HPLC Profile of Longan (cv. Shixia) Pericarp-Sourced Phenolics and Their Antioxidant and Cytotoxic Effects
Abstract
:1. Introduction
2. Results and Discussion
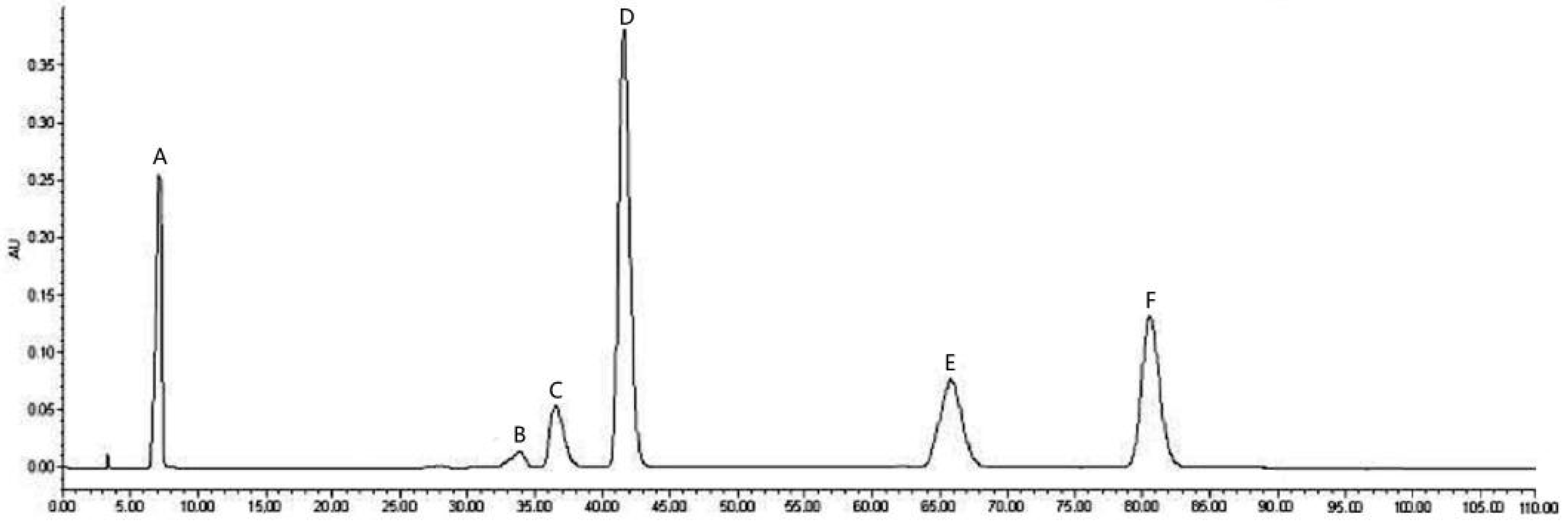
2.1. Total Phenolic Content and HPLC Analysis
2.2. Antioxidant Activity of LPPs and Purified LPE
2.3. Cytotoxic Effect of LPPs and Purified LPE
3. Experimental Section
3.1. General Experimental Procedure
3.2. Preparation of Purified LPE
3.3. Determination of Total Phenolic Content
3.4. HPLC Analysis of LPPs
3.5. Antioxidant Assay of LPPs and Purified LPE
3.5.1. DPPH Radical Scavenging Assay
3.5.2. Hydroxyl Radical (•OH) Scavenging Assay
3.5.3. Ferrous Ion Chelating Assay
3.6. Cytotoxic Effect of LPPs and Purified LPE on Cell Line A549
3.6.1. Inhibitory Activity
3.6.2. Fluorescence Staining of PI
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gonzalez-Gallego, J.; Garcia-Mediavilla, M.V.; Sanchez-Campos, S.; Tunon, M.J. Fruit polyphenols, immunity and inflammation. Br. J. Nutr. 2010, 104, S15–S27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cobb, D.T. Polyphenols-Food Sources, Bioactive Properties and Antioxidant Effects; Nova Science Publishers: New York, NY, USA, 2014; ISBN 978-1-63117-857-3. [Google Scholar]
- Parkar, S.G.; Stevenson, D.E.; Skinner, M.A. The potential influence of fruit polyphenols on colonic microflora and human gut health. Int. J. Food Microbiol. 2008, 124, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Rangkadilok, N.; Sitthimonchai, S.; Worasuttayangkurn, L.; Mahidol, C.; Ruchirawat, M.; Satayavivad, J. Evaluation of free radical scavenging and antityrosinase activities of standardized longan fruit extract. Food Chem. Toxicol. 2007, 45, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Jiang, Y.; Zhao, M.; Chen, F.; Wang, R.; Chen, Y.; Zhang, D. Structural characterisation of polysaccharides purified from longan (Dimocarpus longan Lour.) fruit pericarp. Food Chem. 2009, 115, 609–614. [Google Scholar] [CrossRef]
- Yang, B.; Jiang, Y.; Shi, J.; Chen, F.; Ashraf, M. Extraction and pharmacological properties of bioactive compounds from longan (Dimocarpus longan Lour.) fruit—A review. Food Res. Int. 2011, 44, 1837–1842. [Google Scholar] [CrossRef]
- Zhang, R.F.; Khan, S.A.; Lin, Y.; Guo, D.; Pan, X.; Liu, L.; Wei, Z.; Zhang, Y.; Deng, Y.; Zhang, M. Phenolic profiles and cellular antioxidant activity of longan pulp of 24 representative Chinese cultivars. Int. J. Food Prop. 2018, 21, 746–759. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.L.; Yi, G.J.; Zhou, B.R.; Zeng, J.; Huang, Y.H. The advancement of research on litchi and longan germplasm resources in China. Sci. Hortic. 2007, 114, 143–150. [Google Scholar] [CrossRef]
- Zhang, F. Statistical analysis of China fruit production (II). China Fruit News 2017, 34, 21–34. (In Chinese) [Google Scholar]
- Sun, J.; Shi, J.; Jiang, Y.M.; Xue, S.J.; Wei, X.Y. Identification of two polyphenolic compounds with antioxidant activities in longan pericarp tissues. J. Agr. Food Chem. 2007, 55, 5864–5868. [Google Scholar] [CrossRef]
- Yang, B.; Zhao, M.M.; Shi, J.; Cheng, G.P.; Ruenroengklin, N.; Jiang, Y.M. Variations in water-soluble saccharides and phenols in longan fruit pericarp after drying. J. Food Process Eng. 2008, 31, 66–77. [Google Scholar] [CrossRef]
