Systematic Identification of Thiosemicarbazides for Inhibition of Toxoplasma gondii Growth In Vitro
Abstract
:1. Introduction
2. Results and Discussion
2.1. Rationale and Chemistry
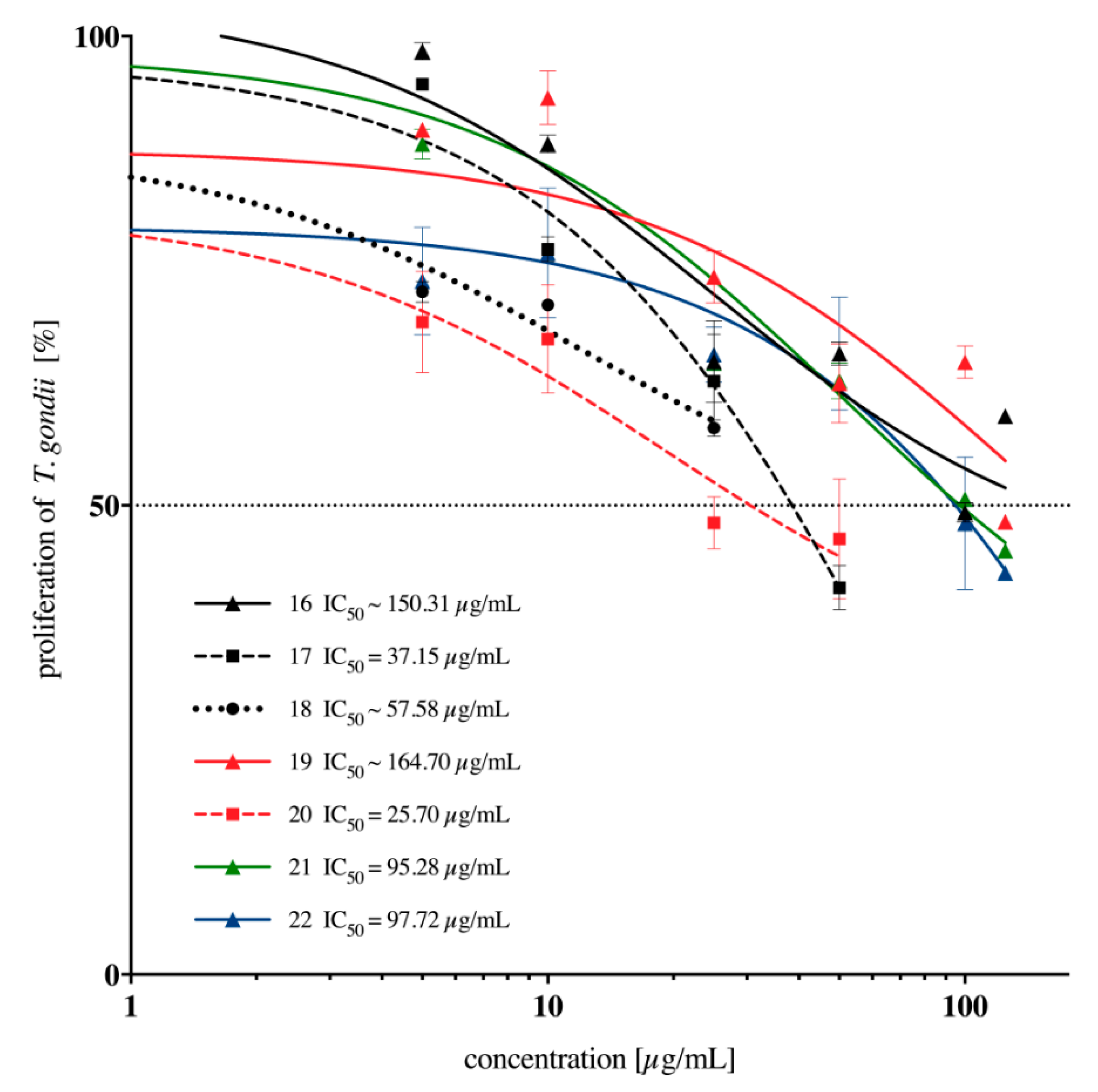
2.2. In Vitro Anti-Toxoplasma gondii Activity of the Imidazole-Thiosemicarbazides 2–22
2.3. Cytotoxicity of the Imidazole-Thiosemicarbazides 2–22 against L929 Cells and Selectivity Index
3. Materials and Methods
3.1. Chemistry
3.2. General Procedure for Synthesis of the Imidazole-Thiosemicarbazides 2–22
3.3. Cytotoxicity Assays
3.3.1. Cell Culture
3.3.2. Preparation of Compounds and Sulfadiazine
3.3.3. Cell Viability Assay
3.4. In Vitro Assay for Anti-T. gondii Activity
3.4.1. Cell Line and Parasite Culture
3.4.2. Influence of 2–22 and Sulfadiazine on T. gondii Proliferation
3.5. Graphs and Statistical Analyses
3.6. Computational Details
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef]
- Tenter, A.M.; Heckeroth, A.R.; Weiss, L.M. Toxoplasma gondii: From animals to humans. Int. J. Parasitol. 2000, 30, 1217–1258. [Google Scholar] [CrossRef]
- Lyons, R.E.; McLeod, R.; Roberts, C.W. Toxoplasma gondii tachyzoite-bradyzoite interconversion. Trends Parasitol. 2002, 18, 198–201. [Google Scholar] [CrossRef]
- Sini, S.; McIntyre, M.K.; Mordue, D.G. Toxoplasma gondii: Determinants of tachyzoite to bradyzoite conversion. Parasitol. Res. 2010, 107, 253–260. [Google Scholar]
- Zaware, N.; Sharma, H.; Yang, J.; Devambatla, R.K.V.; Queener, S.F.; Anderson, K.S.; Gangjee, A. Discovery of potent and selective inhibitors of Toxoplasma gondii thymidylate synthase for opportunistic infections. ACS Med. Chem. Lett. 2013, 4, 1148–1151. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, W.J.; Jeffers, V. Mechanisms of Toxoplasma gondii persistence and latency. FEMS Microbiol. Rev. 2012, 36, 717–733. [Google Scholar] [CrossRef] [PubMed]
- Luft, B.J.; Remington, J.S. Toxoplasmic encephalitis in AIDS. Clin. Infect. Dis. 1992, 15, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Israelski, D.M.; Remington, J.S. Toxoplasmosis in the non-AIDS immunocompromised host. Curr. Clin. Top Infect. Dis. 1993, 13, 322–356. [Google Scholar]
- Dubey, J.P.; Jones, J.L. Toxoplasma gondii infection in humans and animals in the United States. Int. J. Parasitol. 2008, 38, 1257–1278. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, L.; Fangli, L. Current treatment of ocular toxoplasmosis in immunocompetent patients: A network meta-analysis. Acta Trop. 2018, 185, 52–62. [Google Scholar] [CrossRef]
