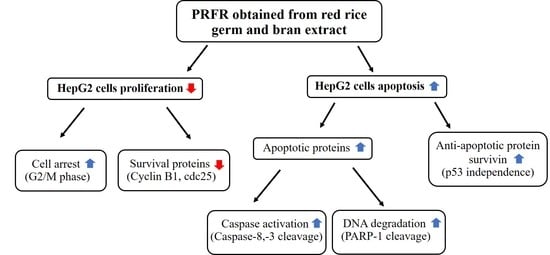
The Proanthocyanidin-Rich Fraction Obtained from Red Rice Germ and Bran Extract Induces HepG2 Hepatocellular Carcinoma Cell Apoptosis
Abstract
:1. Introduction
2. Results
2.1. Effect of PRFR on Hepatocellular Carcinoma cell Viability
2.2. Effect of PRFR on G2/M cell Cycle Arrest in HepG2 Cells
2.3. Effect of PRFR on cell Cycle Regulated Protein Expression in HepG2 Cells
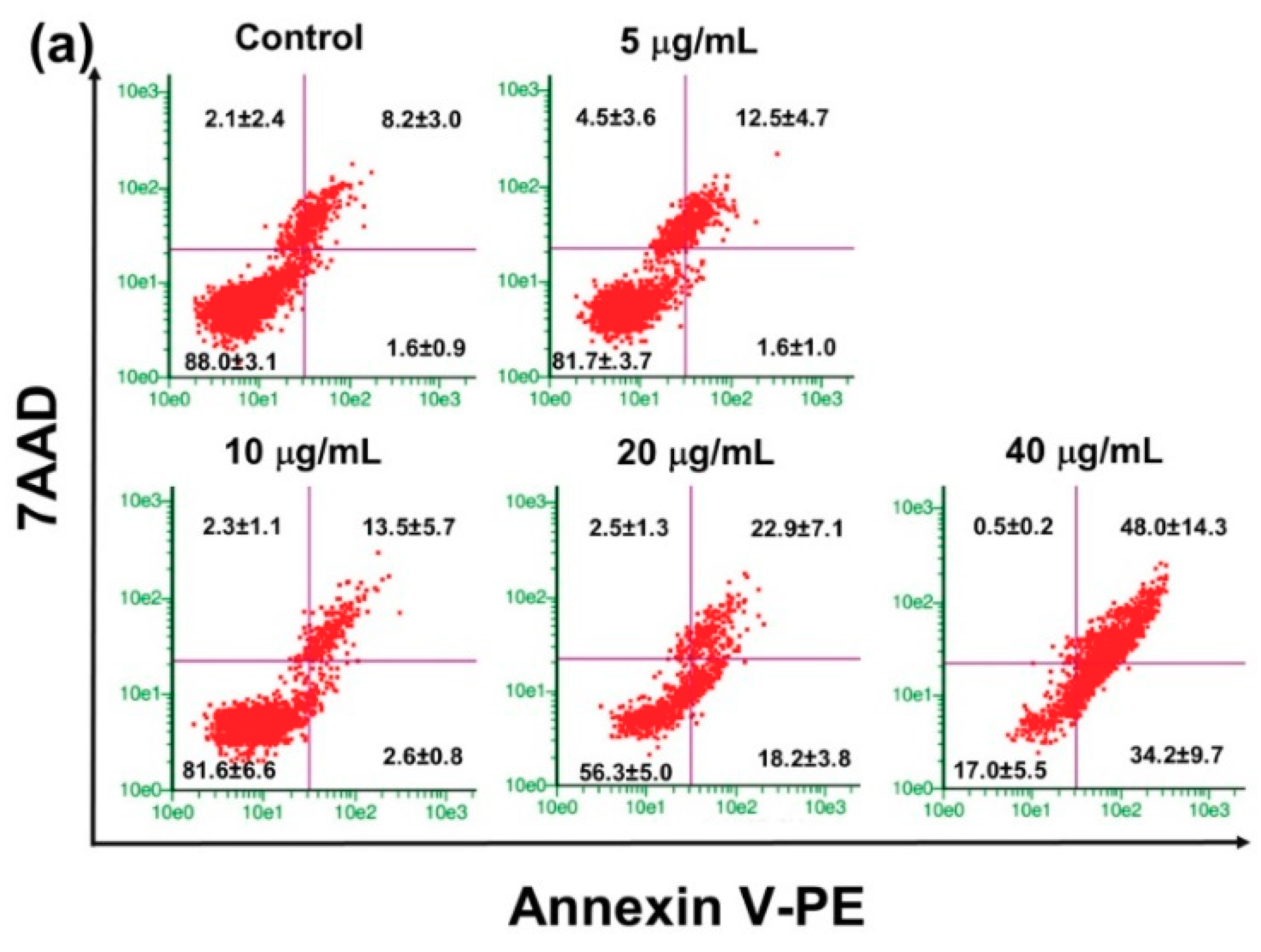
2.4. Effect of PRFR on HepG2 cell Apoptosis
2.5. Effect of PRFR on Apoptotic Proteins, Anti-Apoptotic Protein Survivin and Tumor Suppressor Protein p53 in HepG2 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of Proanthocyanidin Rich Fraction (PRFP) Derived from Red rice Germ and Bran
4.3. Quantification of Proanthocyanidin Content
4.4. Cell Cultures
4.5. Cell Viability Assay
4.6. Cell Cycle Assay
4.7. Apoptosis Assay
4.8. Western Blot Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goufo, P.; Trindade, H. Rice antioxidants: Phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, gamma-oryzanol, and phytic acid. Food Sci. Nutr. 2014, 2, 75–104. [Google Scholar] [CrossRef] [PubMed]
- Limtrakul, P.; Yodkeeree, S.; Pitchakarn, P.; Punfa, W. Suppression of Inflammatory Responses by Black Rice Extract in RAW 264.7 Macrophage Cells via Downregulation of NF-kB and AP-1 Signaling Pathways. Asian Pac. J. Cancer Prev. 2015, 16, 4277–4283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pintha, K.; Yodkeeree, S.; Pitchakarn, P.; Limtrakul, P. Anti-invasive activity against cancer cells of phytochemicals in red jasmine rice (Oryza sativa L.). Asian Pac. J. Cancer Prev. 2014, 15, 4601–4607. [Google Scholar] [CrossRef] [PubMed]
- Pintha, K.; Yodkeeree, S.; Limtrakul, P. Proanthocyanidin in red rice inhibits MDA-MB-231 breast cancer cell invasion via the expression control of invasive proteins. Biol. Pharm. Bull. 2015, 38, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Limtrakul, P.; Yodkeeree, S.; Pitchakarn, P.; Punfa, W. Anti-inflammatory effects of proanthocyanidin-rich red rice extract via suppression of MAPK, AP-1 and NF-kappaB pathways in Raw 264.7 macrophages. Nutr. Res. Pract. 2016, 10, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Gunaratne, A.; Wu, K.; Li, D.; Bentota, A.; Corke, H.; Cai, Y.Z. Antioxidant activity and nutritional quality of traditional red-grained rice varieties containing proanthocyanidins. Food Chem. 2013, 138, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D.; Bagchi, M.; Stohs, S.J.; Das, D.K.; Ray, S.D.; Kuszynski, C.A.; Joshi, S.S.; Pruess, H.G. Free radicals and grape seed proanthocyanidin extract: Importance in human health and disease prevention. Toxicology 2000, 148, 187–197. [Google Scholar] [CrossRef]
