Influence of Silicodactyly in the Preparation of Hybrid Materials
Abstract
:1. Introduction
2. Results and Discussion
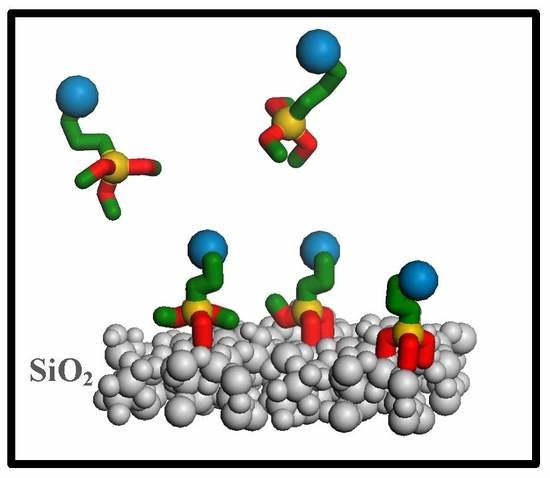
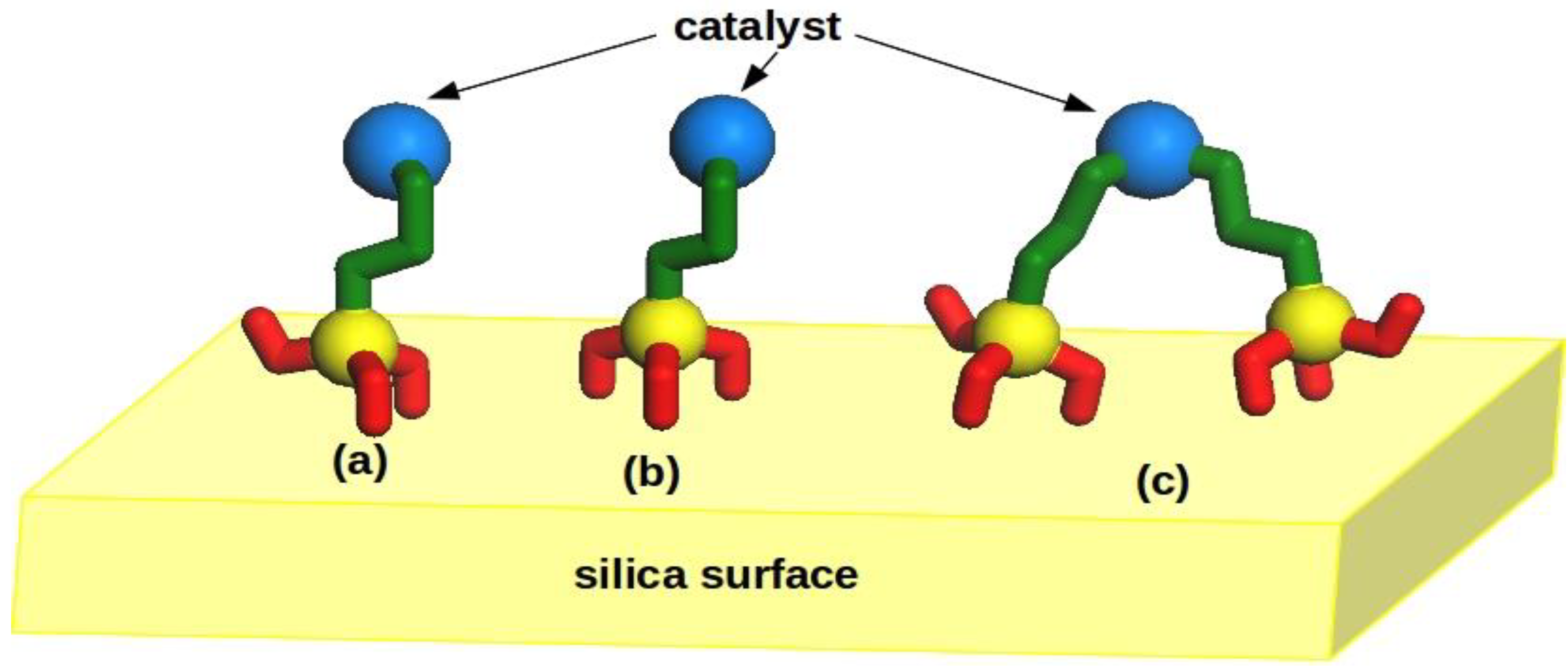
2.1. Hybrid Structures
2.2. Thermogravimetric Analysis
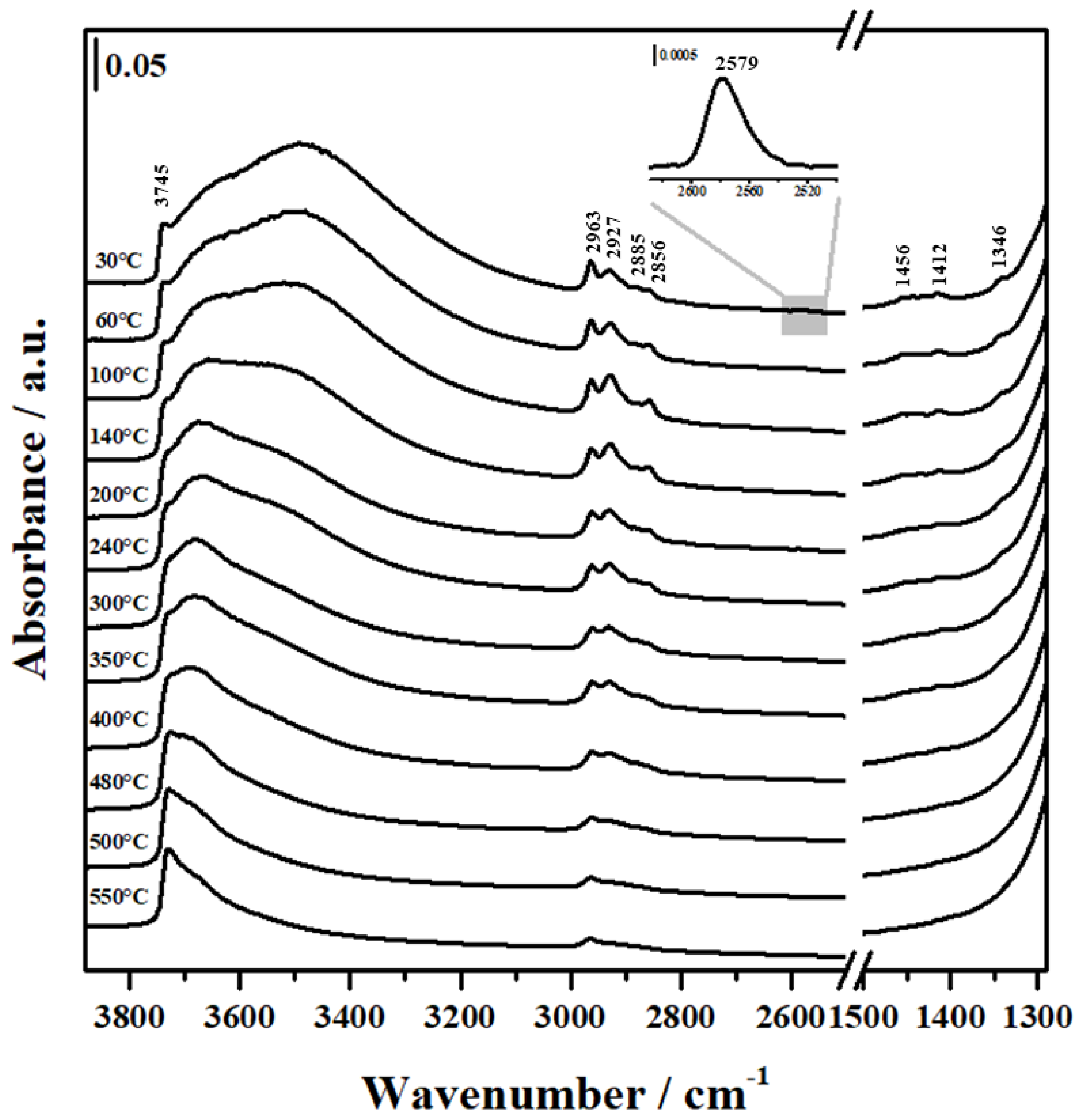
2.3. FTIR Analysis
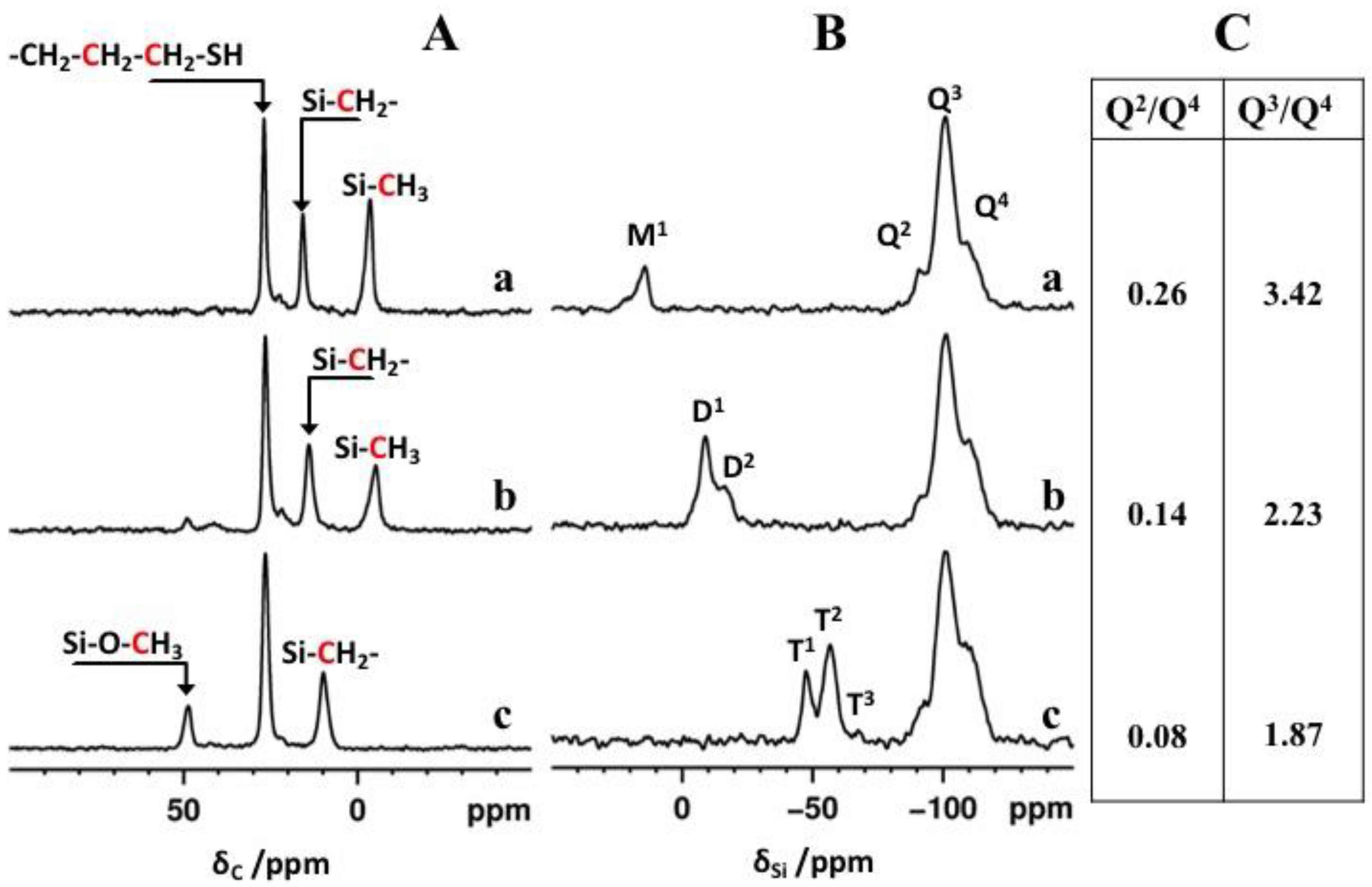
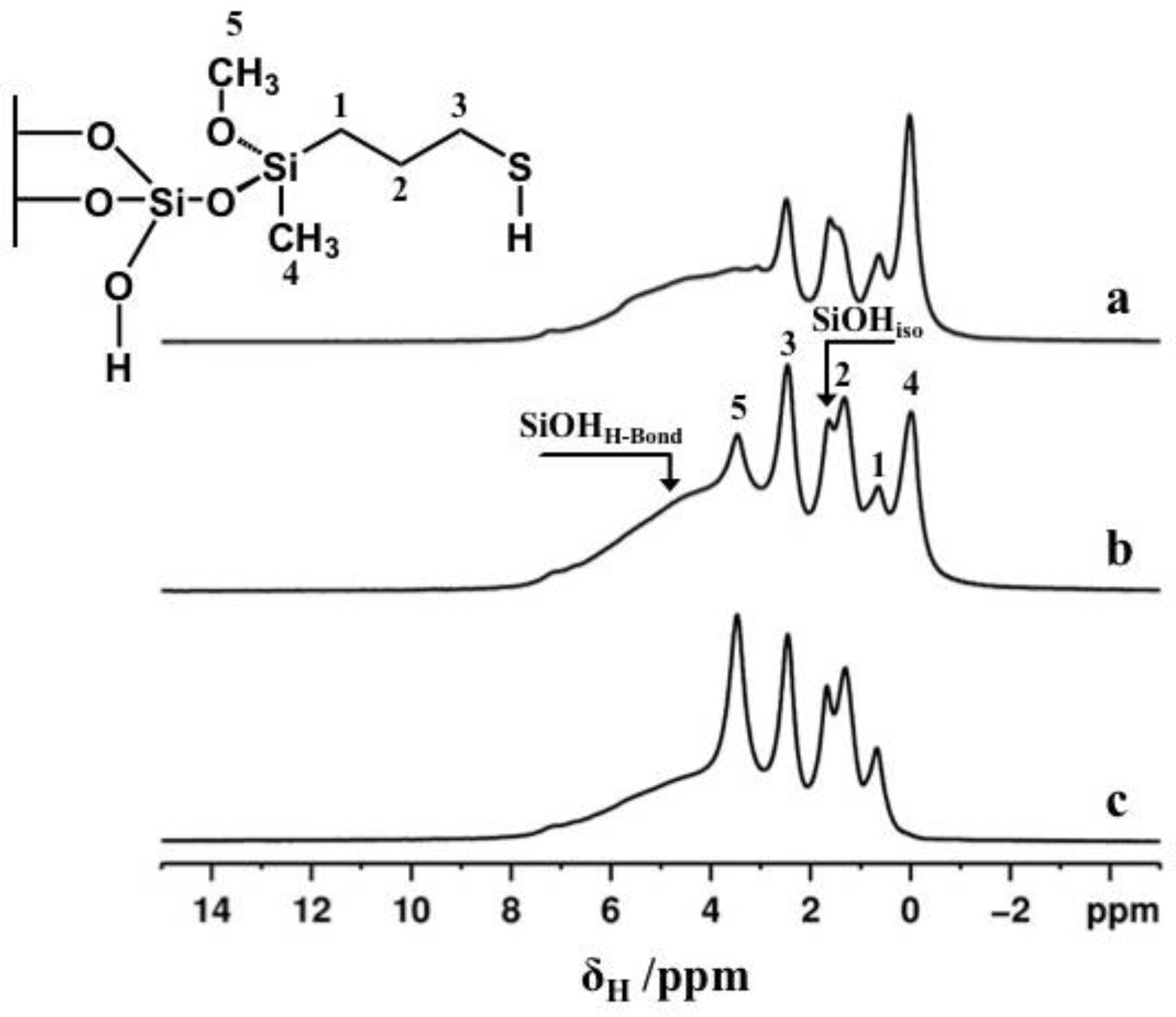
2.4. ss-NMR Analysis
2.5. Computational Modeling
3. Materials and Methods
3.1. General
3.2. 3-Mercaptopropyl(methoxy)dimethylsilane
3.3. Preparation of the Hybrid Materials
3.4. Characterization
3.5. Computational Details
3.5.1. Design of Models
3.5.2. General Settings of Molecular Dynamics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Díaz, U.; Corma, A. Organic-inorganic hybrid materials: Multi-functional solids for multi-step reaction processes. Chem. Eur. J. 2018, 24, 1–16. [Google Scholar] [CrossRef]
- Mir, S.H.; Nagahara, L.A.; Thundat, T.; Mokarian-Tabari, P.; Furukawa, H.; Khosla, A. Organic-inorganic hybrid functional materials: An integrated platform for applied technologies. J. Electrochem. Soc. 2018, 165, B3137–B3156. [Google Scholar] [CrossRef]
- Wight, A.P.; Davis, M.E. Design and preparation of organic−inorganic hybrid catalysts. Chem. Rev. 2002, 102, 3589–3614. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, F.; Cornelius, M.; Morell, J.; Froba, M. Silica-based mesoporous organic-inorganic hybrid materials. Angew. Chem. Int. Ed. 2006, 45, 3216–3251. [Google Scholar] [CrossRef] [PubMed]