- Carlier, Y.; Truyens, C.; Deloron, P.; Peyron, F. Congenital parasitic infections: A review. Acta Trop. 2012, 121, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Speer, C.A.; Shen, S.K.; Kwok, O.C.H.; Blixt, J.A. Oocyst-induced murine Toxoplasmosis: Life cycle, pathogenicity, and stage conversion in mice fed Toxoplasma gondii oocysts. J. Parasitol. 1997, 83, 870–882. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A. Targeting DHFR in parasitic protozoa. Drug Discov. Today 2005, 10, 121–128. [Google Scholar] [CrossRef]
- Neville, A.J.; Zach, S.J.; Wang, X.; Larson, J.J.; Judge, A.K.; Davis, L.A.; Vennerstrom, J.L.; Davis, P.H. Clinically available medicines demonstrating anti-toxoplasma activity. Antimicrob. Agents Chemother. 2015, 59, 7161–7169. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.X.; Wei, S.S.; Lindsay, D.S.; Peng, H.J. A systematic review and meta-analysis of the efficacy of anti-Toxoplasma gondii medicines in humans. PLoS ONE 2015, 10, e0138204. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.E.; Benson, C.; Holmes, K.K.; Brooks, J.T.; Pau, A.; Masur, H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents. Recommendations from CDC, the National Institutes of Health and the HIV Medicine Association of the Infectious Diseases Society of America. CDC MMWR 2009, 58, 1–198. [Google Scholar] [PubMed]
- Chulay, J.D.; Watkins, W.M.; Sixsmith, D.G. Synergistic antimalarial activity of pyrimethamine and sulfadoxine against Plasmodium falciparum in vitro. Am. J. Trop. Med. Hyg. 1984, 33, 325–330. [Google Scholar] [CrossRef]
- Kovacs, J.A.; Allegra, C.J.; Swan, J.C.; Drake, J.C.; Parrillo, J.E.; Chabner, B.A.; Masur, H. Potent antipneumocystis and antitoxoplasma activities of piritrexim, a lipid-soluble antifolate. Antimicrob. Agents Chemother. 1988, 32, 430–433. [Google Scholar] [CrossRef]
- Broughton, M.C.; Queener, S.F. Pneumocystis carinii dihydrofolate reductase used to screen potential antipneumocystis drugs. Antimicrob. Agents Chemother. 1991, 35, 1348–1355. [Google Scholar] [CrossRef]
- Chio, L.C.; Queener, S.F. Identification of highly potent and selective inhibitors of Toxoplasma gondii dihydrofolate reductase. Antimicrob. Agents Chemother. 1993, 37, 1914–1923. [Google Scholar] [CrossRef]
