Gas-Phase Fragmentation Reactions of Protonated Cystine using High-Resolution Tandem Mass Spectrometry
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. High-Resolution Tandem Mass Spectrometry (HR-MS/MS)
Author Contributions
Funding
Conflicts of Interest
References
- Talaty, E.R.; Young, S.M.; Dain, R.P.; Van Stipdonk, M.J. A study of fragmentation of protonated amides of some acylated amino acids by tandem mass spectrometry: Observation of an unusual nitrilium ion. Rapid Commun. Mass Spectrom. 2011, 25, 1119–1129. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Wang, B.; Wang, H.; Liu, J.; Li, Y.; Guo, X. Characteristic NH3 and CO losses from sodiated peptides C-terminated by glutamine residues. Rapid Commun. Mass Spectrom. 2017, 31, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Kotiaho, T.; Eberlin, M.N.; Vainiotalo, P.; Kostiainen, R. Electrospray mass and tandem mass spectrometry identification of ozone oxidation products of amino acids and small peptides. J. Am. Soc. Mass Spectrom. 2000, 11, 526–535. [Google Scholar] [CrossRef] [Green Version]
- Piraud, M.; Vianey-Saban, C.; Petritis, K.; Elfakir, C.; Steghens, J.-P.; Morla, A.; Bouchu, D. ESI-MS/MS analysis of underivatised amino acids: A new tool for the diagnosis of inherited disorders of amino acid metabolism. Fragmentation study of 79 molecules of biological interest in positive and negative ionisation mode. Rapid Commun. Mass Spectrom. 2003, 17, 1297–1311. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Long, Y.C. Crosstalk between cystine and glutathione is critical for the regulation of amino acid signaling pathways and ferroptosis. Sci. Rep. 2016, 6, 30033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, J.M.; Strobel, F.H.; Reed, M.; Pohl, J.; Jones, D.P. A rapid LC-FTMS method for analysis of cysteine, cystine and cysteine/cystine steady-stateredox potential in human plasma. Clin. Chim. Acta 2008, 396, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Wear, J.E.; Keevil, B.G. Measurement of Cystine in Urine by Liquid Chromatography–Tandem Mass Spectrometry. Clin. Chem. 2005, 51, 787–789. [Google Scholar] [CrossRef]
- Bose, N.; Zee, T.; Kapahi, P.; Stoller, M.L. Mass Spectrometry-based in vitro Assay to Identify Drugs that Influence Cystine Solubility. Bio-protocol 2017, 7, e2417. [Google Scholar] [CrossRef]