- Prasad, K.N.; Yang, B.; Zhao, M.M.; Wei, X.Y.; Jiang, Y.M.; Chen, F. High pressure extraction of corilagin from longan (Dimocarpus longan Lour.) fruit pericarp. Sep. Purif. Technol. 2009, 70, 41–45. [Google Scholar] [CrossRef]
- He, N.; Wang, Z.Y.; Yang, C.X.; Lu, Y.H.; Sun, D.H.; Wang, Y.P.; Shao, W.Y.; Li, Q.B.A. Isolation and identification of polyphenolic compounds in longan pericarp. Sep. Purif. Technol. 2009, 70, 219–224. [Google Scholar] [CrossRef]
- Qi, W.E.; Chen, H.B.; Peng, D.F.; Yan, F.F. Present situation, problems and suggestions of the development of Chinese longan industry. Guangdong Agr. Sci. 2016, 43, 169–174. (In Chinese) [Google Scholar]
- Prasad, K.N.; Hao, J.; Shi, J.; Liu, T.; Li, J.; Wei, X.; Qiu, S.; Xue, S.; Jiang, Y. Antioxidant and anticancer activities of high pressure-assisted extract of longan (Dimocarpus longan Lour.) fruit pericarp. Inno. Food Sci. Emerg. Technol. 2009, 10, 412–419. [Google Scholar] [CrossRef]
- Bai, X.; Lai, T.; Zhou, T.; Li, Y.; Li, X.; Zhang, H. In vitro antioxidant activities of phenols and oleanolic acid from mango peel and their cytotoxic effect A549 cell line. Molecules 2018, 23, 1395. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.L.; Yue, T.L.; Yuan, Y.H.; Zhang, H.W. Optimization of microwave-assisted extraction of polyphenols from apple pomace. J. Sep. Sci. 2010, 33, 3571–3578. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, J.; Mu, Y.; Han, L.; Liu, R.; Cai, Y.; Huang, X. Chemical characterization and anti-hyperglycaemic effects of polyphenol enriched longan (Dimocarpus longan Lour.) pericarp extracts. J. Funct. Foods 2015, 13, 314–322. [Google Scholar] [CrossRef]
- Yang, Y.C.; Li, J.; Zu, Y.G.; Fu, Y.J.; Luo, M.; Wu, N.; Liu, X.L. Optimisation of microwave-assisted enzymatic extraction of corilagin and geraniin from Geranium sibiricum Linne and evaluation of antioxidant activity. Food Chem. 2010, 122, 373–380. [Google Scholar] [CrossRef]
- Kwon, S.H.; Wang, Z.; Hwang, S.H.; Kang, Y.H.; Lee, J.Y.; Lim, S.S. Comprehensive evaluation of the antioxidant capacity of Perilla frutescens leaves extract and isolation of free radical scavengers using step-wise HSCCC guided DPPH-HPLC. Int. J. Food Prop. 2017, 20, 921–934. [Google Scholar] [CrossRef]
- Jia, L.; Jin, H.; Zhou, J.; Chen, L.; Lu, Y.; Ming, Y.; Yu, Y. A potential anti-tumor herbal medicine, corilagin, inhibits ovarian cancer cell growth through blocking the TGF-beta signaling pathways. BMC Complem. Altern. Med. 2013, 13, 33. [Google Scholar] [CrossRef]
- Li, N.; Lin, Z.C.; Chen, W.; Zheng, Y.; Ming, Y.L.; Zheng, Z.Z.; Huang, W.; Chen, L.H.; Xiao, J.B.; Lin, H.T. Corilagin from longan seed: Identification, quantification, and synergistic cytotoxicity on SKOv3ip and hey cells with ginsenoside Rh2 and 5-fluorouracil. Food Chem. Toxicol. 2018, 119, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Villaño, D.; Fernández-Pachóna, M.S.; Moyá, M.L.; Troncoso, A.M.; García-Parrilla, M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar]