- Kovacs, J.A.; Allegra, C.J.; Kennedy, S.; Swan, J.C.; Drake, J.; Parrillo, J.E.; Chabner, B.; Masur, H. Efficacy of trimetrexate, a potent lipid-soluble antifolate, in the treatment of rodent Pneumocystis carinii pneumonia. Am. J. Trop. Med. Hyg. 1988, 39, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Radke, J.B.; Burrows, J.N.; Goldberg, D.E.; Sibley, L.D. Evaluation of current and emerging antimalarial medicines for inhibition of Toxoplasma gondii growth in vitro. ACS Infect. Dis. 2018, 10, 1264–1274. [Google Scholar] [CrossRef] [PubMed]
- Rutaganira, F.U.; Barks, J.; Dhason, M.S.; Wang, Q.; Lopez, M.S.; Long, S.; Radke, J.B.; Jones, N.G.; Maddirala, A.R.; Janetka, J.W.; et al. Inhibition of calcium dependent protein kinase 1 (CDPK1) by pyrazolopyrimidine analogs decreases establishment and reoccurrence of central nervous system disease by Toxoplasma gondii. J. Med. Chem. 2017, 60, 9976–9989. [Google Scholar] [CrossRef] [PubMed]
- McCabe, R.E. Antitoxoplasma chemotherapy. In Toxoplasmosis: A Comprehensive Clinical Guide; Joynson, D.H.M., Wreghitt, T.G., Eds.; Cambridge Univ. Press: Cambridge, UK, 2001; pp. 319–359. [Google Scholar]
- Kortagere, S. Novel molecules to treat chronic central nervous system Toxoplasmosis. J. Med. Chem. 2017, 60, 9974–9975. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, J.M.; Davidian, M.; Rainey, P.M.; Hafner, R.; Raasch, R.H.; Luft, B.J. Pyrimethamine pharmacokinetics in human immunodeficiency virus-positive patients seropositive for Toxoplasma gondii. Antimicrob. Agents Chemother. 1996, 40, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Ben-Harari, R.R.; Goodwin, E.; Casoy, J. Adverse event profile of pyrimethamine-based therapy in toxoplasmosis: A systematic review. Drugs R&D 2017, 17, 523–544. [Google Scholar]
- Hernandez, A.V.; Thota, P.; Pellegrino, D.; Pasupuleti, V.; Benites-Zapata, V.A.; Deshpande, A.; Penalva de Oliveira, A.C.; Vidal, J.E. A systematic review and meta-analysis of the relative efficacy and safety of treatment regimens for HIV-associated cerebral toxoplasmosis: Is trimethoprim-sulfamethoxazole a real option? HIV Med. 2017, 18, 115–124. [Google Scholar] [CrossRef]
- Kieffer, F.; Wallon, M. Congenital toxoplasmosis. Handb. Clin. Neurol. 2013, 112, 1099–1101. [Google Scholar]
