Bioactivity Profile of the Diterpene Isosteviol and its Derivatives
Abstract
:1. Introduction
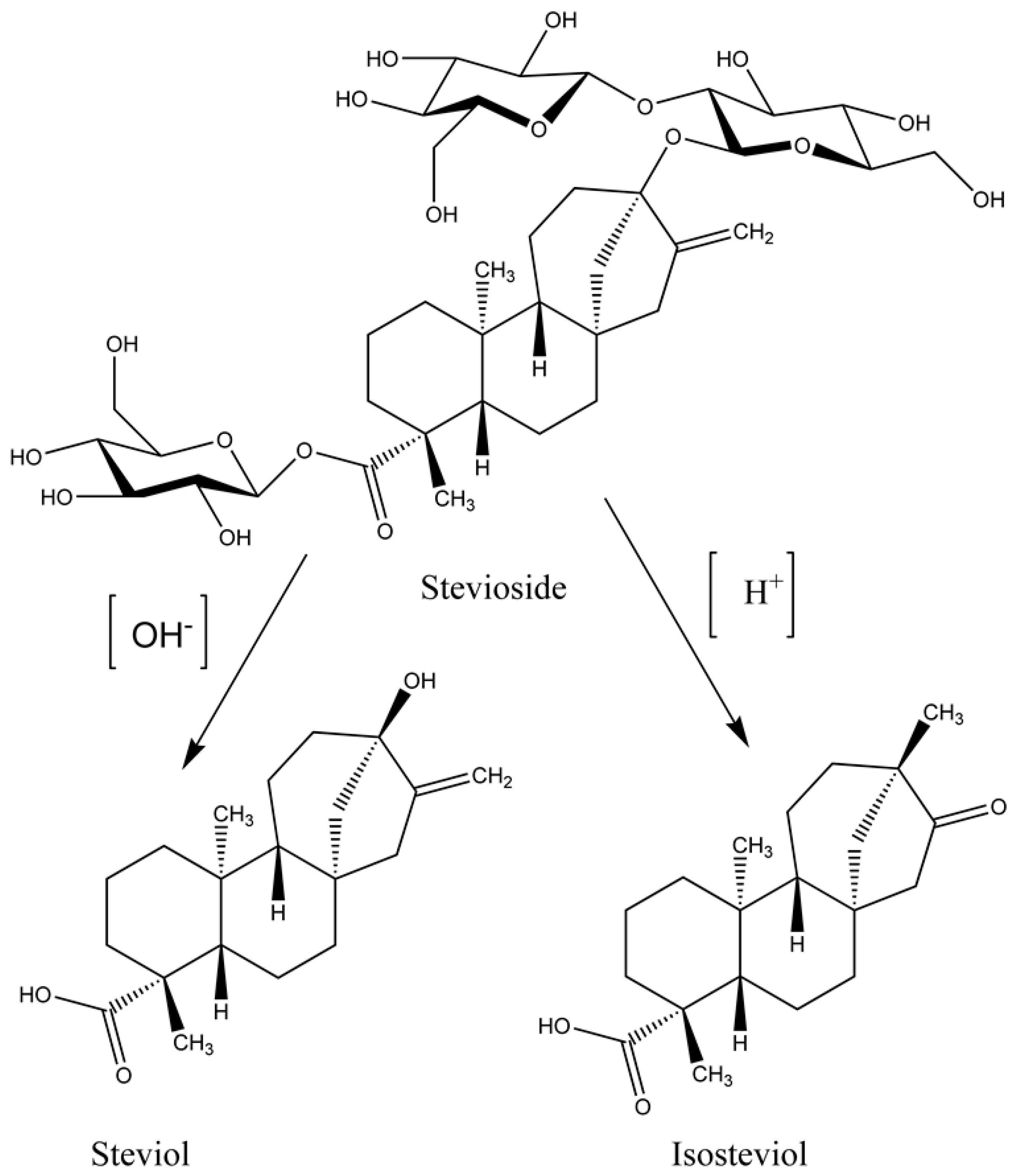
2. Chemistry of Stevia Glycosides
3. Plant Growth Regulator
4. Pharmacological Activities of Isosteviol Derivatives
4.1. Cytotoxic Agents
4.2. DNA Polymerase and DNA Topoisomerase Inhibitors
4.3. Antiviral Agents
Early Antigen Activation of Epstein–Barr Virus (EBV-EA)
4.4. Antibacterial Agents
Anti-Tuberculosis Agents
4.5. Antihypertensive Agent and Cardio Protection
4.6. Neuroprotective Effect
4.7. Antagonists of Angiotensin II
4.8. Anti-Inflammatory Activity
4.9. Anti-Hyperglycemia Effect
4.9.1. Glucose Receptor Sensitization
4.9.2. α-Glucosidase Inhibitor
5. Other Miscellaneous Uses
5.1. Chiral Catalyst
5.2. Anti-Arsenic Contaminator
6. Conclusions
Funding
Conflicts of Interest
References
- Lohoelter, C.; Weckbecker, M.; Waldvogel, S.R. (−)-isosteviol as a versatile ex-chiral-pool building block for organic chemistry. Eur. J. Org. Chem. 2013, 2013, 5539–5554. [Google Scholar] [CrossRef]
- Lohoelter, C.; Schollmeyer, D.; Waldvogel, S.R. Derivatives of (−)-isosteviol with expanded ring D and various oxygen functionalities. Eur. J. Org. Chem. 2012, 2012, 6364–6371. [Google Scholar] [CrossRef]
- Chen, X.; Hermansen, K.; Xiao, J.; Bystrup, S.K.; O’Driscoll, L.; Jeppesen, P.B. Isosteviol has beneficial effects on palmitate-induced α-cell dysfunction and gene expression. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Brandle, J.E.; Telmer, P.G. Steviol glycoside biosynthesis. Phytochemistry 2007. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P.; Klucik, J.; Upreti, M.; Prakash, I. Synthesis of ent-kaurane diterpene monoglycosides. Molecules 2011, 16, 8402–8409. [Google Scholar] [CrossRef]
- Bartholomees, U.; Struyf, T.; Lauwers, O.; Ceunen, S.; Geuns, J.M.C. Validation of an HPLC method for direct measurement of steviol equivalents in foods. Food Chem. 2016, 190, 270–275. [Google Scholar] [CrossRef]
- Wald, J.P.; Morlock, G.E. Quantification of steviol glycosides in food products, Stevia leaves and formulations by planar chromatography, including proof of absence for steviol and isosteviol. J. Chromatogr. A 2017, 1506, 109–119. [Google Scholar] [CrossRef]
- Bridel, M.; Lavieille, R. Le principe à saveur sucrée du Kaà-hê-é (Stevia rebaudiana) Bertoni. Bull. Soc. Chim. Biol. 1931, 13, 636–655. [Google Scholar]
- Avent, A.G.; Hanson, J.R.; Hitchcock, P.B.; De Oliveira, B.H. The influence of a 15-hydroxy group on the rearrangement reactions of steviol and its 16,17-epoxide. J. Chem. Soc. Perkin Trans. 1 1990, 2661–2665. [Google Scholar] [CrossRef]
- Gupta, D. An overview of taxus. J. Drug Discov. Ther. 2015, 3, 1–7. [Google Scholar]
- Takasaki, M.; Konoshima, T.; Kozuka, M.; Tokuda, H.; Takayasu, J.; Nishino, H.; Miyakoshi, M.; Mizutani, K.; Lee, K.H. Cancer preventive agents. Part 8: Chemopreventive effects of stevioside and related compounds. Bioorg. Med. Chem. 2009, 17, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Pariwat, P.; Homvisasevongsa, S.; Muanprasat, C.; Chatsudthipong, V. A natural plant-derived dihydroisosteviol prevents cholera toxin-induced intestinal fluid secretion. J. Pharmacol. Exp. Ther. 2008, 324, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Geuns, J.M.C. Stevioside. Phytochemistry 2003. [Google Scholar] [CrossRef]
- Vouillamoz, J.F.; Wolfram-Schilling, E.; Carron, C.A.; Baroffio, C.A. Agronomical and phytochemical evaluation of Stevia rebaudiana genotypes. Jul. Kühn Arch. 2016, 453, 86–88. [Google Scholar]
- Yadav, A.K.; Singh, S.; Dhyani, D.; Ahuja, P.S. A review on the improvement of stevia [Stevia rebaudiana (Bertoni)]. Can. J. Plant Sci. 2011. [Google Scholar] [CrossRef]
- Espinoza, M.I.; Vincken, J.P.; Sanders, M.; Castro, C.; Stieger, M.; Agosin, E. Identification, quantification, and sensory characterization of steviol glycosides from differently processed Stevia rebaudiana commercial extracts. J. Agric. Food Chem. 2014, 62. [Google Scholar] [CrossRef] [PubMed]
- Philippe, R. Microbial Production of Steviol Glycosides. Eur. Patent EP3215629A1, 27 June 2018. [Google Scholar]
- Markosyan, A. High-Purity Steviol Glycosides. U.S. Patent US20140357588A1, 5 September 2017. [Google Scholar]
- Bomgardner, M. Newcomers head for zero-calorie sweetener market. Chem. Eng. News 2018, 96, 11. [Google Scholar]
- Bondarev, N.; Reshetnyak, O.; Nosov, A. Peculiarities of diterpenoid steviol glycoside production in in vitro cultures of Stevia rebaudiana bertoni. Plant Sci. 2001, 161, 155–163. [Google Scholar] [CrossRef]
- Tavarini, S.; Pagano, I.; Guidi, L.; Angelini, L.G. Impact of nitrogen supply on growth, steviol glycosides and photosynthesis in Stevia rebaudiana Bertoni. Plant Biosyst. 2016, 150, 953–962. [Google Scholar] [CrossRef]
- Parris, C.A.; Shock, C.C.; Qian, M. Soil water tension irrigation criteria affects stevia rebaudiana leaf yield and leaf steviol glycoside composition. Hortscience 2017, 52, 154–161. [Google Scholar] [CrossRef]
- Munza, S.; Prägera, A.; Merktb, N.; Claupeina, W.; Simone, G. Leaf area index, light interception, growth and steviol glycoside formation of Stevia rebaudiana Bertoni under field conditions in southwestern Germany. Ind. Crops Prod. 2018, 111, 520–528. [Google Scholar] [CrossRef]