- Férey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Judeinstein, P.; Sanchez, C. Hybrid organic-inorganic materials: A land of multidisciplinarity. J. Mater.Chem. 1996, 6, 511–525. [Google Scholar] [CrossRef]
- Díaz, U.; Brunel, D.; Corma, A. Catalysis using multifunctional organosiliceous hybrid materials. Chem. Soc. Rev. 2013, 42, 4083–4097. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Zhao, J.; Wickemeyer, B.B.; Dean Toste, F.; Somorjai, G.A. Foundations and strategies of the construction of hybrid catalysts for optimized performances. Nat. Catal. 2018, 1, 318–325. [Google Scholar] [CrossRef]
- Park, J.-W.; Park, Y.J.; Jun, C.-H. Post-grafting of silica surfaces with pre-functionalized organosilanes: New synthetic equivalents of conventional trialkoxysilanes. Chem. Commun. 2011, 47, 4860–4871. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Hartmann, M. Progress in enzyme immobilization in ordered mesoporous materials and related applications. Chem. Soc. Rev. 2013, 42, 3894–3912. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Gai, S.; Lin, J. Functionalized mesoporous silica materials for controlled drug delivery. Chem. Soc. Rev. 2012, 41, 3679–3698. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous silica nanoparticles: A comprehensive review on synthesis and recent advances. Pharmaceutics 2018, 10, 118–167. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Edo, G.; Balmori, A.; Pontón, I.; del Rio, A.M.; Sánchez-García, D. Functionalized ordered mesoporous silicas (MCM-41): Synthesis and applications in catalysis. Catalysts 2018, 8, 617. [Google Scholar] [CrossRef]