- Piper, J.R.; Johnson, C.A.; Krauth, C.A.; Carter, R.L.; Hosmer, C.A.; Queener, S.F.; Borotz, S.E.; Pfefferkorn, E.R. Lipophilic antifolates as agents against opportunistic infections. 1. Agents superior to trimetrexate and piritrexim against Toxoplasma gondii and Pneumocystis carinii in in vitro evaluations. J. Med. Chem. 1996, 39, 1271–1280. [Google Scholar] [CrossRef]
- Araujo, F.G.; Lin, T.; Remington, J.S. The activity of atovaquone (566C80) in murine toxoplasmosis is markedly augmented when used in combination with pyrimethamine or sulfadiazine. J. Infect. Dis. 1993, 167, 494–497. [Google Scholar] [CrossRef]
- Romand, S.; Pudney, M.; Derouin, F. In vitro and in vivo activities of the hydroxynaphthoquinone atovaquone alone or combined with pyrimethamine, sulfadiazine, clarithromycin, or minocycline against Toxoplasma gondii. Antimicrob. Agents Chemother. 1993, 37, 2371–2378. [Google Scholar] [CrossRef] [PubMed]
- Araujo, F.G.; Huskinson-Mark, J.; Gutteridge, W.E.; Remington, J.S. In vitro and in vivo activities of the hydroxynaphthoquinone 566C80 against the cyst form of Toxoplasma gondii. Antimicrob. Agents Chemother. 1992, 36, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Dike, S.Y.; Singh, D.; Thankachen, B.N.; Sharma, B.; Mathur, P.K.; Kore, S.; Kumar, A. A single-pot synthesis of atovaquone: An antiparasitic drug of choice. Org. Process Res. Dev. 2014, 18, 618–625. [Google Scholar] [CrossRef]
- Dziadek, B.; Gatkowska, J.; Grzybowski, M.; Dziadek, J.; Dzitko, K.; Dlugonska, H. Toxoplasma gondii: The vaccine potential of three trivalent antigen-cocktails composed of recombinant ROP2, ROP4, GRA4 and SAG1 proteins against chronic toxoplasmosis in BALB/c mice. Exp. Parasitol. 2012, 131, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Dzitko, K.; Paneth, A.; Plech, T.; Pawełczyk, J.; Stączek, P.; Stefańska, J.; Paneth, P. 1,4-disubstituted thiosemicarbazide derivatives are potent inhibitors of Toxoplasma gondii proliferation. Molecules 2014, 19, 9926–9943. [Google Scholar] [CrossRef] [PubMed]
- Siwek, A.; Świderek, K.; Jankowski, S. Problems with molecular mechanics implementations on the example of 4-benzoyl-1-(4-methyl-imidazol-5-yl)-carbonylthiosemicarbazide. J. Mol. Model. 2012, 18, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Lipiński, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug. Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Logs Calculation. Available online: https://www.organic-chemistry.org/prog/peo/logS.html (accessed on 20 December 2018).