- Li, W.; Zhou, Y.; You, W.; Yang, M.; Ma, Y.; Wang, M.; Wang, Y.; Yuan, S.; Xiao, Y. Development of Photoaffinity Probe for the Discovery of Steviol Glycosides Biosynthesis Pathway in Stevia rebuadiana and Rapid Substrate Screening. ACS Chem. Biol. 2018, 13, 1944–1949. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, Y.; Nakashima, H.; Miyasaka, J.; Ohdoi, K.; Shimizu, H. Impact of blue, red, and far-red light treatments on gene expression and steviol glycoside accumulation in Stevia rebaudiana. Phytochemistry 2017, 137, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Perera, W.H.; Ghiviriga, I.; Rodenburg, D.L.; Alves, K.; Bowling, J.J.; Bharathi, A.; Khan, I.A.; McChesney, J.D. Rebaudiosides T and U, minor C-19 xylopyranosyl and arabinopyranosyl steviol glycoside derivatives from Stevia rebaudiana (Bertoni) Bertoni. Phytochemistry 2017, 135, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Dusek, J.; Carazo, A.; Trejtnar, F.; Hyrsova, L.; Holas, O.; Smutny, T.; Micuda, S.; Pavek, P. Steviol, an aglycone of steviol glycoside sweeteners, interacts with the pregnane X (PXR) and aryl hydrocarbon (AHR) receptors in detoxification regulation. Food Chem. Toxicol. 2017, 109, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Badshah, S.L.; Ullah, A.; Ahmad, N.; Almarhoon, Z.M.; Mabkhot, Y. Increasing the strength and production of artemisinin and its derivatives. Molecules 2018, 23, 100. [Google Scholar] [CrossRef] [PubMed]
- Baldi, A.; Dixit, V.K. Enhanced artemisinin production by cell cultures of Artemisia annua. Sect. Title Ferment. Bioind. Chem. 2008, 2, 341–348. [Google Scholar] [CrossRef]
- Gold, N.; Fossati, E.; Cetti Hansen, C.; Di Falco, M.; Douchin, V.; Martin, V.J.J. A combinatorial approach to study cytochrome P450 enzymes for de novo production of steviol glucosides in baker’s yeast. ACS Synth. Biol. 2018, 7, acssynbio.8b00470. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzano, G.F.; Cantile, T.; Alcidi, B.; Coda, M.; Ingenito, A.; Zarrelli, A.; Di Fabio, G.; Pollio, A. Is stevia rebaudiana bertoni a non-carcinogenic sweetener? A review. Molecules 2016, 21. [Google Scholar]
- Khaybullin, R.N.; Zhang, M.; Fu, J.; Liang, X.; Li, T.; Katritzky, A.R.; Okunieff, P.; Qi, X. Design and synthesis of isosteviol triazole conjugates for cancer therapy. Molecules 2014, 19, 18676–18689. [Google Scholar] [CrossRef]
- Korochkina, M.G.; Nikitashina, A.D.; Khaybullin, R.N.; Petrov, K.A.; Strobykina, I.Y.; Zobov, V.V.; Kataev, V.E. Unfolded and macrocyclic ammonium derivatives of diterpenoids steviol and isosteviol having choline moieties. Synthesis and inhibitory activities toward acetylcholine- and butyrylcholinesterases. Medchemcomm 2012, 3, 1449. [Google Scholar] [CrossRef]
- Korochkina, M.; Fontanella, M.; Casnati, A.; Arduini, A.; Sansone, F.; Ungaro, R.; Latypov, S.; Kataev, V.; Alfonsov, V. Synthesis and spectroscopic studies of isosteviol-calix[4]arene and -calix[6]arene conjugates. Tetrahedron 2005, 61, 5457–5463. [Google Scholar] [CrossRef]
- Rouhani, M. Full structural analysis of steviol: A DFT study. J. Mol. Struct. 2018, 1173, 679–689. [Google Scholar] [CrossRef]
- Mal’Shakova, M.V.; Korochkina, M.G.; Belykh, D.V.; Kataev, V.E.; Kuchin, A.V. Synthesis of conjugates based on chlorin and isosteviol building blocks. Chem. Nat. Compd. 2009, 45, 187–192. [Google Scholar] [CrossRef]
- Kataev, V.E.; Strobykina, I.Y.; Militsina, O.I.; Korochkina, M.G.; Fedorova, O.V.; Ovchinnikova, I.G.; Valova, M.S.; Rusinov, G.L. Isosteviol and some of its derivatives as receptors and carriers of amino acid picrates. Tetrahedron Lett. 2006, 47, 2137–2139. [Google Scholar] [CrossRef]
- Belykh, D.V.; Mal’Shakova, M.V.; Korochkina, M.G.; Kataev, V.E.; Kuchin, A.V. First macrocycle based on chlorin and isosteviol structural elements. Chem. Nat. Compd. 2011, 47, 612–614. [Google Scholar] [CrossRef]
- Khan, K.; Huang, H.; Zheng, Y.-S. Design, Synthesis, and Transport Potential of a New Family of Nonionic Amphiphilic Dendro-calix[4]arene. Curr. Org. Chem. 2012, 16, 2745–2751. [Google Scholar] [CrossRef]
- Khan, K.; Lal Badshah, S.; Ahmad, N.; Rashid, H.U.; Mabkhot, Y. Inclusion complexes of a new family of non-ionic amphiphilic dendrocalix[4]arene and poorly water-soluble drugs naproxen and ibuprofen. Molecules 2017, 22, 783. [Google Scholar] [CrossRef]
- Chatsudthipong, V.; Muanprasat, C. Stevioside and related compounds: Therapeutic benefits beyond sweetness. Pharmacol. Ther. 2009, 121, 41–54. [Google Scholar] [CrossRef]
- Karimi, M.; Hashemi, J.; Ahmadi, A.; Abbasi, A.; Esfahani, M. Study on the bioactivity of steviol and isosteviol in stevia (Stevia rebaudiana Bertoni). Acta Physiol. Plant. 2014, 36, 3243–3248. [Google Scholar] [CrossRef]
- De Oliveira, B.H.; Dos Santos, M.C.; Leal, P.C. Biotransformation of the diperpenoid, isosteviol, by Aspergillus niger, Penicillium chrysogenum and Rhizopus arrhizus. Phytochemistry 1999, 51, 737–741. [Google Scholar] [CrossRef]
- Lin, C.L.; Lin, S.J.; Huang, W.J.; Ku, Y.L.; Tsai, T.H.; Hsu, F.L. Novel ent-beyeran-19-oic acids from biotransformations of isosteviol metabolites by Mortierella isabellina. Planta Med. 2007, 73, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, B.H.; Strapasson, R.A. Biotransformation of isosteviol by Fusarium verticilloides. Phytochemistry 1996, 43, 393–395. [Google Scholar] [CrossRef]
- Parkinson, A.; Ogilvie, B.W.; Paris, B.L.; Hensley, T.N.; Loewen, G.J. Human Biotransformation. Biotransformation and Metabolite Elucidation of Xenobiotics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 1–77. ISBN 9780470504789. [Google Scholar]
