Antibacterial and Biofilm Inhibitory Activity of Medicinal Plant Essential Oils Against Escherichia coli Isolated from UTI Patients
Abstract
:1. Introduction
2. Results
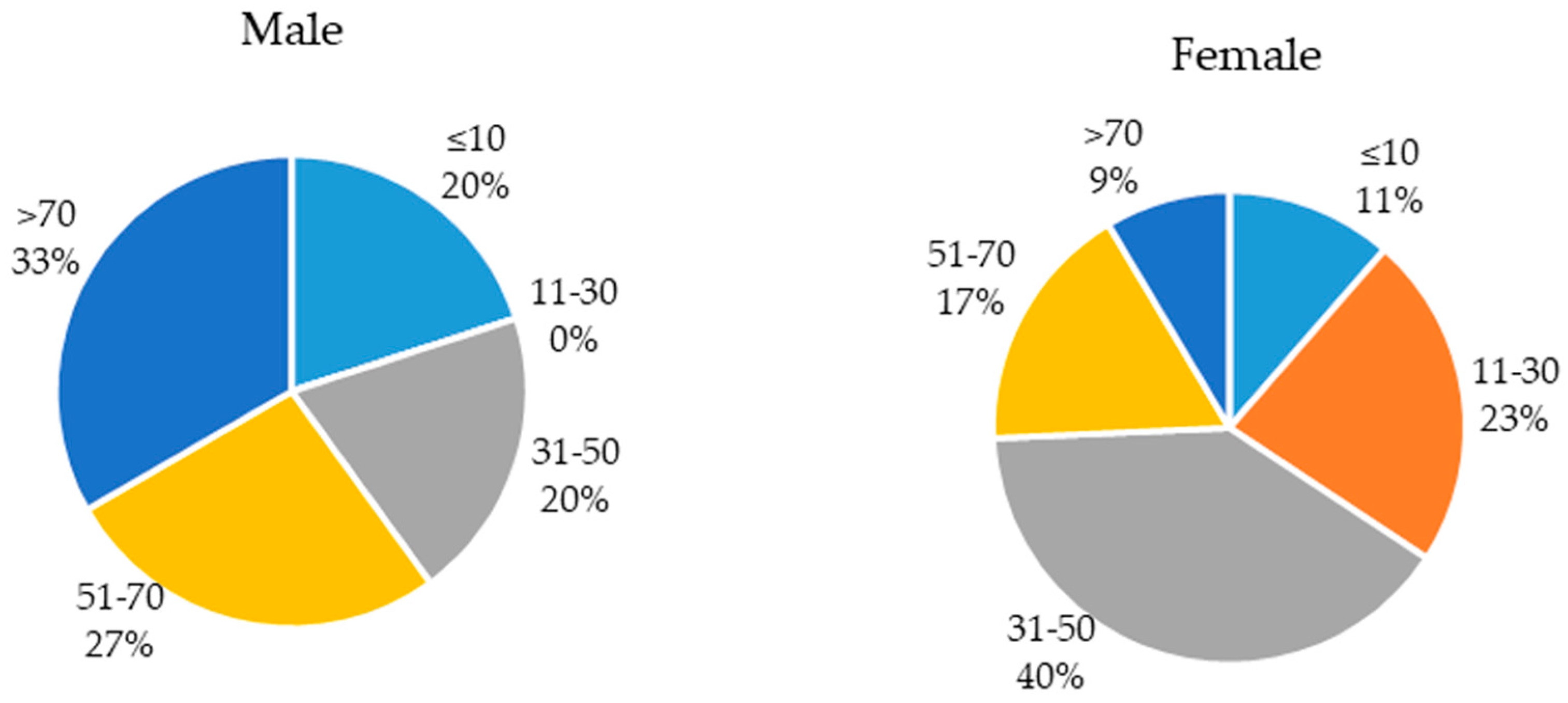
2.1. Population of the Study
2.2. Chemical Composition of the Essential Oils
2.3. Antibacterial Activity of Essential Oils Against E. coli
2.3.1. Disc Diffusion
2.3.2. Antibacterial Activity of MIC and MBC
2.4. Biofilm Formation
2.5. Biofilm Inhibitory Activity of Essentials Oils
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Sampling and Bacterial Strains Identification
5.2. Essential Oils
5.3. Gas Chromatography—Mass Spectrometry Analysis
5.4. Screening for Antibacterial Activity of Essential Oils
5.4.1. Disc Diffusion
5.4.2. Determination of MIC and MBC
5.5. Biofilm Formation
5.6. Inhibition of Biofilm Formation
5.7. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Toro, C.S.; Farfan, M.; Contreras, I.; Flores, O.; Navarro, N.; Mora, G.C.; Prado, V. Genetic analysis of antibiotic-resistance determinants in multidrug-resistant Shigella strains isolated from Chilean children. Epidemiol. Infect. 2005, 133, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Kunin, C.M. Urinary tract infections in females. Clin. Infect. Dis. 1994, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B. Epidemiology of urinary tract infections: Incidence, morbidity, and economic costs. Am. J. Med. 2002, 113, S1–S5. [Google Scholar] [CrossRef]
- El-Kersh, T.A.; Marie, M.A.; Al-Sheikh, Y.A.; Al-Kahtani, S.A. Prevalence and risk factors of community- acquired urinary tract infections due to ESBL- producing Gram negative bacteria in an Armed Forces Hospital in Sothern Saudi Arabia. JMMS 2015, 4, 2315–5159. [Google Scholar]
- Lin, E.; Bhusal, Y.; Horwitz, D.; Shelburne, S.A.; Trautner, B.W. Overtreatment of Enterococcal Bacteriuria. Arch. Intern. Med. 2012, 172, 33–38. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Tabibian, J.H.; Gornbein, J.; Heidari, A.; Dien, S.L.; Lau, V.H.; Chahal, P.; Churchill, B.M.; Haake, D.A. Uropathogens and Host Characteristics. J. Clin. Microbiol. 2008, 46, 3980–3986. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 2002, 292, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Romling, U.; Balsalobre, C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J. Intern. Med. 2012, 272, 541–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pepeljnjak, S.; Kosalec, I.; Kalodera, Z.; Blazevic, N. Antimicrobial activity of juniper berry essential oil (Juniperus communis L., Cupressaceae). Acta Pharm. 2005, 55, 417–422. [Google Scholar] [PubMed]
- Sivasothy, Y.; Chong, W.K.; Hamid, A.; Eldeen, I.M.; Sulaiman, S.F.; Awang, K. Essential oils of Zingiber officinale var. rubrum Theilade and their antibacterial activities. Food Chem. 2011, 124, 514–517. [Google Scholar] [CrossRef]