- Feng, X.; Fryxell, G.E.; Wang, L.-Q.; Kim, A.Y.; Liu, J.; Kemner, K.M. Functionalized monolayers on ordered mesoporous supports. Science 1997, 276, 923–926. [Google Scholar] [CrossRef]
- Mori, Y.; Pinnavaia, T.J. Optimizing organic functionality in mesostructured silica: Direct assembly of mercaptopropyl groups in wormhole framework structures. Chem. Mater. 2001, 13, 2173–2178. [Google Scholar] [CrossRef]
- Brunel, D.; Cauvel, A.; Di Renzo, F.; Fajula, F.; Fubini, B.; Onida, B.; Garrone, E. Prefential grafting of alkoxysilane coupling agents on the hydrophobic portion of the surface of micelle-templated silica. New J. Chem. 2000, 24, 807–813. [Google Scholar] [CrossRef]
- Paul, G.; Musso, G.E.; Bottinelli, E.; Cossi, M.; Marchese, L.; Berlier, G. Investigating the interaction of water vapour with aminopropyl groups on the surface of mesoporous silica nanoparticles. ChemPhysChem 2017, 18, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Margolese, D.; Melero, J.A.; Christiansen, S.C.; Chmelka, B.F.; Stucky, G.D. Direct syntheses of ordered SBA-15 mesoporous silica containing sulfonic acid groups. Chem. Mater. 2000, 12, 2448–2459. [Google Scholar] [CrossRef]
- Shylesh, S.; Sharma, S.; Mirajkar, S.P.; Singh, A.P. Silica functionalised sulphonic acid groups: Synthesis, characterization and catalytic activity in acetalization and acetylation reactions. J. Mol. Catal. A: Chem. 2004, 212, 219–228. [Google Scholar] [CrossRef]