- Parkinson, A.; Ogilvie, B.W.; Buckley, D.B.; Kazmi, F.; Czerwinski, M.; Parkinson, O. Biotransformation of Xenobiotics. In Casarett and Doull’s Toxicology: The Basic Science of Poisons, 8th ed.; McGraw-Hill Education: New York, NY, USA, 2013. [Google Scholar]
- De Oliveira, A.; Adams, S.D.; Lee, L.H.; Murray, S.R.; Hsu, S.D.; Hammond, J.R.; Dickinson, D.; Chen, P.; Chu, T.C. Inhibition of herpes simplex virus type 1 with the modified green tea polyphenol palmitoyl-epigallocatechin gallate. Food Chem. Toxicol. 2013, 52, 207–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, M.S.; Hanson, J.R.; de Oliveira, B.H. The biotransformation of some ent-beyeran-19-oic acids by Gibberella fujikuroi. Phytochemistry 1992, 31, 507–510. [Google Scholar] [CrossRef]
- Wonganan, O.; Tocharus, C.; Puedsing, C.; Homvisasevongsa, S.; Sukcharoen, O.; Suksamrarn, A. Potent vasorelaxant analogs from chemical modification and biotransformation of isosteviol. Eur. J. Med. Chem. 2013, 62, 771–776. [Google Scholar] [CrossRef]
- Milagre, H.M.S.; Martins, L.R.; Takahashi, J.A. Novel agents for enzymatic and fungal hydrolysis of stevioside. Braz. J. Microbiol. 2009, 40, 367–372. [Google Scholar] [CrossRef] [Green Version]
- Hershenhorn, J.; Zohar, M.; Crammer, B.; Ziv, Z.; Weinstein, V.; Kleifeld, Y.; Lavan, Y.; Ikan, R. Plant-growth regulators derived from the sweetener stevioside. Plant Growth Regul. 1997. [Google Scholar] [CrossRef]
- Nevmerzhitskaya, Y.Y.; Timofeeva, O.A.; Mikhaylov, A.L.; Strobykina, A.S.; Strobykina, I.Y.; Mironov, V.F. Stevioside increases the resistance of winter wheat to low temperatures and heavy metals. Dokl. Biol. Sci. 2013, 452, 287–290. [Google Scholar] [CrossRef]
- de Oliveira, B.H.; Stiirmer, J.C.; de Souza Filho, J.D.; Ayub, R.A. Plant growth regulation activity of steviol and derivatives. Phytochemistry 2008, 69. [Google Scholar] [CrossRef]
- Fribert, P.; Paulová, L.; Patáková, P.; Rychtera, M.; Melzoch, K. Alternativní metody separace kapalných biopaliv z média při fermentaci. Chem. Listy 2013, 107, 843–847. [Google Scholar] [CrossRef]
- Asia, S.; Asia, S. All cancers. Int. Agency Res. Cancer 2018, 876, 1–2. [Google Scholar] [CrossRef]
- Stats, F. Globocan 2008. Stat 2008, 1–8. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2018. [Google Scholar] [CrossRef] [PubMed]
- Moons, N.; De Borggraeve, W.; Dehaen, W. Isosteviol as a Starting Material in Organic Synthesis. Curr. Org. Chem. 2011, 15, 2731–2741. [Google Scholar] [CrossRef]
- Badshah, S.L.; Mabkhot, Y. Arresting kinase suppressor of Ras in an inactive state. Chin. J. Cancer 2017, 36, 5. [Google Scholar] [CrossRef] [PubMed]
- Vasas, A.; Rédei, D.; Csupor, D.; Molnár, J.; Hohmann, J. Diterpenes from European Euphorbia species serving as prototypes for natural-product-based drug discovery. Eur. J. Org. Chem. 2012, 5115–5130. [Google Scholar] [CrossRef]
- Altmann, K.-H.; Gertsch, J.J. Anticancer drugs from nature-natural products as a unique source of new microtubule-stabilizing agents. Nat. Prod. Rep. 2007, 24, 327–357. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Shi, Q.W.; Dong, M.; Kiyota, H.; Gu, Y.C.; Cong, B. Natural taxanes: Developments since 1828. Chem. Rev. 2011, 111, 7652–7709. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Wu, J.; Shi, L.; Wang, K.; Zhou, B.; Tang, Y.; Zhang, D.; Wu, Y.; Hua, W.; Wu, X. Synthesis and evaluation of cytotoxic effects of novel α-methylenelactone tetracyclic diterpenoids. Bioorg. Med. Chem. Lett. 2012, 22, 1922–1925. [Google Scholar] [CrossRef] [PubMed]
- Nofal, Z.M.; Srour, A.M.; El-Eraky, W.I.; Saleh, D.O.; Girgis, A.S. Rational design, synthesis and QSAR study of vasorelaxant active 3-pyridinecarbonitriles incorporating 1H-benzimidazol-2-yl function. Eur. J. Med. Chem. 2013, 63, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Prachayasittikul, V.; Pingaew, R.; Worachartcheewan, A.; Nantasenamat, C.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Synthesis, anticancer activity and QSAR study of 1,4-naphthoquinone derivatives. Eur. J. Med. Chem. 2014, 84, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Hari Narayana Moorthy, N.S.; Ramos, M.J.; Fernandes, P.A. QSAR analysis of isosteviol derivatives as α-glucosidase inhibitors with element count and other descriptors. Lett. Drug Des. Discov. 2011, 8. [Google Scholar] [CrossRef]
- Shah, B.A.; Kumar, A.; Gupta, P.; Sharma, M.; Sethi, V.K.; Saxena, A.K.; Singh, J.; Qazi, G.N.; Taneja, S.C. Cytotoxic and apoptotic activities of novel amino analogues of boswellic acids. Bioorg. Med. Chem. Lett. 2007, 17, 6411–6416. [Google Scholar] [CrossRef]
- Zhang, T.; Lu, L.H.; Liu, H.; Wang, J.W.; Wang, R.X.; Zhang, Y.X.; Tao, J.C. D-ring modified novel isosteviol derivatives: Design, synthesis and cytotoxic activity evaluation. Bioorg. Med. Chem. Lett. 2012, 22, 5827–5832. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhou, J.; Zhang, T.; Zhu, H.; Qian, H.; Zhang, H.; Huang, W.; Gust, R. Design and synthesis of thiourea derivatives containing a benzo[5,6]cyclohepta[1,2-b]pyridine moiety as potential antitumor and anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2012, 22, 2701–2704. [Google Scholar] [CrossRef] [PubMed]