- Jamalpoor, A.; Sparidans, R.W.; Pou Casellas, C.; Rood, J.J.M.; Joshi, M.; Masereeuw, R.; Janssen, M.J. Quantification of cystine in human renal proximal tubule cells using liquid chromatography-tandem mass spectrometry. Biomed. Chromatogr. 2018, 32, e4238. [Google Scholar] [CrossRef] [Green Version]
- Piraud, M.; Vianey-Saban, C.; Bourdin, C.; Acquaviva-Bourdain, C.; Boyer, S.; Elfakir, C.; Bouchu, D. A new reversed-phase liquid chromatographic/tandem mass spectrometric method for analysis of underivatised amino acids: Evaluation for the diagnosis and the management of inherited disorders of amino acid metabolism. Rapid Commun. Mass Spectrom. 2005, 19, 3287–3297. [Google Scholar] [CrossRef]
- Zee, T.; Bose, N.; Zee, J.; Beck, J.N.; Yang, S.; Parihar, J.; Yang, M.; Damodar, S.; Hall, D.; O’Leary, M.N.; et al. α-Lipoic acid treatment prevents cystine urolithiasis in a mouse model of cystinuria. Nat. Med. 2017, 23, 288–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, Y.P.; Torrente, L.; Liu, M.; Asara, J.M.; Dibble, C.C.; DeNicola, G.M. Cysteine dioxygenase 1 is a metabolic liability for non-small cell lung cancer. bioRxiv 2018, 459602. [Google Scholar] [CrossRef]
- Choi, M.S.; Rehman, S.U.; Kim, I.S.; Park, H.-J.; Song, M.-Y.; Yoo, H.H. Development of a mixed-mode chromatography with tandem mass spectrometry method for the quantitative analysis of 23 underivatized amino acids in human serum. J. Pharm. Biomed. Anal. 2017, 145, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.; Siu, K.M.; Hopkinson, A.C. Transnitrosylation products of the dipeptide cysteinyl-cysteine: An examination by tandem mass spectrometry and density functional theory. Phys. Chem. Chem. Phys. 2016, 18, 6047–6052. [Google Scholar] [CrossRef] [PubMed]
- Lioe, H.; O’Hair, R.A.J. A novel salt bridge mechanism highlights the need for nonmobile proton conditions to promote disulfide bond cleavage in protonated peptides under low-energy collisional activation. J. Am. Soc. Mass Spectrom. 