Columbianadin Suppresses Lipopolysaccharide (LPS)-Induced Inflammation and Apoptosis through the NOD1 Pathway
Abstract
:1. Introduction
2. Results
2.1. CBN Reduced the Expression of Inflammatory Cytokines Induced by LPS in THP-1
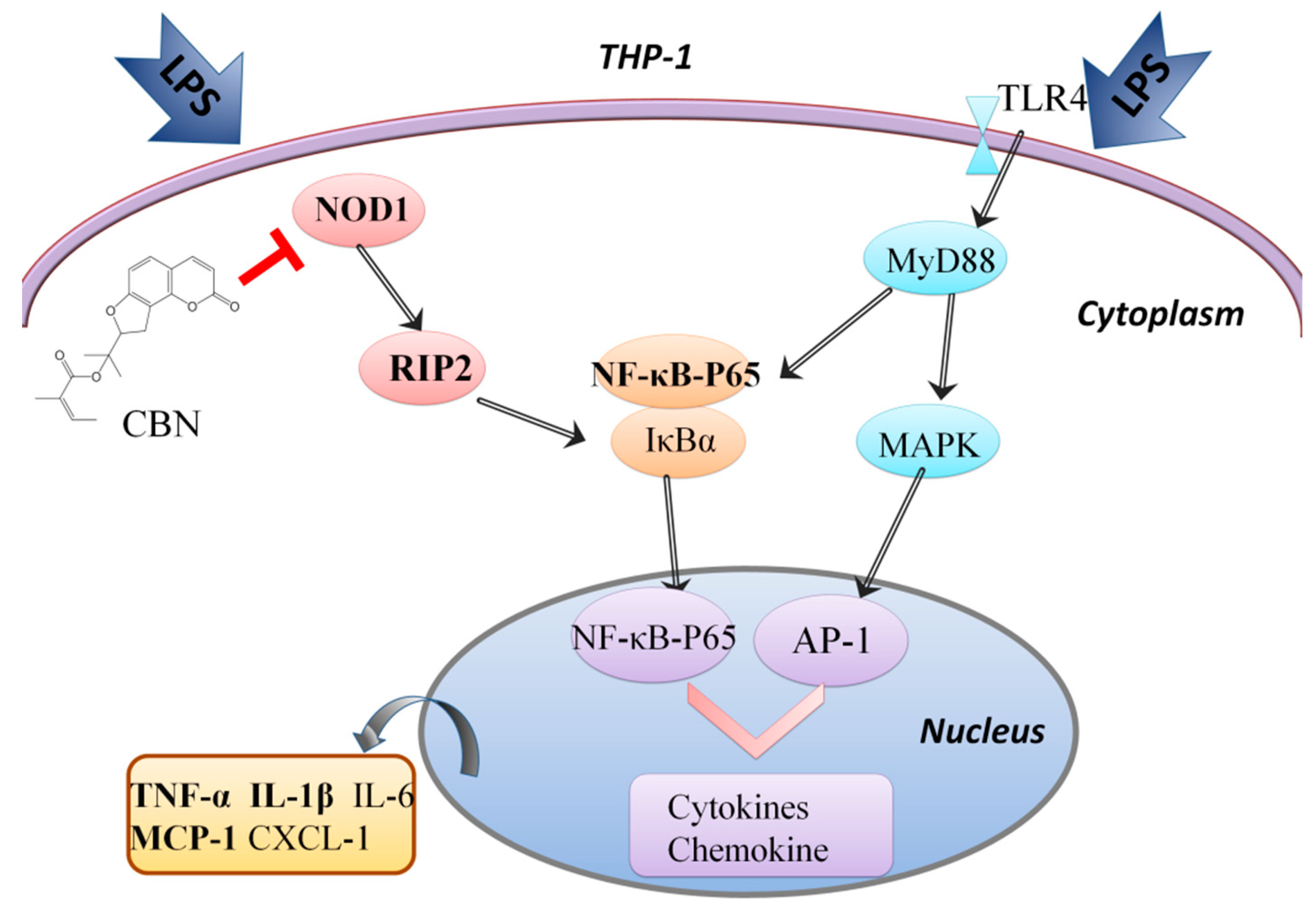
2.2. CBN Targeted the NOD1 Signaling Pathway in LPS-Induced Inflammation
2.3. CBN Failed to Inhibit the Inflammatory Response in NOD1 Knockdown
2.4. CBN Inhibited the Expression of Pro-Inflammatory Cytokine Production
2.5. CBN Suppressed LPS-Induced Apoptosis via Inhibiting the NOD1 Pathway
3. Discussion
4. Method
4.1. Cell Culture and Treatment
4.2. siRNA and Expression Plasmid Transfection
4.3. PCR Array
4.4. Enrichment and Pathway Analysis of Differentially Expressed Genes
4.5. qPCR Analysis
4.6. Immunoblot
4.7. ELISA Analysis
4.8. Apoptosis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lu, J.; Fang, K.; Wang, S.; Xiong, L.; Zhang, C.; Liu, Z.; Guan, X.; Zheng, R.; Wang, G.; Zheng, J.; et al. Anti-Inflammatory Effect of Columbianetin on Lipopolysaccharide-Stimulated Human Peripheral Blood Mononuclear Cells. Mediat. Inflamm. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Wang, F.; Ai, Y.; Ma, W.; Bian, Q.; Lee, D.Y.; Dai, R. Simultaneous determination of seven coumarins by UPLC-MS/MS: Application to a comparative pharmacokinetic study in normal and arthritic rats after oral administration of Huo Luo Xiao Ling Dan or single-herb extract. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 991, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Hoult, J.R.; Paya, M. Pharmacological and biochemical actions of simple coumarins: Natural products with therapeutic potential. Gen. Pharmacol. 1996, 27, 713–722. [Google Scholar] [CrossRef]
- Khetwal, K.S.; Pathak, R.P. Columbianadin: A Novel Coumarin from Heracleum brunonis. Planta Med. 1987, 53, 581. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.F.; Zhang, L.; Zhang, Y.B.; Yang, X.W. Simultaneous assessment of absorption characteristics of coumarins from Angelicae Pubescentis Radix: In vitro transport across Caco-2 cell and in vivo pharmacokinetics in rats after oral administration. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1060, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Tsai, H.Y.; Wu, T.S. Anti-inflammatory and analgesic activities from roots of Angelica pubescens. Planta Med. 1995, 61, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.J.; Lee, J.H.; Choi, J.S.; Lee, S.K.; Kim, Y.S.; Kim, H.P. Inhibition of airway inflammation by the roots of Angelica decursiva and its constituent, columbianadin. J. Ethnopharmacol. 