- Manjula, S.N.; Malleshappa Noolvi, N.; Vipan Parihar, K.; Manohara Reddy, S.A.; Ramani, V.; Gadad, A.K.; Singh, G.; Gopalan Kutty, N.; Mallikarjuna Rao, C. Synthesis and antitumor activity of optically active thiourea and their 2-aminobenzothiazole derivatives: A novel class of anticancer agents. Eur. J. Med. Chem. 2009, 44, 2923–2929. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, T.; Yu, S.; Dai, X.; Wu, Y.; Tao, J. Synthesis, cytotoxic activity, and 2D- and 3D-QSAR studies of 19-carboxyl-modified novel isosteviol derivatives as potential anticancer agents. Chem. Biol. Drug Des. 2017, 89, 870–887. [Google Scholar] [CrossRef]
- Zhu, S.L.; Wu, Y.; Liu, C.J.; Wei, C.Y.; Tao, J.C.; Liu, H.M. Synthesis and in vitro cytotoxic activity evaluation of novel heterocycle bridged carbothioamide type isosteviol derivatives as antitumor agents. Bioorg. Med. Chem. Lett. 2013, 23, 1343–1346. [Google Scholar] [CrossRef]
- Chaaban, I.; El-Khawass, E.-S.; Mahran, M.; El-Sayed, O.; El-Saidi, H.; Aboul-Enen, H. Design, synthesis, and in vitro evaluation of cytotoxic activity of new substituted 1,4-benzoquinones and hydroquinones. Med. Chem. Res. 2007, 16. [Google Scholar] [CrossRef]
- Dewang, P.M.; Kim, D.-K. Synthesis and biological evaluation of 2-pyridyl-substituted pyrazoles and imidazoles as transforming growth factor-β type 1 receptor kinase inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 4228–4232. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.R.; Elmegeed, G.A.; Abd-Elmalek, H.A.; Younis, M. Synthesis of biologically active steroid derivatives by the utility of Lawesson’s reagent. Steroids 2005, 70, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Abdelhalim, M.M.; Kamel, E.M.; Rabie, S.T.; Mohamed, N.R. Synthesis and biological evaluation of some nitrogen containing steroidal heterocycles. Steroids 2011, 76, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.H.; Krishnaiah, M.; Sreenu, D.; Subrahmanyam, V.B.; Rao, K.S.; Mohan, A.V.N.; Park, C.-Y.; Son, J.-Y.; Sheen, Y.Y.; Kim, D.-K. Synthesis and biological evaluation of 1-substituted-3-(6-methylpyridin-2-yl)-4-([1,2,4]triazolo[1,5-a]pyridin-6-yl)pyrazoles as transforming growth factor-β type 1 receptor kinase inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 6049–6053. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.H.; Krishnaiah, M.; Sreenu, D.; Rao, K.S.; Subrahmanyam, V.B.; Park, C.-Y.; Son, J.-Y.; Sheen, Y.Y.; Kim, D.-K. Synthesis and biological evaluation of 1-substituted-3(5)-(6-methylpyridin-2-yl)-4-(quinolin-6-yl)pyrazoles as transforming growth factor-β type 1 receptor kinase inhibitors. Bioorg. Med. Chem. 2011, 19, 2633–2640. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Dai, G.F.; Yang, J.H.; Zhang, Y.X.; Zhu, Y.; Tao, J.C. Stereoselective synthesis of 15- and 16-substituted isosteviol derivatives and their cytotoxic activities. Bioorg. Med. Chem. Lett. 2009, 19, 1818–1821. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.L.; Wu, Y.; Liu, C.J.; Wei, C.Y.; Tao, J.C.; Liu, H.M. Design and stereoselective synthesis of novel isosteviol-fused pyrazolines and pyrazoles as potential anticancer agents. Eur. J. Med. Chem. 2013, 65, 70–82. [Google Scholar] [CrossRef]
- Chen, J.M.; Zhang, J.; Xia, Y.M.; Wang, X.X.; Li, J. The natural sweetener metabolite steviol inhibits the proliferation of human osteosarcoma U2OS cell line. Oncol. Lett. 2018. [Google Scholar] [CrossRef] [Green Version]
- Zou, M.; Yu, S.S.; Wang, K.; Zhang, D.Y.; Wu, X.M.; Hua, W.Y. Glycosylation of ent-kaurene derivatives and an evaluation of their cytotoxic activities. Chin. J. Nat. Med. 2013. [Google Scholar] [CrossRef]
- Garifullin, B.F.; Andreeva, O.V.; Strobykina, I.Y.; Babaev, V.M.; Kataev, V.E. Macrocyclic derivatives of diterpenoid isosteviol with hydrazide and hydrazone moieties. Macroheterocycles 2013, 6, 184–191. [Google Scholar] [CrossRef]
- Wang, T.T.; Liu, Y.; Chen, L. Synthesis and cytotoxic activity of nitric oxide-releasing isosteviol derivatives. Bioorg. Med. Chem. Lett. 2014, 24, 2198–2201. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, T.; Ling, Y.; Bao, N.; Shi, W.; Chen, L.; Sun, J. Design, synthesis and cytotoxic evaluation of nitric oxide-releasing derivatives of isosteviol. Chem. Biol. Drug Des. 2017, 90, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Ukiya, M.; Sawada, S.; Kikuchi, T.; Kushi, Y.; Fukatsu, M.; Akihisa, T. Cytotoxic and apoptosis-inducing activities of steviol and isosteviol derivatives against human cancer cell lines. Chem. Biodivers. 2013, 10, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, O.V.; Sharipova, R.R.; Strobykina, I.Y.; Kravchenko, M.A.; Strobykina, A.S.; Voloshina, A.D.; Musin, R.Z.; Kataeva, V.E. Development of synthetic approaches to macrocyclic glycoterpenoids on the basis of glucuronic acid and diterpenoid isosteviol. Russ. J. Org. Chem. 2015, 51. [Google Scholar] [CrossRef]
- Malki, A.; Laha, R.; Bergmeier, S.C. Synthesis and cytotoxic activity of MOM-ether analogs of isosteviol. Bioorg. Med. Chem. Lett. 2014, 24, 1184–1187. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-H.; Lee, L.-W.; Sheu, S.-Y.; Lin, P.-Y. Study on the stevioside analogues of steviolbioside, steviol, and isosteviol 19-alkyl amide dimers: synthesis and cytotoxic and antibacterial activity. Chem. Pharm. Bull. (Tokyo) 2004, 52, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Xiao, Q.; Pan, J.Y.; Wu, J. Natural products from semi-mangrove flora: Source, chemistry and bioactivities. Nat. Prod. Rep. 2009, 26, 281–298. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, Q.; Xu, J.; Li, M.Y.; Pan, J.Y.; Yang, M. Natural products from true mangrove flora: source, chemistry and bioactivities. Nat. Prod. Rep. 2008, 5. [Google Scholar] [CrossRef]