- Gianotti, E.; Diaz, U.; Velty, A.; Corma, A. Designing bifunctional acid–base mesoporous hybrid catalysts for cascade reactions. Catal. Sci. Technol. 2013, 3, 2677–2688. [Google Scholar] [CrossRef] [Green Version]
- Bayer, E.; Albert, K.; Reiners, J.; Nieder, M.; Müller, D. Characterization of chemically modified silica gels by 29Si and 13C cross-polarization and magic angle spinning nuclear magnetic resonance. J. Chromatogr. 1983, 264, 197–213. [Google Scholar] [CrossRef]
- Garcìa, N.; Benito, E.; Guzmàn, J.; Tiemblo, P. Use of p-toluenesulfonic acid for the controlled grafting of alkoxysilanes onto silanol containing surfaces: Preparation of tunable hydrophilic, hydrophobic, and super-hydrophobic silica. J. Am. Chem. Soc. 2007, 129, 5052–5060. [Google Scholar] [CrossRef] [PubMed]
- Paul, G.; Bisio, C.; Braschi, I.; Cossi, M.; Gatti, G.; Gianotti, E.; Marchese, L. Combined solid-state NMR, FT-IR and computational studies on layered and porous materials. Chem. Soc Rev. 2018, 47, 5684–5739. [Google Scholar] [CrossRef] [PubMed]
- Borrego, T.; Andrade, M.; Pinto, M.L.; Silva, A.R.; Carvalho, A.P.; Rocha, J.; Freire, C.; Pires, J. Physicochemical characterization of silylated functionalized materials. J. Colloid Interface Sci. 2010, 344, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Paul, G.; Steuernagel, S.; Koller, H. Non-covalent interactions of a drug molecule encapsulated in a hybrid silica gel. Chem. Commun. 2007, 5194–5196. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, G.; Michel, D. High-Resolution Solid State NMR of Silicates and Zeolites; John Wiley & Sons Ltd.: New York, NY, USA, 1987; pp. 1–485. [Google Scholar]