- Vayalil, P.K.; Mittal, A.; Katiyar, S.K. Proanthocyanidins from grape seeds inhibit expression of matrix metalloproteinases in human prostate carcinoma cells, which is associated with the inhibition of activation of MAPK and NF kappa B. Carcinogenesis 2004, 25, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Uchino, R.; Madhyastha, R.; Madhyastha, H.; Dhungana, S.; Nakajima, Y.; Omura, S.; Maruyama, M. NFkappaB-dependent regulation of urokinase plasminogen activator by proanthocyanidin-rich grape seed extract: Effect on invasion by prostate cancer cells. Blood Coagul. Fibrinolysis 2010, 21, 528–533. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Pan, Q.H.; Shi, Y.; Duan, C.Q. Chemical synthesis of proanthocyanidins in vitro and their reactions in aging wines. Molecules 2008, 13, 3007–3032. [Google Scholar] [CrossRef] [PubMed]
- Limtrakul, P.; Yodkeeree, S.; Punfa, W.; Srisomboon, J. Inhibition of the MAPK Signaling Pathway by Red Rice Extract in UVB-irradiated Human Skin Fibroblasts. Nat. Prod. Commun. 2016, 11, 1877–1882. [Google Scholar] [PubMed]
- Yodkeeree, S.; Thippraphan, P.; Punfa, W.; Srisomboon, J.; Limtrakul, P. Skin Anti-aging Assays of Proanthocyanidin Rich Red Rice Extract, Oryzanol and Other Phenolic Compounds. Nat. Prod. Commun. 2018, 13, 967–972. [Google Scholar]
- Tsim, N.C.; Frampton, A.E.; Habib, N.A.; Jiao, L.R. Surgical treatment for liver cancer. World J. Gastroenterol. 2010, 16, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.; Bilheem, S.; Chansaard, W.; Chitapanarux, I.; Daoprasert, K.; Khuanchana, S.; Leklob, A.; Pongnikorn, D.; Rozek, L.S.; Siriarechakul, S.; et al. National and Subnational Population-Based Incidence of Cancer in Thailand: Assessing Cancers with the Highest Burdens. Cancers 2017, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Srivatanakul, P. Epidemiology of Liver Cancer in Thailand. Asian Pac. J. Cancer Prev. 2001, 2, 117–121. [Google Scholar] [PubMed]
- Daher, S.; Massarwa, M.; Benson, A.A.; Khoury, T. Current and Future Treatment of Hepatocellular Carcinoma: An Updated Comprehensive Review. J. Clin. Transl. Hepatol. 2018, 6, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Sherman, M.; Llovet, J.M.; Beaugrand, M.; Lencioni, R.; Burroughs, A.K.; Christensen, E.; Pagliaro, L.; Colombo, L.; Rodés, J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J. Hepatol 2001, 35, 421–430. [Google Scholar] [CrossRef]