- Alijani, H.Q.; Pourseyedi, S.; Torkzadeh Mahani, M.; Khatami, M. Green synthesis of zinc sulfide (ZnS) nanoparticles using Stevia rebaudiana Bertoni and evaluation of its cytotoxic properties. J. Mol. Struct. 2019, 1175, 214–218. [Google Scholar] [CrossRef]
- Lange, S.S.; Takata, K.I.; Wood, R.D. DNA polymerases and cancer. Nat. Rev. Cancer 2011, 11, 96–110. [Google Scholar] [CrossRef] [Green Version]
- Stewart, L.; Redinbo, M.R.; Qiu, X.; Hol, W.G.; Champoux, J.J. A model for the mechanism of human topoisomerase I. Science 1998, 279, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Champoux, J.J. DNA topoisomerases: structure, function, and mechanism. Annu. Rev. Biochem. 2001, 70, 369–413. [Google Scholar] [CrossRef] [PubMed]
- Nitiss, J.L. Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer 2009, 9, 338–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitiss, J.L.; Soans, E.; Rogojina, A.; Seth, A.; Mishina, M. Topoisomerase assays. Curr. Protoc. Pharmacol. 2012. [Google Scholar] [CrossRef]
- Akihisa, T.; Kikuchi, T.; Nagai, H.; Ishii, K.; Tabata, K.; Suzuki, T. 4-Hydroxyderricin from Angelica keiskei roots induces caspase-dependent apoptotic cell death in HL60 human leukemia cells. J. Oleo Sci. 2011, 60, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Badshah, S.L.; Muska, M.; Ahmad, N.; Khan, K. The current case of quinolones: Synthetic approaches and antibacterial activity. Molecules 2016, 21, 268. [Google Scholar] [CrossRef] [PubMed]
- Badshah, S.L.; Ullah, A. New developments in non-quinolone-based antibiotics for the inhibition of bacterial gyrase and topoisomerase IV. Eur. J. Med. Chem. 2018, 152, 393–400. [Google Scholar] [CrossRef]
- Mizushina, Y.; Akihisa, T.; Ukiya, M.; Hamasaki, Y.; Murakami-Nakai, C.; Kuriyama, I.; Takeuchi, T.; Sugawara, F.; Yoshida, H. Structural analysis of isosteviol and related compounds as DNA polymerase and DNA topoisomerase inhibitors. Life Sci. 2005, 77, 2127–2140. [Google Scholar] [CrossRef]
- Lavanchy, D. Worldwide epidemiology of HBV infection, disease burden, and vaccine prevention. J. Clin. Virol. 2005. [Google Scholar] [CrossRef]
- Kane, A.; Lloyd, J.; Zaffran, M.; Simonsen, L.; Kane, M. Transmission of hepatitis B, hepatitis C and human immunodeficiency viruses through unsafe injections in the developing world: Model-based regional estimates. Bull. World Health Organ. 1999, 77, 801–807. [Google Scholar] [CrossRef]
- Simonsen, L.; Kane, A.; Lloyd, J.; Zaffran, M.; Kane, M. Unsafe injections in the developing world and transmission of bloodborne pathogens: A review. Bull. World Health Organ. 1999. [Google Scholar] [CrossRef]
- Shepard, C.W.; Simard, E.P.; Finelli, L.; Fiore, A.E.; Bell, B.P. Hepatitis B Virus Infection: Epidemiology and Vaccination. Epidemiol. Rev. 2006. [Google Scholar] [CrossRef] [PubMed]
- Gish, R.G. Current treatment and future directions in the management of chronic hepatitis B viral infection. Clin. Liver Dis. 2005. [Google Scholar] [CrossRef] [PubMed]
- Iavarone, M.; Colombo, M. Management of hepatocellular carcinoma. In Viral Hepatitis, 4th ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; ISBN 9781118637272. [Google Scholar]
- Liu, C.J.; Yu, S.L.; Liu, Y.P.; Dai, X.J.; Wu, Y.; Li, R.J.; Tao, J.C. Synthesis, cytotoxic activity evaluation and HQSAR study of novel isosteviol derivatives as potential anticancer agents. Eur. J. Med. Chem. 2016, 115, 26–40. [Google Scholar] [CrossRef]
- Huang, T.J.; Chou, B.-H.; Lin, C.-W.; Weng, J.H.; Chou, C.-H.; Yang, L.M.; Lin, S.-J. Synthesis and antiviral effects of isosteviol-derived analogues against the hepatitis B virus. Phytochemistry 2014, 99, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.J.; Yang, C.L.; Kuo, Y.C.; Chang, Y.C.; Yang, L.M.; Chou, B.H.; Lin, S.J. Synthesis and anti-hepatitis B virus activity of C4 amide-substituted isosteviol derivatives. Bioorg. Med. Chem. 2015, 23, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Shibukawa, K.; Hamada, Y.; Kuruma, T.; Kawabata, A.; Masuyama, A. Syntheses of (−)-Tripterifordin and (−)-Neotripterifordin from Stevioside. J. Org. Chem. 2018, 83, 1606–1613. [Google Scholar] [CrossRef]
- Baliga, M.S.; Katiyar, S.K. Chemoprevention of photocarcinogenesis by selected dietary botanicals. Photochem. Photobiol. Sci. 2006. [Google Scholar] [CrossRef]
- Balunas, M.J.; Kinghorn, A.D. Drug discovery from medicinal plants. Life Sci. 2005, 78, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.; Generalov, R.; Pereira, M.D.C.; Peres, I.; Juzenas, P.; Coelho, M.A.N. Epigallocatechin gallate-loaded polysaccharide nanoparticles for prostate cancer chemoprevention. Nanomedicine (Lond). 2011, 6, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003. [Google Scholar] [CrossRef]
- Chatterjee, S.; Biswas, G.; Basu, S.K.; Acharya, K. Antineoplastic effect of mushrooms: A review. Aust. J. Crop Sci. 2011, 5, 904–911. [Google Scholar]
- Gupta, E.; Purwar, S.; Sundaram, S.; Rai, G.K. Nutritional and therapeutic values of Stevia rebaudiana: A review. Acad. J. 2013. [Google Scholar] [CrossRef]
- Chang, S.F.; Chou, B.H.; Yang, L.M.; Hsu, F.L.; Lin, W.K.; Ho, Y.; Lin, S.J. Microbial transformation of isosteviol oxime and the inhibitory effects on NF-κB and AP-1 activation in LPS-stimulated macrophages. Bioorg. Med. Chem. 2009, 17, 6348–6353. [Google Scholar] [CrossRef] [PubMed]