- Marquesa, J.L.; Volcãob, L.M.; Funcka, G.D.; Kroninga, I.S.; da Silvaa, W.P.; Fiorentinia, Â.M.; Ribeiroca, G.A. Antimicrobial activity of essential oils of Origanum vulgare L. and Origanum majorana L. against Staphylococcus aureus isolated from poultry meat. Ind. Crops. Prod. 2015, 77, 444–450. [Google Scholar] [CrossRef]
- Yakoubi, S.; Cherrat, A.; Diouri, M.; EL Hilali, F.; Zair, T. Chemical composition and antibacterial activity of Thymus zygis subsp. gracilis (Boiss.) R. Morales essential oils from Morocco. Med. J. Chem. 2014, 3, 746–758. [Google Scholar]
- Jardak, M.; Elloumi-Mseddi, J.; Aifa, S.; Mnif, S. Chemical composition, anti-biofilm activity and potential cytotoxic effect on cancer cells of Rosmarinus officinalis L. essential oil from Tunisia. Lipids Health Dis. 2017, 16, 190. [Google Scholar] [CrossRef] [PubMed]
- Abdalá, A.E.; Roozen, J.P. The effects of stabilized extracts of sage and oregano on the oxidation of salad dressings. Eur. Food Res. Technol. 2011, 212, 551–560. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods-a review. Inter. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–565. [Google Scholar] [CrossRef] [PubMed]
- Kordali, S.; Kotan, R.; Mavi, A.; Cakir, A.; Ala, A.; Yildirim, A. Determination of the chemical composition and antioxidant activity of the essential oil of Artemisia dracunculus and of the antifungal and antibacterial activities of turkish Artemisia absinthium, A. dracunculus, Artemisia santonicum, and Artemisia spicigera essential oils. J. Agric. Food Chem. 2005, 53, 9452–9458. [Google Scholar] [PubMed]
- Prabuseenivasan, S.; Jayakumar, M.; Ignacimuthu, S. In vitro antibacterial activity of some plant essential oils. BMC Compl. Altern. Med. 2006, 6, 39. [Google Scholar] [CrossRef]
- Wojnicz, D.; Kucharska, A.Z.; Sokól-Letowska, A.; Kicia, M.; Tichaczek-Goska, D. Medicinal plants extracts affect virulence factors expression and biofilm formation by the uropathogenic Escherichia coli. Urol. Res. 2012, 40, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Park, H.D. Ginger extract inhibits biofilm formation by Pseudomonas aeruginosa PA14. PLoS ONE 2013, 8, e76106. [Google Scholar] [CrossRef] [PubMed]
- Huma, J.; Fohad, M.H.; Iqbal, A. Antibacterial and antibiofilm activity of some Essential oils and compounds against clinical strains of Staphylococcus aureus. J. Biomed. 2014, 1, 65–71. [Google Scholar]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.K.; Valdramidis, V.P.; O’Donnel, C.P.; Muthukumarappan, K.; Bourke, P.; Cullen, P.J. Application of natural antimicrobials for food preservation. J. Food Chem. 2009, 57, 5987–6000. [Google Scholar] [CrossRef] [PubMed]