- Zhao, Y.; Abraham, M.H.; Lee, J.; Hersey, A.; Luscombe, N.C.; Beck, G.; Sherborne, B.; Cooper, I. Rate-limited steps of human oral absorption and QSAR studies. Pharm. Res. 2002, 19, 1446–1457. [Google Scholar] [CrossRef]
- Plech, T.; Wujec, M.; Siwek, A.; Kosikowska, U.; Malm, A. Synthesis and antimicrobial activity of thiosemicarbazides, s-triazoles and their Mannich bases bearing 3-chlorophenyl moiety. Eur. J. Med. Chem. 2011, 46, 241–248. [Google Scholar] [CrossRef]
- Liesen, A.P.; de Aquino, T.M.; Carvalho, C.S.; Lima, V.T.; de Araujo, J.M.; de Lima, J.G.; de Faria, A.R.; de Melo, E.J.T.; Alves, A.J.; Alves, E.W.; et al. Synthesis and evaluation of anti-Toxoplasma gondii and antimicrobial activities of thiosemicarbazides, 4-thiazolidinones and 1,3,4-thiadiazoles. Eur. J. Med. Chem. 2010, 45, 3685–3691. [Google Scholar] [CrossRef] [PubMed]
- Siwek, A.; Wujec, M.; Dobosz, M.; Wawrzycka-Gorczyca, I. Study of direction of cyclization of 1-azolyl-4-aryl/alkyl-thiosemicarbazides. Heteroatom Chem. 2010, 21, 521–532. [Google Scholar] [CrossRef]
- Siwek, A.; Stączek, P.; Stefańska, J. Synthesis and structure-activity relationship studies of 4-arylthiosemicarbazides as topoisomerase IV inhibitors with Gram-positive antibacterial activity. Search for molecular basis of antibacterial activity of thiosemicarbazides. Eur. J. Med. Chem. 2011, 46, 5717–5726. [Google Scholar] [CrossRef] [PubMed]
- Case, R.J.; Franzblau, S.G.; Wang, Y.; Cho, S.H.; Soejarto, D.D.; Pauli, G.F. Ethnopharmacological evaluation of the informant consensus model on anti-tuberculosis claims among the Manus. J. Ethnopharmacol. 2006, 106, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.C.S.; de Morais, S.A.L.; de Aquino, F.J.T.; Chang, R.; de Oliveira, A.; Martins, M.M.; Martins, C.H.G.; Sousa, L.C.F.; Barros, T.T.; da Silva, C.V.; et al. Bioassay-guided fractionation and antimicrobial and cytotoxic activities of Cassia bakeriana extracts. Rev. Bras. Farmacogn. 2017, 27, 91–98. [Google Scholar] [CrossRef]
- Hyperchem 8.0.3; HyperCube Inc.: Gainsville, FL, USA, 2007.
- Calculation of Molecular Physicochemical Properties—Molinspiration. Available online: http://www.molinspiration.com/cgi-bin/properties (accessed on 20 December 2018).