- Chou, B.H.; Yang, L.M.; Chang, S.F.; Hsu, F.L.; Lo, C.H.; Lin, W.K.; Wang, L.H.; Liu, P.C.; Lin, S.J. Fungal transformation of isosteviol lactone and its biological evaluation for inhibiting the AP-1 transcription factor. Phytochemistry 2009, 70, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Chou, B.H.; Yang, L.M.; Chang, S.F.; Hsu, F.L.; Lo, C.H.; Liaw, J.H.; Liu, P.C.; Lin, S.J. Microbial transformation of isosteviol lactone and evaluation of the transformation products on androgen response element. J. Nat. Prod. 2008, 71, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.F.; Yang, L.M.; Lo, C.H.; Liaw, J.H.; Wang, L.H.; Lin, S.J. Microbial transformation of isosteviol and bioactivities against the glucocorticoid/androgen response elements. J. Nat. Prod. 2008, 71, 87–92. [Google Scholar] [CrossRef]
- Akihisa, T.; Hamasaki, Y.; Tokuda, H.; Ukiya, M.; Kimura, Y.; Nishino, H. Microbial Transformation of Isosteviol and Inhibitory Effects on Epstein-Barr Virus Activation of the Transformation Products. J. Nat. Prod. 2004, 67, 407–410. [Google Scholar] [CrossRef]
- Sánchez-Osuna, M.; Cortés, P.; Barbé, J.; Erill, I. Origin of the Mobile Dihydro-Pteroate Synthase Gene Determining Sulfonamide Resistance in Clinical Isolates. Front. Microbiol. 2019, 9, 3332. [Google Scholar] [CrossRef]
- Mayer, C.; Janin, Y.L. Non-quinolone inhibitors of bacterial type IIA topoisomerases: A feat of bioisosterism. Chem. Rev. 2014, 114, 2313–2342. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, C.-J.; Liu, X.; Dai, G.-F.; Du, J.-Y.; Tao, J.-C. Stereoselective Synthesis, Characterization, and Antibacterial Activities of Novel Isosteviol Derivatives with D-Ring Modification. Helv. Chim. Acta 2010, 93, 2052–2069. [Google Scholar] [CrossRef]
- Korochkina, M.G.; Sharipova, R.R.; Strobykina, I.Y.; Lantsova, A.D.; Voloshina, A.D.; Kulik, N.V.; Zobov, V.V.; Kataev, V.E.; Mironov, V.F. Synthesis and antimicrobial and antifungal activity of derivatives of the diterpenoid isosteviol and the glycoside steviolbioside containing onium nitrogen atoms. Pharm. Chem. J. 2011, 44, 597–600. [Google Scholar] [CrossRef]
- Korochkina, M.G.; Babaev, V.M.; Strobykina, I.Y.; Voloshina, A.D.; Kulik, N.V.; Kataev, V.E. Synthesis and antimicrobial activity of several bis-quaternized ammonium derivatives of the diterpenoid isosteviol. Chem. Nat. Compd. 2012, 47, 914–917. [Google Scholar] [CrossRef]
- Garifullin, B.F.; Chestnova, R.V.; Mironov, V.F.; Kataev, V.E. Synthesis and antituberculosis activity of conjugates of the diterpenoid isosteviol and the drug dimephosphon. Chem. Nat. Compd. 2012, 48, 794–798. [Google Scholar] [CrossRef]
- Sharipova, R.R.; Andreeva, O.V.; Garifullin, B.F.; Strobykina, I.Y.; Strobykina, A.S.; Voloshina, A.D.; Kravchenko, M.A.; Kataev, V.E. Synthesis and Antimicrobial and Antituberculosis Activity of the First Conjugates of the Diterpenoid Isosteviol and D-Arabinofuranose. Chem. Nat. Compd. 2018, 54, 92–97. [Google Scholar] [CrossRef]
- Garifullin, B.F.; Strobykina, I.Y.; Mordovskoi, G.G.; Mironov, V.F.; Kataev, V.E. Synthesis and antituberculosis activity of derivatives of the diterpenoid isosteviol with azine, hydrazide, and hydrazone moieties. Chem. Nat. Compd. 2011, 47, 55–58. [Google Scholar] [CrossRef]
- Kataev, V.E.; Khaybullin, R.N.; Garifullin, B.F.; Sharipova, R.R. New Targets for Growth Inhibition of Mycobacterium tuberculosis: Why Do Natural Terpenoids Exhibit Antitubercular Activity? Russ. J. Bioorg. Chem. 2018. [Google Scholar] [CrossRef]
- Kataev, V.E.; Strobykina, I.Y.; Andreeva, O.V.; Garifullin, B.F.; Sharipova, R.R.; Mironov, V.F.; Chestnova, R.V. Synthesis and antituberculosis activity of derivatives of Stevia rebaudiana glycoside steviolbioside and diterpenoid isosteviol containing hydrazone, hydrazide, and pyridinoyl moieties. Russ. J. Bioorg. Chem. 2011, 37, 483–491. [Google Scholar] [CrossRef]
- Kataev, V.E.; Militsina, O.I.; Strobykina, I.Y.; Kovylyaeva, G.I.; Musin, R.Z.; Fedorova, O.V.; Rusinov, G.L.; Zueva, M.N.; Mordovskoi, G.G.; Tolstikov, A.G. Synthesis and anti-tuberculous activity of diesters based on isosteviol and dicarboxylic acids. Pharm. Chem. J. 2006, 40, 473–475. [Google Scholar] [CrossRef]
- Khaybullin, R.N.; Strobykina, I.Y.; Gubskaya, V.P.; Fazleeva, G.M.; Latypov, S.K.; Kataev, V.E. New malonate macrocycle bearing two isosteviol moieties and its adduct with fullerene C60. Mendeleev Commun. 2011, 21, 134–136. [Google Scholar] [CrossRef]
- Garifullin, B.F.; Strobykina, I.Y.; Sharipova, R.R.; Kravchenko, M.A.; Andreeva, O.V.; Bazanova, O.B.; Kataev, V.E. Synthesis and antituberculosis activity of the first macrocyclic glycoterpenoids comprising glucosamine and diterpenoid isosteviol. Carbohydr. Res. 2016, 431, 15–24. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Ordway, D.; Arroz, M.J.; Costa, J.; Delgado, R. Activity against Mycobacterium tuberculosis with concomitant induction of cellular immune responses by a tetraaza-macrocycle with acetate pendant arms. Res. Microbiol. 2001. [Google Scholar] [CrossRef]
