peak Area [% of Total] |
|---|---|---|---|---|
| C8H8O | Coumaran | 120 | 8.22 | 4.02 |
| C9H10O2 | Benzyl acetate | 150 | 9.58 | 2.54 |
| C8H8O3 | Vanillin | 152 | 10.73 | 8.22 |
| C11H22O2 | Undecanoic acid | 186 | 12.73 | 2.56 |
| C13H18O | Megastigmatrienone | 190 | 13.02 | 3.20 |
| C14H28O2 | Myristic acid | 228 | 15.00 | 3.26 |
| C18H36O | 2-Pentadecanone | 268 | 15.89 | 2.13 |
| C10H20O2 | Capric acid | 172 | 16.05 | 2.17 |
| C16H32O2 | Palmitic acid | 256 | 17.13 | 17.34 |
| C18H30O2 | Linolenic acid | 278 | 18.80 | 11.16 |
| C19H36O3 | Methyl ricinoleate | 312 | 19.46 | 27.86 |
| C16H34 | Hexadecane | 226 | 20.22 | 3.82 |
| Rice leaf EO | UPLC/ESI-MS | |
|---|---|---|
| MA | MB | |
| Retention time (min) | 4.00 ± 0.04 | 2.45 ± 0.06 |
| LOD (ng/mL) | 0.097 | 0.157 |
| LOQ (ng/mL) | 0.293 | 0.476 |
| Yield (ng/g FW) | 9.80 ± 0.22 | 4.93 ± 0.13 |
| % of Total EO | 7.35 | 3.70 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minh, T.N.; Xuan, T.D.; Van, T.M.; Andriana, Y.; Viet, T.D.; Khanh, T.D.; Tran, H.-D. Phytochemical Analysis and Potential Biological Activities of Essential Oil from Rice Leaf. Molecules 2019, 24, 546. https://doi.org/10.3390/molecules24030546
Minh TN, Xuan TD, Van TM, Andriana Y, Viet TD, Khanh TD, Tran H-D. Phytochemical Analysis and Potential Biological Activities of Essential Oil from Rice Leaf. Molecules. 2019; 24(3):546. https://doi.org/10.3390/molecules24030546
Chicago/Turabian StyleMinh, Truong Ngoc, Tran Dang Xuan, Truong Mai Van, Yusuf Andriana, Tran Duc Viet, Tran Dang Khanh, and Hoang-Dung Tran. 2019. "Phytochemical Analysis and Potential Biological Activities of Essential Oil from Rice Leaf" Molecules 24, no. 3: 546. https://doi.org/10.3390/molecules24030546
APA StyleMinh, T. N., Xuan, T. D., Van, T. M., Andriana, Y., Viet, T. D., Khanh, T. D., & Tran, H.-D. (2019). Phytochemical Analysis and Potential Biological Activities of Essential Oil from Rice Leaf. Molecules, 24(3), 546. https://doi.org/10.3390/molecules24030546