- On-line Software—VCCLab. Available online: http://www.vcclab.org/online.html (accessed on 20 December 2018).
Sample Availability: Samples of the compounds 2–22 are available from the authors. |



| Compound | Mass | logP | HBA | HBD | logS | PSA | %ABS |
|---|---|---|---|---|---|---|---|
| 2 | 320.33 | 0.50 | 9 | 4 | −4.15 | 127.66 | 64.96 |
| 3 | 320.33 | 0.53 | 9 | 4 | −4.17 | 127.66 | 64.96 |
| 4 | 320.33 | 0.55 | 9 | 4 | −4.18 | 127.66 | 64.96 |
| 5 | 305.36 | 0.60 | 7 | 4 | −4.05 | 91.07 | 77.58 |
| 6 | 305.36 | 0.62 | 7 | 4 | −4.06 | 91.07 | 77.58 |
| 7 | 305.36 | 0.65 | 7 | 4 | −4.06 | 91.07 | 77.58 |
| 8 | 289.36 | 0.93 | 6 | 4 | −4.04 | 81.83 | 80.77 |
| 9 | 261.74 | 0.14 | 6 | 4 | −3.29 | 81.83 | 80.77 |
| 10 | 309.78 | 1.25 | 6 | 4 | −4.28 | 81.83 | 80.77 |
| 11 | 293.33 | 0.73 | 6 | 4 | −4.19 | 81.83 | 80.77 |
| 12 | 317.37 | 0.47 | 7 | 4 | −4.24 | 98.91 | 74.88 |
| 13 | 335.39 | 0.24 | 8 | 4 | −4.21 | 100.30 | 74.40 |
| 14 | 323.81 | 1.65 | 6 | 4 | −4.50 | 81.83 | 80.77 |
| 15 | 311.32 | 0.85 | 6 | 4 | −4.22 | 81.83 | 80.77 |
| 16 | 289.36 | 0.99 | 6 | 4 | −4.09 | 81.83 | 80.77 |
| 17 | 289.36 | 1.02 | 6 | 4 | −4.10 | 81.83 | 80.77 |
| 18 | 289.36 | 1.04 | 6 | 4 | −4.11 | 81.83 | 80.77 |
| 19 | 318.41 | 0.69 | 7 | 4 | −3.88 | 85.07 | 79.65 |
| 20 | 346.46 | 1.45 | 7 | 4 | −3.99 | 85.07 | 79.65 |
| 21 | 319.39 | 1.02 | 7 | 4 | −4.23 | 91.07 | 77.58 |
| 22 | 359.50 | 1.64 | 6 | 4 | −5.07 | 81.83 | 80.77 |
| Compound | Concentration [µg/mL] | CC30 [µg/mL] | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 500.00 | 250.00 | 125.00 | 62.50 | 31.25 | 15.63 | 7.81 | 3.91 | ||
| 2 | 29.12 | 53.38 | 75.33 | 89.73 | 94.61 | 96.73 | 100.57 | 102.91 | 181.55 |
| 2.17 | 5.01 | 3.36 | 3.34 | 4.82 | 5.88 | 1.44 | 2.54 | ||
| 3 | 15.27 | 55.81 | 72.38 | 79.00 | 89.38 | 94.61 | 94.64 | 95.37 | 141.25 |
| 0.77 | 3.57 | 0.53 | 1.12 | 1.07 | 2.18 | 0.54 | 2.85 | ||
| 4 | 23.19 | 66.91 | 79.58 | 88.86 | 93.11 | 95.08 | 95.71 | 98.52 | 238.78 |
| 0.70 | 1.52 | 3.46 | 3.30 | 2.35 | 1.81 | 2.11 | 5.26 | ||
| 5 | 53.64 | 63.12 | 69.69 | 78.51 | 86.52 | 89.00 | 91.87 | 95.80 | 138.04 |
| 6.97 | 2.25 | 6.41 | 7.89 | 2.83 | 1.17 | 3.39 | 4.83 | ||
| 6 | 56.79 | 67.32 | 68.79 | 79.66 | 83.28 | 86.17 | 88.60 | 92.82 | 165.96 |
| 0.65 | 1.50 | 1.42 | 1.61 | 0.40 | 1.58 | 1.16 | 1.56 | ||