- Fields, L.E.; Burt, V.L.; Cutler, J.A.; Hughes, J.; Roccella, E.J.; Sorlie, P. The burden of adult hypertension in the United States 1999 to 2000: A rising tide. Hypertension 2004. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, I.; Kotchen, T.A. Trends in Prevalence, Awareness, Treatment, and Control of Hypertension in the United States, 1988-2000. J. Am. Med. Assoc. 2003. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.; Hayes, P.C. Beta-blockers in portal hypertension: New developments and controversies. Liver Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Bankston, J.R.; Kass, R.S. Molecular determinants of local anesthetic action of beta-blocking drugs: Implications for therapeutic management of long QT syndrome variant 3. J. Mol. Cell. Cardiol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, M.; Pittman, Q.J. Nifedipine facilitates neurotransmitter release independently of calcium channels. Proc. Natl. Acad. Sci. USA 2003. [Google Scholar] [CrossRef]
- Shepherd, G. Treatment of poisoning caused by β-adrenergic and calcium-channel blockers. Am. J. Health Pharm. 2006. [Google Scholar] [CrossRef]
- Engebretsen, K.M.; Kaczmarek, K.M.; Morgan, J.; Holger, J.S. High-dose insulin therapy in beta-blocker and calcium channel-blocker poisoning. Clin. Toxicol. 2011. [Google Scholar] [CrossRef]
- Alomar, M.J. Factors affecting the development of adverse drug reactions (Review article). Saudi Pharm. J. 2014. [Google Scholar] [CrossRef]
- Weinstein, R.S.; Cole, S.; Knaster, H.B.; Dahlbert, T. Beta blocker overdose with propranolol and with atenolol. Ann. Emerg. Med. 1985. [Google Scholar] [CrossRef]
- Barron, T.I.; Connolly, R.M.; Sharp, L.; Bennett, K.; Visvanathan, K. Beta blockers and breast cancer mortality: A population-based study. J. Clin. Oncol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.-L.; Yang, H.-Y.; Chan, P.; Cheng, T.-H.; Liu, J.-C.; Hsu, F.-L.; Liu, I.-M.; Cheng, Y.-W.; Cheng, J.-T. Isosteviol as a potassium channel opener to lower intracellular calcium concentrations in cultured aortic smooth muscle cells. Planta Med. 2004, 70, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.T.; Quayle, J.M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am. J. Physiol. 1995, 268, 799–822. [Google Scholar] [CrossRef] [PubMed]
- Boucherat, O.; Chabot, S.; Antigny, F.; Perros, F.; Provencher, S.; Bonnet, S. Potassium channels in pulmonary arterial hypertension. Eur. Respiratory J. 2015, 46, 1167–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, K.L.; Chan, P.; Yang, H.Y.; Hsu, F.L.; Liu, I.M.; Cheng, Y.W.; Cheng, J.T. Isosteviol acts on potassium channels to relax isolated aortic strips of Wistar rat. Life Sci. 2004, 74, 2379–2387. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.C.; Kao, P.F.; Hsieh, M.H.; Chen, Y.J.; Chan, P. The antihypertensive effect of stevioside derivative isosteviol in spontaneously hypertensive rats. Acta Cardiol. Sin. 2001, 17, 133–140. [Google Scholar]
- Xu, D.; Zhang, S.; Foster, D.; Wang, J. The effects of isosteviol against myocardium injury induced by ischaemia-reperfusion in the isolated guinea pig heart. Clin. Exp. Pharmacol. Physiol. 2007, 34, 488–493. [Google Scholar] [CrossRef]
- Xu, D.; Du, W.; Zhao, L.; Davey, A.K.; Wang, J. The neuroprotective effects of isosteviol against focal cerebral ischemia injury induced by middle cerebral artery occlusion in rats. Planta Med. 2008, 74, 816–821. [Google Scholar] [CrossRef]
- Hu, H.; Sun, X.O.; Tian, F.; Zhang, H.; Liu, Q.; Tan, W. Neuroprotective effects of isosteviol sodium injection on acute focal cerebral ischemia in rats. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef]
- Jin, H.; Gerber, J.P.; Wang, J.; Ji, M.; Davey, A.K. Oral and i.v. pharmacokinetics of isosteviol in rats as assessed by a new sensitive LC-MS/MS method. J. Pharm. Biomed. Anal. 2008, 48, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.L.; Lin, J.W.; Liu, J.C.; Yang, H.Y.; Kao, P.F.; Chen, C.H.; Loh, S.H.; Chiu, W.T.; Cheng, T.H.; Lin, J.G.; et al. Antiproliferative effect of isosteviol on angiotensin-II-treated rat aortic smooth muscle cells. Pharmacology 2006, 76, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.J.; Kim, H.J.; Lee, Y.K.; Yang, H.S. Effects of 5-hydroxytryptamine on rocuronium-induced neuromuscular blockade in the rat phrenic nerve-hemidiaphragm preparation. Korean J. Anaesthesiol. 2007, 52, 438–442. [Google Scholar] [CrossRef]
- Wong, K.L.; Wu, K.C.; So, E.C.; Wu, R.S.C.; Cheng, T.H. The anti-oxidative effect of isosteviol on angiotensin-II-induced reactive oxygen species generation in hypertensive injury of aortic smooth muscle cells. Eur. J. Anaesthesiol. 2007, 24, 125. [Google Scholar] [CrossRef]
- Yang, L.M.; Chang, S.F.; Lin, W.K.; Chou, B.H.; Wang, L.H.; Liu, P.C.; Lin, S.J. Oxygenated compounds from the bioconversion of isostevic acid and their inhibition of TNF-α and COX-2 expressions in LPS-stimulated RAW264.7 cells. Phytochemistry 2012. [Google Scholar] [CrossRef]
- Dinarello, C.A. Anti-inflammatory Agents: Present and Future. Cell 2010. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Tovar, E.; Hernández-Aquino, E.; Casas-Grajales, S.B.; Montaño, L.D.; Galindo-Gómez, S.; Camacho, J.; Muriel, P. Stevia prevents acute and chronic liver injury induced by carbon tetrachloride by blocking oxidative stress through Nrf2 upregulation. Oxid. Med. Cell. Longev. 2018, 12. [Google Scholar] [CrossRef]
- Ramos-Tovar, E.; Flores-Beltrán, R.; Galindo-Gómez, S.; Vera-Aguilar, E.; Diaz-Ruiz, A.; Montes, S.; Camacho, J.; Tsutsumi, V.; Muriel, P. Stevia rebaudiana tea prevents experimental cirrhosis via regulation of NF-κB, Nrf2, transforming growth factor beta, Smad7, and hepatic stellate cell activation. Phyther. Res. 2018, 32, 2568–2576. [Google Scholar] [CrossRef]