- Agina, E.V.; Sizov, A.S.; Yablokov, M.Y.; Borschev, O.V.; Bessonov, A.A.; Kirikova, M.N.; Bailey, M.J.A.; Ponomarenko, S.A. Polymer surface engineering for efficient printing of highly conductive metal nanoparticle inks. ACS Appl. Mater. Interfaces 2015, 7, 11755–11764. [Google Scholar] [CrossRef] [PubMed]
- Ugliengo, P.; Sodupe, M.; Musso, F.; Bush, I.J.; Orlando, R.; Dovesi, R. Realistic models of hydroxylated amorphous silica surfaces and MCM-41 mesoporous material simulated by large-scale periodic B3LYP calculations. Adv. Mater. 2008, 20, 4579–4583. [Google Scholar] [CrossRef]
- Materials Studio 6.0; Accelrys Software Inc.: San Diego, CA, USA, 2011.
- Rappe, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A., III; Skiff, W.M. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Schneider, T.; Stoll, E. Molecular-dynamics study of a three-dimensional one-component model for distortive phase transitions. Phys. Rev. B 1978, 17, 1302–1322. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comp. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
Sample Availability: Compound 3-Mercaptopropyl(methoxy)dimethylsilane is available from the authors. |





| Acronyms | Mercapto Precursor Grafted on MCM-41 |
|---|---|
| MeO-MCM-41 | 3-mercaptopropyl(methoxy)dimethylsilane |
| diMeO-MCM-41 | 3-mercaptopropyl(dimethoxy)methylsilane |
| triMeO-MCM-41 | 3-mercaptopropyl(trimethoxy)silane |
