Polyalcohols as Hydrogen-Bonding Donors in Choline Chloride-Based Deep Eutectic Solvents for Extraction of Xanthones from the Pericarp of Garcinia mangostana L.
Abstract
1. Introduction
2. Results and Discussion
2.1. Density, Polarity and Viscosity of ChCl-Polyalcohol DESs
2.2. Optimum Extraction Time
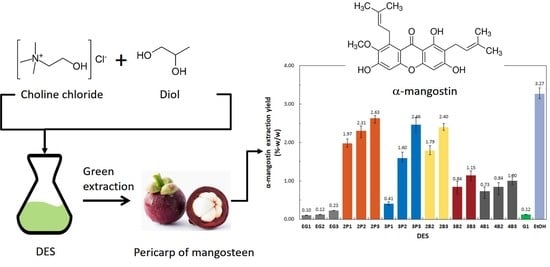
2.3. α-Mangostin Extraction Yields
2.3.1. Effect of ChCl to Polyalcohol Mole Ratio
2.3.2. Effect of Molecular Structure of Polyalcohols
2.3.3. Effect of Polarity of DESs
2.3.4. Effect of Viscosity of DESs
2.4. Composition of Xanthones in Ethanolic and DES Extracts
2.5. ChCl-1,2-Propanediol DES as a Designer Solvent for Green Extraction
3. Materials and Methods
3.1. Chemicals and Plant Material
3.2. Preparation of Mangosteen Powder and ChCl-Polyalcohol DESs
3.3. Physicochemical Properties of DESs
3.4. Extraction of α-Mangostin Using Polyalcohol DESs and Ethanol
3.5. HPLC Analysis of α-Mangostin Extracted into DESs
3.6. Recovery of Xanthones from DESs
3.7. LC-MS Analysis of α-Mangostin Extracted Using DES and Ethanol
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kanchanapom, K.; Kanchanapom, M. Tropical and Subtropical Fruits; Ag Science Inc.: Auburndale, FL, USA, 1998. [Google Scholar]
- Matsumoto, K.; Akao, Y.; Ohguchi, K.; Ito, T.; Tanaka, T.; Iinuma, M.; Nozawa, Y. Xanthones induce cell-cycle arrest and apoptosis in human colon cancer DLD-1 cells. Bioorg. Med. Chem. 2005, 13, 6064–6069. [Google Scholar] [CrossRef] [PubMed]
- Akao, Y.; Nakagawa, Y.; Nozawa, Y. Anti-Cancer Effects of Xanthones from Pericarps of Mangosteen. Int. J. Mol. Sci. 2008, 9, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhao, M.; Yang, B.; Zhao, Q.; Jiang, Y. Phenolics from hull of Garcinia mangostana fruit and their antioxidant activities. Food Chem. 2007, 104, 176–181. [Google Scholar] [CrossRef]
- Kondo, M.; Zhang, L.; Ji, H.; Kou, Y.; Ou, B. Bioavailability and antioxidant effects of a xanthone-rich Mangosteen (Garcinia mangostana) product in humans. J. Agric. Food Chem. 2009, 57, 8788–8792. [Google Scholar] [CrossRef] [PubMed]
- Wittenauer, J.; Falk, S.; Schweiggert-Weisz, U.; Carle, R. Characterisation and quantification of xanthones from the aril and pericarp of mangosteens (Garcinia mangostana L.) and a mangosteen containing functional beverage by HPLC–DAD–MSn. Food Chem. 2012, 134, 445–452. [Google Scholar] [CrossRef]
- Pedraza-Chaverri, J.; Cárdenas-Rodríguez, N.; Orozco-Ibarra, M.; Pérez-Rojas, J.M. Medicinal properties of mangosteen (Garcinia mangostana). Food Chem. Toxicol. 2008, 46, 3227–3239. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.Y.; Ng, J.W.; Ng, W.K.; Forde, C.G.; Henry, C.J. Plant polyphenols to enhance the nutritional and sensory properties of chocolates. Food Chem. 2016, 200, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as a new extraction media for phenolic metabolites in Carthamus tinctorius L. Anal. Chem. 2013, 85, 6272–6278. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.; Rahman, M.; Sharif, K.; Mohamed, A.; Sahena, F.; Jahurul, M.; Ghafoor, K.; Norulaini, N.; Omar, A. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Earle, M.J.; Esperança, J.M.; Gilea, M.A.; Lopes, J.N.C.; Rebelo, L.P.; Magee, J.W.; Seddon, K.R.; Widegren, J.A. The distillation and volatility of ionic liquids. Nature 2006, 439, 831. [Google Scholar] [CrossRef]