| 7 | 68.56 | 70.70 | 83.97 | 85.77 | 87.65 | 89.47 | 90.31 | 95.08 | 389.05 |
| 2.33 | 2.04 | 3.30 | 2.66 | 3.44 | 2.38 | 2.17 | 2.23 | ||
| 8 | 46.58 | 53.26 | 64.92 | 88.77 | 92.04 | 96.52 | 99.36 | 100.77 | 123.03 |
| 5.33 | 2.57 | 11.22 | 2.60 | 3.92 | 2.13 | 1.44 | 1.48 | ||
| 9 | 87.44 | 92.82 | 94.44 | 95.57 | 95.97 | 97.07 | 98.34 | 100.37 | >500.00 |
| 4.13 | 2.87 | 2.43 | 1.91 | 2.17 | 2.82 | 2.85 | 4.66 | ||
| 10 | 22.09 | 61.82 | 69.89 | 72.12 | 74.63 | 93.54 | 94.09 | 95.74 | 112.20 |
| 0.67 | 1.35 | 2.32 | 0.89 | 1.33 | 0.87 | 1.01 | 0.51 | ||
| 11 | 46.32 | 57.77 | 66.05 | 75.56 | 92.73 | 99.82 | 101.38 | 103.55 | 95.50 |
| 5.94 | 5.36 | 3.52 | 3.25 | 2.41 | 3.16 | 4.18 | 4.22 | ||
| 12 | 56.21 | 76.77 | 96.70 | 99.30 | 101.38 | 102.71 | 104.30 | 106.32 | 206.54 |
| 0.57 | 2.63 | 2.35 | 2.00 | 2.15 | 1.00 | 1.38 | 3.04 | ||
| 13 | 77.00 | 94.18 | 95.74 | 97.51 | 99.85 | 102.62 | 105.08 | 107.45 | >500.00 |
| 4.51 | 2.70 | 2.82 | 3.18 | 2.78 | 2.64 | 3.60 | 3.17 | ||
| 14 | 10.41 | 21.05 | 49.27 | 61.53 | 67.49 | 76.63 | 91.49 | 101.21 | 28.18 |
| 0.40 | 3.40 | 11.56 | 1.68 | 0.48 | 3.12 | 2.92 | 4.46 | ||
| 15 | 51.18 | 65.18 | 74.84 | 84.32 | 96.20 | 100.77 | 101.96 | 104.65 | 158.49 |
| 0.69 | 3.28 | 2.03 | 3.56 | 2.33 | 0.35 | 1.05 | 2.38 | ||
| 16 | 63.38 | 71.48 | 79.69 | 91.23 | 93.83 | 99.96 | 102.28 | 103.23 | 288.40 |
| 4.14 | 1.77 | 1.29 | 2.91 | 2.62 | 3.23 | 1.75 | 1.35 | ||
| 17 | 40.66 | 50.05 | 56.53 | 64.89 | 74.78 | 85.74 | 88.72 | 94.09 | 50.12 |
| 8.30 | 2.40 | 6.98 | 4.98 | 2.22 | 4.54 | 5.67 | 2.69 | ||
| 18 | 52.95 | 60.44 | 63.73 | 64.60 | 69.05 | 82.04 | 91.69 | 99.10 | 34.67 |
| 2.69 | 2.04 | 1.01 | 0.45 | 2.20 | 2.35 | 0.61 | 1.48 | ||
| 19 | 53.32 | 60.78 | 68.47 | 78.57 | 80.76 | 85.71 | 91.75 | 93.08 | 112.20 |
| 1.55 | 2.37 | 0.63 | 1.03 | 2.41 | 3.61 | 4.92 | 5.38 | ||
| 20 | 12.87 | 53.35 | 65.96 | 67.92 | 71.31 | 85.65 | 93.60 | 101.55 | 63.10 |
| 0.20 | 15.09 | 1.34 | 1.38 | 3.07 | 4.03 | 6.55 | 5.20 | ||
| 21 | 72.03 | 82.27 | 84.35 | 100.83 | 106.18 | 111.59 | 113.96 | 114.94 | 500.00 |
| 4.17 | 1.30 | 0.63 | 3.08 | 5.33 | 2.84 | 3.30 | 3.43 | ||
| 22 | 58.84 | 68.18 | 76.60 | 79.81 | 80.71 | 101.27 | 110.20 | 115.81 | 190.55 |
| 2.96 | 2.65 | 1.19 | 2.27 | 2.48 | 3.40 | 3.49 | 3.10 | ||
| Compound | Compound Structure | CC30 [µg/mL] | IC50 [µg/mL] | SI |