2007, 18, 1109–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lioe, H.; O’Hair, R.A.J. Comparison of collision-induced dissociation and electron-induced dissociation of singly protonated aromatic amino acids, cystine and related simple peptides using a hybrid linear ion trap-FT-ICR mass spectrometer. Anal. Bioanal. Chem. 2007, 389, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Clemen, M.; Gernert, C.; Peters, J.; Grotemeyer, J. Fragmentation reactions of labeled and untabeled Rhodamine B in a high-resolution Fourier transform ion cyclotron resonance mass spectrometer. Eur. J. Mass Spectrom. 2013, 19, 135–139. [Google Scholar] [CrossRef]
- Thurman, E.M.; Ferrer, I.; Benotti, M.; Heine, C.E. Intramolecular Isobaric Fragmentation: A Curiosity of Accurate Mass Analysis of Sulfadimethoxine in Pond Water. Anal. Chem. 2004, 76, 1228–1235. [Google Scholar] [CrossRef]
- Winkler, H.U.; Beckey, H.D. Field desorption mass spectrometry of amino acids. Org. Mass Spectrom. 1972, 6, 655–660. [Google Scholar] [CrossRef]
- Verbueken, A.H.; Van Grieken, R.E.; De Broe, M.E.; Wedeen, R.P. Identification of inorganic and organic microliths in kidney sections by laser microprobe mass spectrometry. Anal. Chim. Acta 1987, 195, 97–115. [Google Scholar] [CrossRef]
- Dookeran, N.N.; Yalcin, T.; Harrison, A.G. Fragmentation Reactions of Protonated α-Amino Acids. J. Mass Spectrom. 1996, 31, 500–508. [Google Scholar] [CrossRef]
- McGaw, E.A.; Phinney, K.W.; Lowenthal, M.S. Comparison of orthogonal liquid and gas chromatography-mass spectrometry platforms for the determination of amino acid concentrations in human plasma. J. Chromatogr. A 2010, 1217, 5822–5831. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
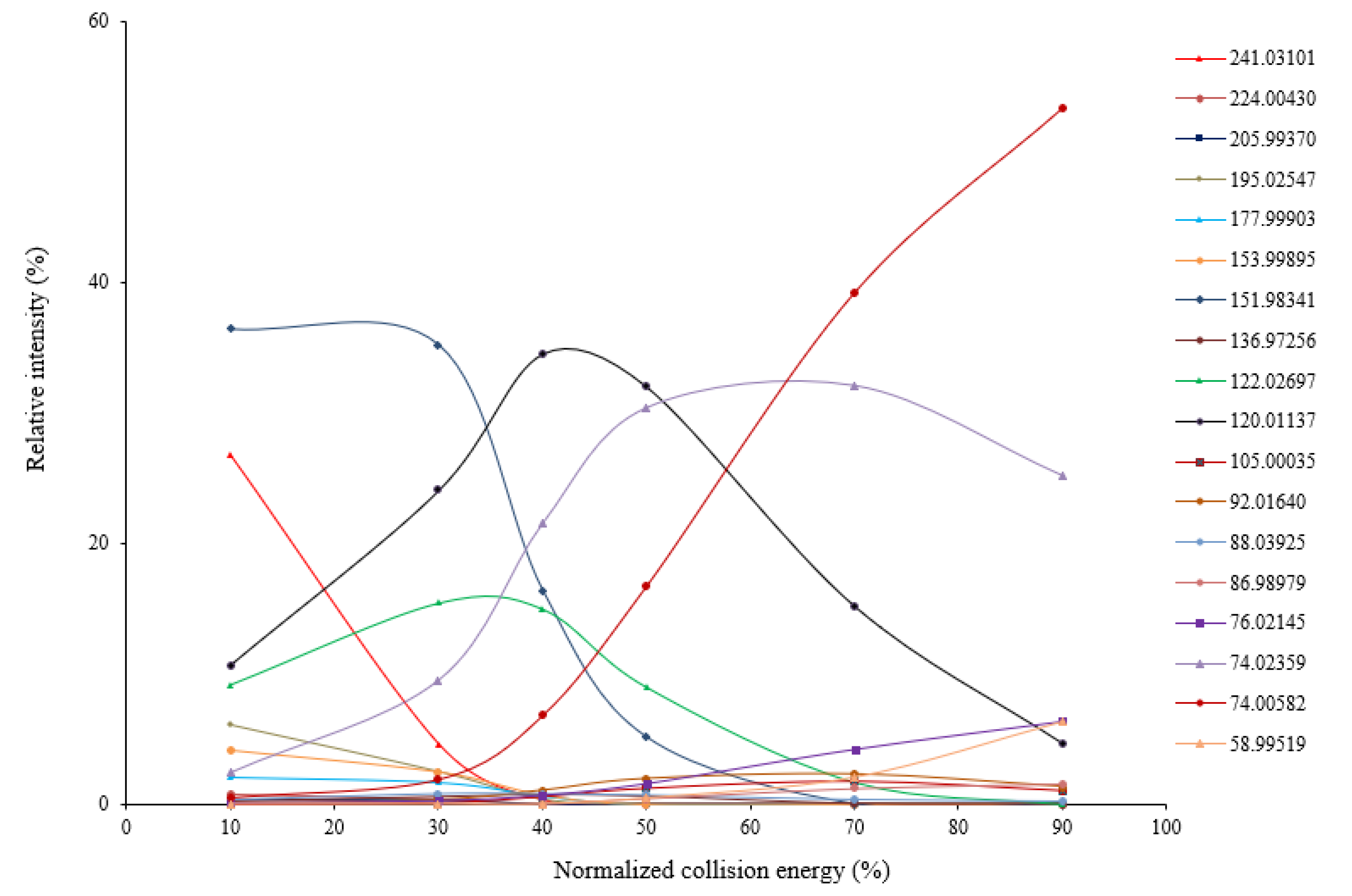


| Protonated Cystine (Theoretical m/z) | Observed m/z Values of Fragment Ions (n = 3) a | Proposed Chemical Identity | Theoretical m/z | Mass Error (ppm) | Reported in Previous Studies [Reference] c | |
|---|---|---|---|---|---|---|
| Mean | SEM | |||||
| Cystine (241.03113) | 224.00430 | 0.00007 | [M + H − NH3]+ | 224.00458 | −1.2 | CID [4,15,16], others [19] |
| 205.99370 | 0.00015 | [M + H − NH3 − H2O]+ | 205.99401 | −1.5 | unreported | |
| 195.02547 | 0.00006 | [M + H − H2O − CO]+ | 195.02565 | −0.9 | CID [4,14,15,16] | |
| 177.99903 | 0.00006 | [M + H − H2O − CO − NH3]+ | 177.99910 | −0.4 | CID [14,15,16] | |
| 153.99895 | 0.00007 | [M + H − C3H5NO2]+ | 153.99910 | −1.0 | CID [4,15,16] | |