- Earle, M.J.; Seddon, K.R. Ionic liquids. Green solvents for the future. Pure Appl. Chem. 2000, 72, 1391–1398. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.; Witkamp, G.-J.; Verpoorte, R. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Angell, C.A.; Ansari, Y.; Zhao, Z. Ionic liquids: Past, present and future. Faraday Discuss. 2012, 154, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep eutectic solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef] [PubMed]
- Pena-Pereira, F.; Namieśnik, J. Ionic liquids and deep eutectic mixtures: Sustainable solvents for extraction processes. ChemSusChem 2014, 7, 1784–1800. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, Z.; Leitner, W.; de María, P.D. Practical separation of alcohol–ester mixtures using Deep-Eutectic-Solvents. Tetrahedron Lett. 2012, 53, 6968–6971. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; Hayyan, A.; Al-Saadi, M.A.; AlNashef, I.M.; Mirghani, M.E.; Saheed, O.K. Are deep eutectic solvents benign or toxic? Chemosphere 2013, 90, 2193–2195. [Google Scholar] [CrossRef]
- Abbott, A.P.; Harris, R.C.; Ryder, K.S.; D’Agostino, C.; Gladden, L.F.; Mantle, M.D. Glycerol eutectics as sustainable solvent systems. Green Chem. 2011, 13, 82–90. [Google Scholar] [CrossRef]
- Harris, R.C. Physical Properties of Alcohol Based Deep Eutectic Solvents; University of Leicester: Leicester, UK, 2009. [Google Scholar]
- Stefanovic, R.; Ludwig, M.; Webber, G.B.; Atkin, R.; Page, A.J. Nanostructure, hydrogen bonding and rheology in choline chloride deep eutectic solvents as a function of the hydrogen bond donor. Phys. Chem. Chem. Phys. 2017, 19, 3297–3306. [Google Scholar] [CrossRef]
- Mulia, K.; Krisanti, E.; Terahadi, F.; Putri, S. Selected natural deep eutectic solvents for the extraction of α-mangostin from mangosteen (Garcinia mangostana L.) pericarp. Int. J. Technol. 2015, 7, 1211–1220. [Google Scholar] [CrossRef]
- Deye, J.F.; Berger, T.A.; Anderson, A.G. Nile Red as a solvatochromic dye for measuring solvent strength in normal liquids and mixtures of normal liquids with supercritical and near critical fluids. Anal. Chem. 1990, 62, 615–622. [Google Scholar] [CrossRef]
- Bi, W.; Tian, M.; Row, K.H. Evaluation of alcohol-based deep eutectic solvent in extraction and determination of flavonoids with response surface methodology optimization. J. Chromatogr. A 2013, 1285, 22–30. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Scientific Opinion on safety and efficacy of choline chloride as a feed additive for all animal species. EFSA J. 2011, 9, 2353. [Google Scholar]
- Fowles, J.R.; Banton, M.I.; Pottenger, L.H. A toxicological review of the propylene glycols. Crit. Rev. Toxicol. 2013, 43, 363–390. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=184.1666 (1,2-propanediol) and https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=182.8252 (choline chloride); (accessed on 30 January 2019).