2014, 155, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Hsu, A.C.; Dua, K.; Starkey, M.R.; Haw, T.J.; Nair, P.M.; Nichol, K.; Zammit, N.; Grey, S.T.; Baines, K.J.; Foster, P.S.; et al. MicroRNA-125a and -b inhibit A20 and MAVS to promote inflammation and impair antiviral response in COPD. JCI Insight 2017, 2, e90443. [Google Scholar] [CrossRef]
- Lin, M.; Tang, S.C. Toll-like receptors: Sensing and reacting to diabetic injury in the kidney. Nephrol. Dial. Transpl. 2014, 29, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Nishio, H.; Kanno, S.; Onoyama, S.; Ikeda, K.; Tanaka, T.; Kusuhara, K.; Fujimoto, Y.; Fukase, K.; Sueishi, K.; Hara, T. Nod1 ligands induce site-specific vascular inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Navarro, R.; Delgado-Wicke, P.; Nunez-Prado, N.; Compte, M.; Blanco-Toribio, A.; Nunez, G.; Alvarez-Vallina, L.; Sanz, L. Role of nucleotide-binding oligomerization domain 1 (NOD1) in pericyte-mediated vascular inflammation. J. Cell. Mol. Med. 2016, 20, 980–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barlow, G.M.; Yu, A.; Mathur, R. Role of the Gut Microbiome in Obesity and Diabetes Mellitus. Nutr. Clin. Pract. 2015, 30, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Han, J.M.; Lee, E.K.; Gong, S.Y.; Sohng, J.K.; Kang, Y.J.; Jung, H.J. Sparassis crispa exerts anti-inflammatory activity via suppression of TLR-mediated NF-kappaB and MAPK signaling pathways in LPS-induced RAW264.7 macrophage cells. J. Ethnopharmacol. 2018, 231, 10–18. [Google Scholar] [CrossRef]
- Jeong, H.J.; Na, H.J.; Kim, S.J.; Rim, H.K.; Myung, N.Y.; Moon, P.D.; Han, N.R.; Seo, J.U.; Kang, T.H.; Kim, J.J.; et al. Anti-inflammatory effect of Columbianetin on activated human mast cells. Biol. Pharm. Bull. 2009, 32, 1027–1031. [Google Scholar] [CrossRef]
- Dinarello, C.A. A clinical perspective of IL-1beta as the gatekeeper of inflammation. Eur. J. Immunol. 2011, 41, 1203–1217. [Google Scholar] [CrossRef]
- McIlwain, D.R.; Lang, P.A.; Maretzky, T.; Hamada, K.; Ohishi, K.; Maney, S.K.; Berger, T.; Murthy, A.; Duncan, G.; Xu, H.C.; et al. iRhom2 regulation of TACE controls TNF-mediated protection against Listeria and responses to LPS. Science 2012, 335, 229–232. [Google Scholar] [CrossRef]