|---|---|---|---|---|
| 2 |  | 181.55 | 43.05 | 0.63 |
| 3 |  | 141.25 | 14.78 | 0.98 |
| 4 |  | 238.78 | 21.62 | 1.05 |
| 5 |  | 138.04 | 33.11 | 0.62 |
| 6 |  | 165.96 | 79.65 | 0.54 |
| 7 |  | 389.05 | 113.99 | 0.53 |
| 8 | 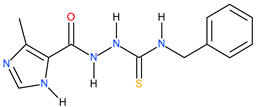 | 123.03 | ~149.59 | −0.08 |
| 9 | 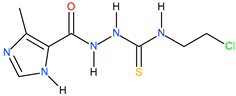 | >500 | >125 | - |
| 10 | 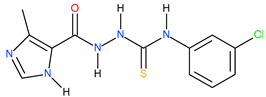 | 112.20 | 25.70 | 0.64 |
| 11 | 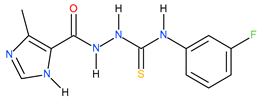 | 95.50 | ~117.92 | −0.09 |
| 12 | 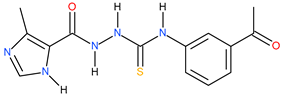 | 206.54 | 95.50 | 0.34 |
| 13 | 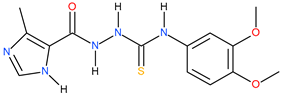 | >500 | ~174.98 | <0.46 |
| 14 | 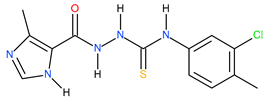 | 28.18 | 18.62 | 0.18 |
| 15 | 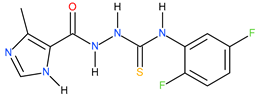 | 158.49 | 53.70 | 0.47 |
| 16 | 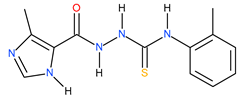 | 288.40 | ~150.31 | 0.28 |
| 17 |  | 50.12 | 37.15 | 0.13 |
| 18 | 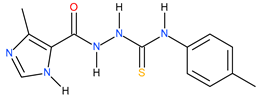 | 34.67 | ~57.58 | −0.22 |
| 19 | 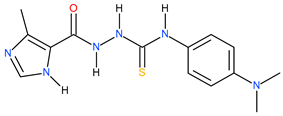 | 112.20 | ~164.70 | −0.17 |
| 20 | 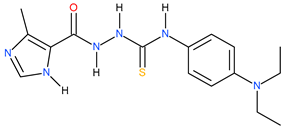 | 63.10 | 25.70 | 0.39 |
| 21 | 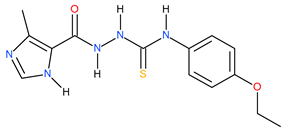 | 500 | 95.28 | 0.72 |
| 22 | 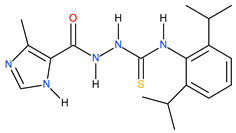 | 190.55 | 97.72 | 0.29 |
| Sulfadiazine | 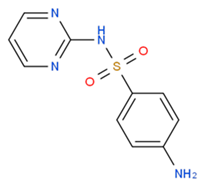 | 1251.12 | ~2721.45 | −0.34 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paneth, A.; Węglińska, L.; Bekier, A.; Stefaniszyn, E.; Wujec, M.; Trotsko, N.; Dzitko, K. Systematic Identification of Thiosemicarbazides for Inhibition of Toxoplasma gondii Growth In Vitro. Molecules 2019, 24, 614. https://doi.org/10.3390/molecules24030614
Paneth A, Węglińska L, Bekier A, Stefaniszyn E, Wujec M, Trotsko N, Dzitko K. Systematic Identification of Thiosemicarbazides for Inhibition of Toxoplasma gondii Growth In Vitro. Molecules. 2019; 24(3):614. https://doi.org/10.3390/molecules24030614
Chicago/Turabian StylePaneth, Agata, Lidia Węglińska, Adrian Bekier, Edyta Stefaniszyn, Monika Wujec, Nazar Trotsko, and Katarzyna Dzitko. 2019. "Systematic Identification of Thiosemicarbazides for Inhibition of Toxoplasma gondii Growth In Vitro" Molecules 24, no. 3: 614. https://doi.org/10.3390/molecules24030614