- Balogh, J.; Victor, D.; Asham, E.H.; Burroughs, S.G.; Boktour, M.; Saharia, A.; Li, X.; Ghobrial, R.M.; Monsour, H.P., Jr. Hepatocellular carcinoma: A review. J. Hepatocell. Carcinoma. 2016, 3, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Rodriguez, E.O.; Dia, V.P.; Yousef, G.G.; Garcia-Saucedo, P.A.; Lopez-Medina, J.; Paredes-Lopez, O.; de Mejia, E.G.; Lila, M.A. Inhibition of pro-inflammatory responses and antioxidant capacity of Mexican blackberry (Rubus spp.) extracts. J. Agric. Food Chem. 2010, 58, 9542–9548. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fan, M.; Candas, D.; Zhang, TQ.; Qin, L.; Eldridge, A.; Wachsmann-Hogiu, S.; Ahmed, K.M.; Chromy, B.A.; Nantajit, D.; et al. Cyclin B1/Cdk1 coordinates mitochondrial respiration for cell-cycle G2/M progression. Dev. Cell. 2014, 29, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Berry, L.D.; Gould, K.L. Regulation of Cdc2 activity by phosphorylation at T14/Y15. Prog. Cell Cycle Res. 1996, 2, 99–105. [Google Scholar] [PubMed]
- Nagata, S. Apoptosis and Clearance of Apoptotic Cells. Ann. Rev. Immunol. 2018, 36, 489–517. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Soldani, C.; Scovassi, A.I. Poly(ADP-ribose) polymerase-1 cleavage during apoptosis: An update. Apoptosis. 2002, 7, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, W.H.; Biade, S.; Zilfou, JT.; Chen, J.; Murphy, M. Transcriptional repression of the anti-apoptotic survivin gene by wild type p53. J. Biol. Chem. 2002, 277, 3247–3257. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.; McGuirk, M.; Hockenberry, T.N.; Wu, Q.; Ashar, H.; Black, S.; Wen, S.F.; Wang, L.; Kirschmeier, P.; Bishop, W.R.; et al. Human survivin is negatively regulated by wild-type p53 and participates in p53-dependent apoptotic pathway. Oncogene 2002, 21, 2613–2622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mita, A.C.; Mita, M.M.; Nawrocki, S.T.; Giles, F.J. Survivin: Key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin. Cancer Res. 2008, 14, 5000–5005. [Google Scholar] [CrossRef] [PubMed]
- Cressey, R.; Chotpadiwetkul, R.; Lertprasertsuke, N.; Kasinrerk, W. Expression of apoptosis inhibitor survivin: Common and independent of p53 aberration in Thai cancer patients. CMU J. Nat. Sci. 2007, 6, 185–194. [Google Scholar]