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sc. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Jessop, P.G.; Jessop, D.A.; Fu, D.; Phan, L. Solvatochromic parameters for solvents of interest in green chemistry. Green Chem. 2012, 14, 1245–1259. [Google Scholar] [CrossRef]
- Pothitirat, W.; Gritsanapan, W. Thin-layer chromatography-densitometric analysis of alpha-mangostin content in Garcinia mangostana fruit rind extracts. J. AOAC Int. 2008, 91, 1145–1148. [Google Scholar] [PubMed]
Sample Availability: No samples are available from the authors. |





| Polyalcohol (HBD) | ChCl:HBD Mole Ratio | DES | Density (g/mL) | ENR (kcal/mol) * | Viscosity (cP) | α-Mangostin Extraction Yield (%) ** |
|---|---|---|---|---|---|---|
| Ethylene glycol | 1:1 | EG1 | 1.08 | 47.4 | 31.2 | 0.10 ± 0.01 |
| 1:2 | EG2 | 1.05 | 47.4 | 30.4 | 0.12 ± 0.01 | |
| 1:3 | EG3 | 1.04 | 47.5 | 29.7 | 0.23 ± 0.01 | |
| 1,2-Propanediol | 1:1 | 2P1 | 1.05 | 56.5 | 37.1 | 1.97 ± 0.11 |
| 1:2 | 2P2 | 1.03 | 56.7 | 35.2 | 2.31 ± 0.16 | |
| 1:3 | 2P3 | 1.01 | 56.6 | 31.6 | 2.63 ± 0.11 | |
| 1,3-Propanediol | 1:1 | 3P1 | 1.04 | 56.1 | 40.1 | 0.41 ± 0.05 |
| 1:2 | 3P2 | 1.03 | 56.7 | 39.2 | 1.60 ± 0.13 | |
| 1:3 | 3P3 | 1.10 | 56.8 | 38.1 | 2.46 ± 0.19 | |
| 1,2-Butanediol | 1:2 | 2B2 | 1.06 | 50.6 | 27.6 | 1.79 ± 0.12 |
| 1:3 | 2B3 | 1.06 | 50.9 | 25.8 | 2.40 ± 0.09 | |
| 1,3-Butanediol | 1:2 | 3B2 | 1.05 | 50.6 | 67.0 | 0.84 ± 0.17 |
| 1:3 | 3B3 | 1.04 | 50.1 | 62.2 | 1.15 ± 0.13 | |
| 1,4-Butanediol | 1:1 | 4B1 | 1.10 | 57.8 | 44.8 | 0.73 ± 0.17 |
| 1:2 | 4B2 | 1.05 | 58.8 | 43.9 | 0.84 ± 0.17 | |
| 1:3 | 4B3 | 1.05 | 60.6 | 43.7 | 1.00 ± 0.13 | |
| Glycerol | 1:1 | G1 | 1.14 | 55.6 | 46.8 | 0.12 ± 0.01 |
| Ethanol | - | EtOH | - | 51.6 | - | 3.27 ± 0.14 |
| Compound | Xanthone | Formula | [M + H]+ m/z | Composition * (%) | |
|---|---|---|---|---|---|
| Ethanol | DES | ||||
| 1 | 1,7-dihydroxy-3-methoxy-2-(3-methylbut-2-enyl)xanthon | C19H18O5 | 327 | - | 5.8 |
| 2 | γ-mangostin | C23H24O6 | 397 | 13.0 | 12.8 |
| 3 | gartanin | C23H24O6 | 397 | 16.0 | 12.3 |
| 4 | α-mangostin | C24H26O6 | 411 | 53.6 | 52.4 |
| 5 | garcinone E | C28H32O6 | 465 | 9.5 | 10.4 |
| 6 | garcimangosone B | C24H24O6 | 409 | 4.7 | 4.2 |
| 7 | β-mangostin | C25H28O6 | 425 | 3.3 | 2.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulia, K.; Fauzia, F.; Krisanti, E.A. Polyalcohols as Hydrogen-Bonding Donors in Choline Chloride-Based Deep Eutectic Solvents for Extraction of Xanthones from the Pericarp of Garcinia mangostana L. Molecules 2019, 24, 636. https://doi.org/10.3390/molecules24030636
Mulia K, Fauzia F, Krisanti EA. Polyalcohols as Hydrogen-Bonding Donors in Choline Chloride-Based Deep Eutectic Solvents for Extraction of Xanthones from the Pericarp of Garcinia mangostana L. Molecules. 2019; 24(3):636. https://doi.org/10.3390/molecules24030636
Chicago/Turabian StyleMulia, Kamarza, Farah Fauzia, and Elsa Anisa Krisanti. 2019. "Polyalcohols as Hydrogen-Bonding Donors in Choline Chloride-Based Deep Eutectic Solvents for Extraction of Xanthones from the Pericarp of Garcinia mangostana L." Molecules 24, no. 3: 636. https://doi.org/10.3390/molecules24030636
APA StyleMulia, K., Fauzia, F., & Krisanti, E. A. (2019). Polyalcohols as Hydrogen-Bonding Donors in Choline Chloride-Based Deep Eutectic Solvents for Extraction of Xanthones from the Pericarp of Garcinia mangostana L. Molecules, 24(3), 636. https://doi.org/10.3390/molecules24030636