- Haller, H.; Bertram, A.; Nadrowitz, F.; Menne, J. Monocyte chemoattractant protein-1 and the kidney. Curr. Opin. Nephrol. Hypertens. 2016, 25, 42–49. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 2009, 21, 317–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Khan, P.M.; Correa, R.G.; Divlianska, D.B.; Peddibhotla, S.; Sessions, E.H.; Magnuson, G.; Brown, B.; Suyama, E.; Yuan, H.; Mangravita-Novo, A.; et al. Identification of Inhibitors of NOD1-Induced Nuclear Factor-kappaB Activation. ACS Med. Chem. Lett. 2011, 2, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Inohara, N.; Koseki, T.; del Peso, L.; Hu, Y.; Yee, C.; Chen, S.; Carrio, R.; Merino, J.; Liu, D.; Ni, J.; et al. Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-kappaB. J. Biol. Chem. 1999, 274, 14560–14567. [Google Scholar] [CrossRef] [PubMed]
- Baeuerle, P.A.; Baltimore, D. NF-kappa B: Ten years after. Cell 1996, 87, 13–20. [Google Scholar] [CrossRef]
- Yang, L.; Tang, Z.; Zhang, H.; Kou, W.; Lu, Z.; Li, X.; Li, Q.; Miao, Z. PSMA7 directly interacts with NOD1 and regulates its function. Cell. Physiol. Biochem. 2013, 31, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds Columbianadin are available from the authors. |





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Hsu, A.C.-Y.; Pan, H.; Gu, Y.; Zuo, X.; Dong, B.; Wang, Z.; Zheng, J.; Lu, J.; Zheng, R.; et al. Columbianadin Suppresses Lipopolysaccharide (LPS)-Induced Inflammation and Apoptosis through the NOD1 Pathway. Molecules 2019, 24, 549. https://doi.org/10.3390/molecules24030549
Zhang C, Hsu AC-Y, Pan H, Gu Y, Zuo X, Dong B, Wang Z, Zheng J, Lu J, Zheng R, et al. Columbianadin Suppresses Lipopolysaccharide (LPS)-Induced Inflammation and Apoptosis through the NOD1 Pathway. Molecules. 2019; 24(3):549. https://doi.org/10.3390/molecules24030549
Chicago/Turabian StyleZhang, Chao, Alan Chen-Yu Hsu, He Pan, Yinuo Gu, Xu Zuo, Bing Dong, Ziyan Wang, Jingtong Zheng, Junying Lu, Ruipeng Zheng, and et al. 2019. "Columbianadin Suppresses Lipopolysaccharide (LPS)-Induced Inflammation and Apoptosis through the NOD1 Pathway" Molecules 24, no. 3: 549. https://doi.org/10.3390/molecules24030549
APA StyleZhang, C., Hsu, A. C.-Y., Pan, H., Gu, Y., Zuo, X., Dong, B., Wang, Z., Zheng, J., Lu, J., Zheng, R., & Wang, F. (2019). Columbianadin Suppresses Lipopolysaccharide (LPS)-Induced Inflammation and Apoptosis through the NOD1 Pathway. Molecules, 24(3), 549. https://doi.org/10.3390/molecules24030549