- Ma, J.; Ma, Z.; Wang, J.; Milne, R.W.; Xu, D.; Davey, A.K.; Evans, A.M. Isosteviol reduces plasma glucose levels in the intravenous glucose tolerance test in Zucker diabetic fatty rats 322. Diabetes Obes. Metab. 2007, 9, 597–599. [Google Scholar] [CrossRef]
- Xu, D.; Xu, M.; Lin, L.; Rao, S.; Wang, J.; Davey, A.K. The effect of isosteviol on hyperglycemia and dyslipidemia induced by lipotoxicity in rats fed with high-fat emulsion. Life Sci. 2012, 90, 30–38. [Google Scholar] [CrossRef]
- Nordentoft, I.; Jeppesen, P.B.; Hong, J.; Abudula, R.; Hermansen, K. Isosteviol increases insulin sensitivity and changes gene expression of key insulin regulatory genes and transcription factors in islets of the diabetic KKAy mouse. Diabetes Obes. MeTab. 2008, 10, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Bertram, H.C.; Jeppesen, P.B.; Hermansen, K. An NMR-based metabonomic investigation on effects of supplementation with isosteviol or soy protein to diabetic KKAy mice. Diabetes Obes. MeTab. 2009, 11, 992–995. [Google Scholar] [CrossRef] [PubMed]
- Moons, N.; De Borggraeve, W.; Dehaen, W. Stevioside and Steviol as Starting Materials in Organic Synthesis. Curr. Org. Chem. 2012, 16, 1986–1995. [Google Scholar] [CrossRef]
- Dinh Ngoc, T.; Moons, N.; Kim, Y.; De Borggraeve, W.; Mashentseva, A.; Andrei, G.; Snoeck, R.; Balzarini, J.; Dehaen, W. Synthesis of triterpenoid triazine derivatives from allobetulone and betulonic acid with biological activities. Bioorg. Med. Chem. 2014. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.C.; Philipson, L.H. Update on Diabetes Classification. Med. Clin. North Am. 2015, 99, 1–16. [Google Scholar] [CrossRef] [PubMed]
- El-Mesallamy, A.; Mahmoud, S.A.; Elazab, K.M.; Hussein, S.A.M.; Hussein, A.M. Attenuation of metabolic dysfunctions in the skeletal muscles of type 1 diabetic rats by Stevia rebaudiana extracts, via AMPK upregulation and antioxidant activities. Acta Sci. Pol. Technol. Aliment. 2018, 17, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Benalla, W.; Bellahcen, S.; Bnouham, M. Antidiabetic Medicinal Plants as a Source of Alpha Glucosidase Inhibitors. Curr. Diabetes Rev. 2010. [Google Scholar] [CrossRef]
- Van de Laar, F.A.; Lucassen, P.L.; Akkermans, R.P.; Van de Lisdonk, E.H.; Rutten, G.E.; Van Weel, C. Alpha-glucosidase inhibitors for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2005, 18. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, J.-H.; Dai, G.-F.; Liu, C.-J.; Tian, G.-Q.; Ma, W.-Y.; Tao, J.-C. Stereoselective synthesis of bioactive isosteviol derivatives as alpha-glucosidase inhibitors. Bioorg. Med. Chem. 2009, 17, 1464–1473. [Google Scholar] [CrossRef]
- An, Y.J.; Zhang, Y.X.; Wu, Y.; Liu, Z.M.; Pi, C.; Tao, J.C. Simple amphiphilic isosteviol-proline conjugates as chiral catalysts for the direct asymmetric aldol reaction in the presence of water. Tetrahedron Asymmetry 2010, 21, 688–694. [Google Scholar] [CrossRef]
- An, Y.J.; Wang, C.C.; Xu, Y.Z.; Wang, W.J.; Tao, J.C. Highly enantioselective α-aminoxylation reactions catalyzed by isosteviol-proline conjugates in buffered aqueous media. Catal. Lett. 2011, 141, 1123–1129. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Ma, Z.-W.; Li, Y.-X.; Tao, J.-C. New prolinamides with isosteviol skeleton as efficient organocatalysts for the direct asymmetric aldol reaction. Lett. Org. Chem. 2018. [Google Scholar] [CrossRef]
- An, Y.J.; Wang, C.C.; Liu, Z.P.; Tao, J.C. Isosteviol-proline conjugates as highly efficient amphiphilic organocatalysts for asymmetric three-component Mannich reactions in the presence of water. Helv. Chim. Acta 2012, 95, 43–51. [Google Scholar] [CrossRef]
- An, Y.; Qin, Q.; Wang, C.; Tao, J. Isosteviol-amino acid conjugates as highly efficient organocatalysts for the asymmetric one-pot three-component mannich reactions. Chin. J. Chem. 2011, 29, 1511–1517. [Google Scholar] [CrossRef]
- Ma, Z.W.; Liu, Y.X.; Huo, L.J.; Gao, X.; Tao, J.C. Doubly stereocontrolled asymmetric Michael addition of acetylacetone to nitroolefins promoted by an isosteviol-derived bifunctional thiourea. Tetrahedron Asymmetry 2012, 23, 443–448. [Google Scholar] [CrossRef]
- Song, Z.T.; Zhang, T.; Du, H.L.; Ma, Z.W.; Zhang, C.H.; Tao, J.C. Highly enantioselective michael addition promoted by a new diterpene-derived bifunctional thiourea catalyst: A doubly stereocontrolled approach to chiral succinimide derivatives. Chirality 2014. [Google Scholar] [CrossRef] [PubMed]
- Samadder, A.; Das, J.; Das, S.; Khuda-Bukhsh, A.R. Dihydroxy-isosteviol-methyl-ester, an active biological component of Pulsatilla nigricans, reduces arsenic induced cellular dysfunction in testis of male mice. Environ. Toxicol. Pharmacol. 2012, 34, 743–752. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, A.; Munir, S.; Mabkhot, Y.; Badshah, S.L. Bioactivity Profile of the Diterpene Isosteviol and its Derivatives. Molecules 2019, 24, 678. https://doi.org/10.3390/molecules24040678
Ullah A, Munir S, Mabkhot Y, Badshah SL. Bioactivity Profile of the Diterpene Isosteviol and its Derivatives. Molecules. 2019; 24(4):678. https://doi.org/10.3390/molecules24040678
Chicago/Turabian StyleUllah, Asad, Sidra Munir, Yahia Mabkhot, and Syed Lal Badshah. 2019. "Bioactivity Profile of the Diterpene Isosteviol and its Derivatives" Molecules 24, no. 4: 678. https://doi.org/10.3390/molecules24040678
APA StyleUllah, A., Munir, S., Mabkhot, Y., & Badshah, S. L. (2019). Bioactivity Profile of the Diterpene Isosteviol and its Derivatives. Molecules, 24(4), 678. https://doi.org/10.3390/molecules24040678