| 151.98341 | 0.00005 | [M + H − C3H7NO2]+ | 151.98345 | −0.2 | CID [4,15,16], others [16,20] | |
| 136.97256 | 0.00006 | [M + H − C3H5NO2 − NH3]+ | 136.97255 | 0.1 | Unreported | |
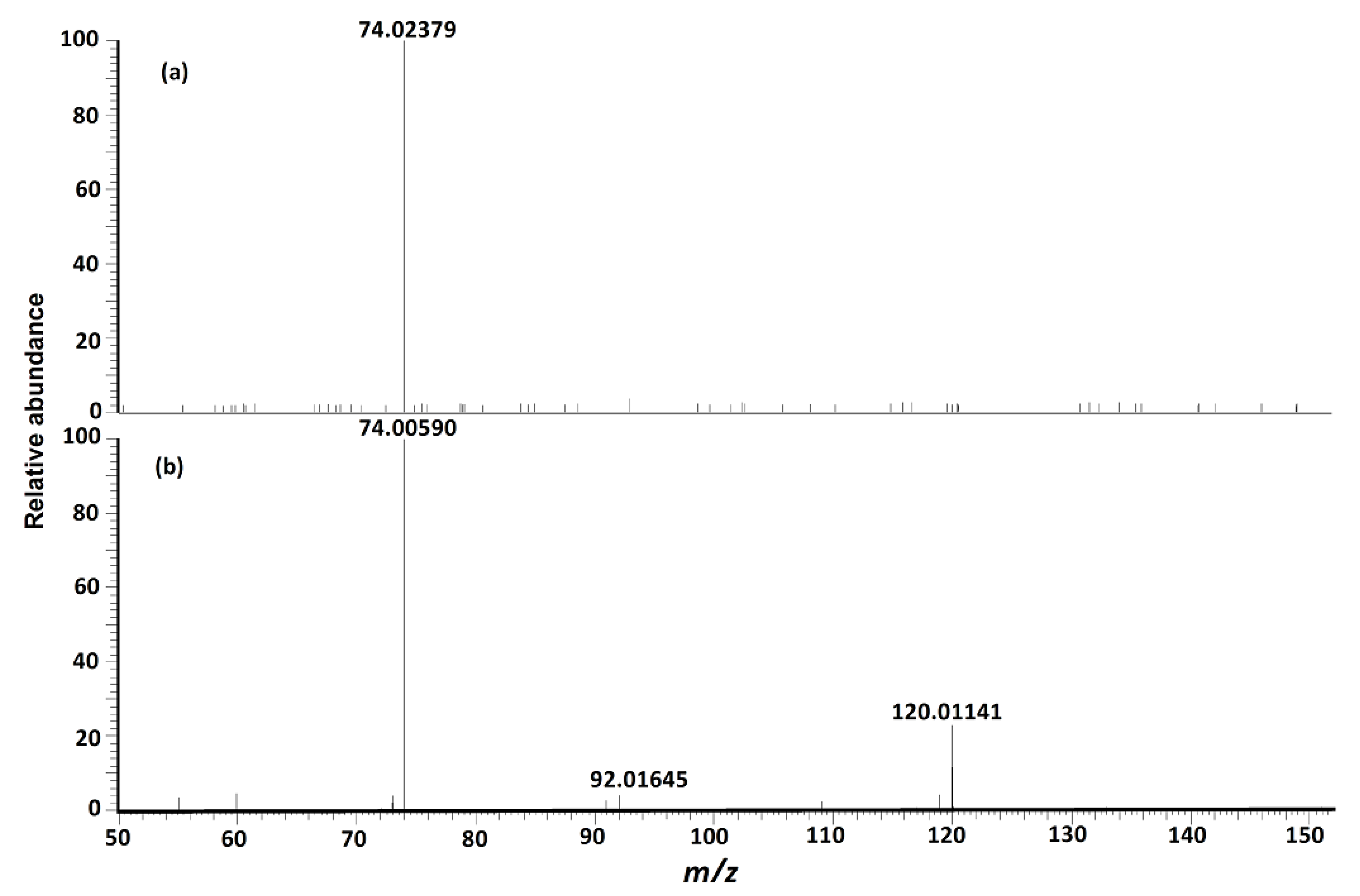
| 122.02697 | 0.00006 | [M + H − C3H5NO2S]+ | 122.02703 | −0.5 | CID [4,14,15,16], others [16,20] | |
| 120.01137 | 0.00006 | [M + H − C3H7NO2S]+ | 120.01138 | −0.1 | CID [4,15,16], others [16,20] | |
| 105.00035 | 0.00006 | [M + H − C3H5NO2S − NH3]+ | 105.00048 | −1.3 | unreported | |
| 92.01640 | 0.00005 | [M + H − C3H7NO2S − CO]+ | 92.01646 | −0.7 | unreported | |
| 88.03925 | 0.00004 | [M + H − C3H5NO2S − H2S]+ | 88.03930 | −0.6 | others [16,20] | |
| 86.98979 | 0.00005 | [M + H − C3H5NO2S − NH3 − H2O]+ | 86.98989 | −1.4 | unreported | |
| 76.02145 | 0.00003 | [M + H − C3H5NO2S − H2O − CO]+ | 76.02155 | −1.3 | unreported | |
| 74.02359 b | 0.00003 | [M + H − C3H7NO2 − CH2S2]+ | 74.02365 | −0.9 | CID [4], others [16,20] | |
| 74.00582 | 0.00003 | [M + H − C3H7NO2S − H2O − CO]+ | 74.00590 | −1.1 | unreported | |
| 58.99519 | 0.00002 | [M + H − C3H5NO2S − NH3 − H2O − CO]+ | 58.99500 | 3.2 | unreported | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Chan, W.; Ang, I.L.; Wei, R.; Lam, M.M.T.; Lei, K.M.K.; Poon, T.C.W. Gas-Phase Fragmentation Reactions of Protonated Cystine using High-Resolution Tandem Mass Spectrometry. Molecules 2019, 24, 747. https://doi.org/10.3390/molecules24040747
Zhang P, Chan W, Ang IL, Wei R, Lam MMT, Lei KMK, Poon TCW. Gas-Phase Fragmentation Reactions of Protonated Cystine using High-Resolution Tandem Mass Spectrometry. Molecules. 2019; 24(4):747. https://doi.org/10.3390/molecules24040747
Chicago/Turabian StyleZhang, Pengwei, Wan Chan, Irene L. Ang, Rui Wei, Melody M. T. Lam, Kate M. K. Lei, and Terence C. W. Poon. 2019. "Gas-Phase Fragmentation Reactions of Protonated Cystine using High-Resolution Tandem Mass Spectrometry" Molecules 24, no. 4: 747. https://doi.org/10.3390/molecules24040747
APA StyleZhang, P., Chan, W., Ang, I. L., Wei, R., Lam, M. M. T., Lei, K. M. K., & Poon, T. C. W. (2019). Gas-Phase Fragmentation Reactions of Protonated Cystine using High-Resolution Tandem Mass Spectrometry. Molecules, 24(4), 747. https://doi.org/10.3390/molecules24040747