- Altieri, D.C. Survivin and apoptosis control. Adv. Cancer Res. 2003, 88, 31–52. [Google Scholar] [PubMed]
- Tamm, I.; Wang, Y.; Sausville, E.; Scudiero, D.A.; Vigna, N.; Oltersdorf, T.; Reed, J.C. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998, 58, 5315–5320. [Google Scholar] [PubMed]
- Lluís, L.; Muñoz, M.; Nogués, M.R.; Sánchez-Martos, V.; Romeu, M.; Giralt, M.; Valls, J.; Solà, R. Toxicology evaluation of a procyanidin-rich extract from grape skins and seeds. Food Chem. Toxicol. 2011, 49, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Yamakoshi, J.; Saito, M.; Kataoka, S.; Kikuchi, M.J.F.; Toxicology, C. Safety evaluation of proanthocyanidin-rich extract from grape seeds. Food Chem. Toxicol. 2002, 40, 599–607. [Google Scholar] [CrossRef]
- Sano, A. Safety assessment of 4-week oral intake of proanthocyanidin-rich grape seed extract in healthy subjects. Food Chem. Toxicol. 2017, 108, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Brooker, S.; Martin, S.; Pearson, A.; Bagchi, D.; Earl, J.; Gothard, L.; Hall, E.; Porter, L.; Yarnold, J. Double-blind, placebo-controlled, randomised phase II trial of IH636 grape seed proanthocyanidin extract (GSPE) in patients with radiation-induced breast induration. Radiother. Oncol. 2006, 79, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Herald, T.J.; Gadgil, P.; Perumal, R.; Bean, S.R.; Wilson, J.D. High-throughput micro-plate HCI-vanillin assay for screening tannin content in sorghum grain. J. Sci. Food Agric. 2014, 94, 2133–2136. [Google Scholar] [CrossRef] [PubMed]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Upanan, S.; Yodkeeree, S.; Thippraphan, P.; Punfa, W.; Wongpoomchai, R.; Limtrakul, P. The Proanthocyanidin-Rich Fraction Obtained from Red Rice Germ and Bran Extract Induces HepG2 Hepatocellular Carcinoma Cell Apoptosis. Molecules 2019, 24, 813. https://doi.org/10.3390/molecules24040813
Upanan S, Yodkeeree S, Thippraphan P, Punfa W, Wongpoomchai R, Limtrakul P. The Proanthocyanidin-Rich Fraction Obtained from Red Rice Germ and Bran Extract Induces HepG2 Hepatocellular Carcinoma Cell Apoptosis. Molecules. 2019; 24(4):813. https://doi.org/10.3390/molecules24040813
Chicago/Turabian StyleUpanan, Supranee, Supachai Yodkeeree, Pilaiporn Thippraphan, Wanisa Punfa, Rawiwan Wongpoomchai, and Pornngarm Limtrakul (Dejkriengkraikul). 2019. "The Proanthocyanidin-Rich Fraction Obtained from Red Rice Germ and Bran Extract Induces HepG2 Hepatocellular Carcinoma Cell Apoptosis" Molecules 24, no. 4: 813. https://doi.org/10.3390/molecules24040813