| Samples | Δwt% Due to H2O a | Δwt% Due to Mercapto Silane b |
|---|---|---|
| MCM-41 | 1.25 | - |
| MeO-MCM-41 | 1.13 | 6.30 |
| diMeO-MCM-41 | 0.45 | 8.20 |
| triMeO-MCM-41 | 0.74 | 8.90 |
| Hybrid Structures | Average Distance (±stdev)/Å |
|---|---|
| tri-dactyl | 4.09 (±0.35) |
| di-dactyl(MeO) | 3.90 (±0.29) |
| di-dactyl(Me) | 4.42 (±0.63) |
| mono-dactyl(MeO,MeO) | 3.42 (±0.44) |
| mono-dactyl(MeO,Me) | 4.29 (±1.05) |
| mono-dactyl(Me,Me) | 3.75 (±0.03) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivaldi, C.; Miletto, I.; Paul, G.; Giovenzana, G.B.; Fraccarollo, A.; Cossi, M.; Marchese, L.; Gianotti, E. Influence of Silicodactyly in the Preparation of Hybrid Materials. Molecules 2019, 24, 848. https://doi.org/10.3390/molecules24050848
Ivaldi C, Miletto I, Paul G, Giovenzana GB, Fraccarollo A, Cossi M, Marchese L, Gianotti E. Influence of Silicodactyly in the Preparation of Hybrid Materials. Molecules. 2019; 24(5):848. https://doi.org/10.3390/molecules24050848
Chicago/Turabian StyleIvaldi, Chiara, Ivana Miletto, Geo Paul, Giovanni B. Giovenzana, Alberto Fraccarollo, Maurizio Cossi, Leonardo Marchese, and Enrica Gianotti. 2019. "Influence of Silicodactyly in the Preparation of Hybrid Materials" Molecules 24, no. 5: 848. https://doi.org/10.3390/molecules24050848
APA StyleIvaldi, C., Miletto, I., Paul, G., Giovenzana, G. B., Fraccarollo, A., Cossi, M., Marchese, L., & Gianotti, E. (2019). Influence of Silicodactyly in the Preparation of Hybrid Materials. Molecules, 24(5), 848. https://doi.org/10.3390/molecules24050848