- Yang, X.N.; Yan, F.; Huang, S.R.; Fu, C.L. Antioxidant activities of fractions from longan pericarps. Food Sci. Technol. 2014, 34, 341–345. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.Q.; Wang, C.H.; Shi, Q.J.; Ma, C.H. Preparation of different molecular weight polysaccharides from Porphyridium cruentum and their antioxidant activities. Int. J. Biol. Macromol. 2009, 45, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.L.; Wu, Z.W.; Zhao, T.H.; Sun, Y.; Xu, R.J. Characterization, antioxidant and hepatoprotective activities of polysaccharides from Ilex latifolia Thunb. Carbohyd. Polym. 2014, 101, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Jia, J.X.; Ren, X.J.; Li, B.H.; Zhang, Q. Extraction, preliminary characterization and in vitro antioxidant activity of polysaccharides from Oudemansiella radicata mushroom. Int. J. Biol. Macromol. 2018, 120, 1760–1769. [Google Scholar] [CrossRef] [PubMed]
- Chuen, T.L.K.; Vuong, Q.V.; Hirun, S.; Bowyer, M.C.; Predebon, M.J.; Goldsmith, C.D.; Sakoff, J.A.; Scarlett, C.J. Antioxidant and anti-proliferative properties of Davidson’s plum (Davidsonia pruriens F. Muell) phenolic-enriched extracts as affected by different extraction solvents. J. Herb. Med. 2016, 6, 187–192. [Google Scholar] [CrossRef]
- Shen, J.; Ma, H.L.; Zhang, T.C.; Liu, H.; Yu, L.H.; Li, G.S.; Li, H.S.; Hu, M.C. Magnolol inhibits the growth of non-small cell lung cancer via inhibiting microtubule polymerization. Cell. Physiol. Biochem. 2017, 42, 1789–1801. [Google Scholar] [CrossRef]
- Castellani, S.; Trapani, A.; Spagnoletta, A.; Toma1, L.; Magrone, T.; Gioia, S.D.; Mandracchia, D.; Trapani, G.; Jirillo, E.; Conese, M. Nanoparticle delivery of grape seed-derived proanthocyanidins to airway epithelial cells dampens oxidative stress and infammation. J. Transl. Med. 2018, 140, 1–15. [Google Scholar]
Sample Availability: Samples of the compounds A–F are available from the authors. |



| Compound | Antioxidant Activity (%) | |||
|---|---|---|---|---|
| DPPH• Scavenging Rate | •OH Inhibition Rate | Ferrous Ion Chelation Effect | ||
| A * | protocatechuic acid | 59.6 ± 0.5 | 56.9 ± 0.5 | 36.4 ± 0.7 |
| B * | isoscopoletin | 48.9 ± 0.8 | 43.2 ± 0.1 | 28.9 ± 0.2 |
| C * | quercetin | 53.6 ± 0.4 | 18.3 ± 0.5 | 22.3 ± 0.5 |
| D * | ellagic acid | 45.6 ± 0.4 | 51.3 ± 0.5 | 33.1 ± 0.2 |
| E * | corilagin | 71.8 ± 0.5 | 75.9 ± 0.3 | 32.3 ± 0.5 |
| F * | proanthocyanidins C1 | 28.8 ± 0.1 | 36.9 ± 0.2 | 5.3 ± 0.4 |
| LPE ※ | longan pericarp extract | 55.5 ± 0.3 | 60.2 ± 0.3 | 23.9 ± 0.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, X.; Pan, R.; Li, M.; Li, X.; Zhang, H. HPLC Profile of Longan (cv. Shixia) Pericarp-Sourced Phenolics and Their Antioxidant and Cytotoxic Effects. Molecules 2019, 24, 619. https://doi.org/10.3390/molecules24030619
Bai X, Pan R, Li M, Li X, Zhang H. HPLC Profile of Longan (cv. Shixia) Pericarp-Sourced Phenolics and Their Antioxidant and Cytotoxic Effects. Molecules. 2019; 24(3):619. https://doi.org/10.3390/molecules24030619
Chicago/Turabian StyleBai, Xuelian, Rui Pan, Mingzhu Li, Xiuting Li, and Huawei Zhang. 2019. "HPLC Profile of Longan (cv. Shixia) Pericarp-Sourced Phenolics and Their Antioxidant and Cytotoxic Effects" Molecules 24, no. 3: 619. https://doi.org/10.3390/molecules24030619
APA StyleBai, X., Pan, R., Li, M., Li, X., & Zhang, H. (2019). HPLC Profile of Longan (cv. Shixia) Pericarp-Sourced Phenolics and Their Antioxidant and Cytotoxic Effects. Molecules, 24(3), 619. https://doi.org/10.3390/molecules24030619