- Bano, K.; Khan, J.; Begum, R.H.; Munir, S.; Akbar, N. Patterns of antibiotic sensitivity of bacterial pathogens among urinary tract infections (UTI) patients in a Pakistani population. J. Microbiol. Res. 2012, 6, 414–420. [Google Scholar]
- Ramanath, K.; Shafiya, S. Prescription pattern of antibiotic usage for urinary tract infection treated in a rural tertiary care hospital. J. Pharm. 2011, 2, 57–63. [Google Scholar]
- Iqbal, T.; Naqvi, R.; Akhter, S.F. Frequency of urinary tract infection in renal transplant recipients and effect on graft function. J. Pak. Med. 2010, 10, 826–829. [Google Scholar]
- Daoud, Z.; Afif, C. Escherichia coli Isolated from Urinary Tract Infections of Lebanese Patients between 2000 and 2009: Epidemiology and Profiles of Resistance. Chemother. Res. Pract. 2011, 2011, 218431. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Lakshmi, V.; Rajagopalan, R. Occurrence of extended spectrum β-lactamases among Enterobacteriaceae spp. isolated at a tertiary care Institute. J. Med. Microbiol. 2006, 24, 208–211. [Google Scholar]
- Pattnaik, S.; Subramanyam, V.R.; Bapaji, M.; Kole, C.R. Antibacterial and antifungal activity of aromatic constituents of essential oils. Microbios 1997, 89, 39–46. [Google Scholar]
- Ouedrhiria, W.; Mounyr, B.; Harkib, H.; Mojac, S.; Grechea, H. Synergistic antimicrobial activity of two binary combinations of marjoram, lavender, and wild thyme essential oils. Int. J. Food Prop. 2017, 12, 3149–3158. [Google Scholar] [CrossRef]
- Santoyo, S.; Cavero, S.; Jaime, L.; Ibanez, E.; Senorans, F.J.; Reglero, G. Chemical composition and antimicrobial activity of Rosmarinus officinalis L. essential oil obtained via supercritical fluidextraction. J. Food Prot. 2005, 68, 790–795. [Google Scholar] [CrossRef]
- Li, L.; Li, Z.W.; Yin, Z.Q.; Wei, Q.; Jia, R.Y.; Zhou, L.J.; Xu, J.; Song, X.; Zhou, Y.; Du, Y.H.; et al. Antibacterial activity of leaf essential oil and its constituents from Cinnamomum longepaniculatum. Int. J. Clin. Exp. Med. 2014, 7, 1721–1727. [Google Scholar] [PubMed]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Faleiro, M.L.; Miguel, M.G.; Ladeiro, F.; Venâncio, F.; Tavares, R.; Brito, J.C.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Antimicrobial activity of essential oils isolated from Portuguese endemic species of Thymus. Lett. Appl. Microbiol. 2002, 36, 35–40. [Google Scholar] [CrossRef]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents. Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef] [PubMed]
- Tenke, P.; Kovacs, B.; Jackel, M.; Nagy, E. The role of biofilm infection in urology. World. J. Urol. 2006, 24, 13–20. [Google Scholar] [CrossRef]
- Aminov, R.I. Horizontal gene exchange in environmental microbiota. Front. Microbiol. 2011, 2, 158. [Google Scholar] [CrossRef]
- Sandasi, M.; Leonard, C.M.; Viljoen, A.M. The effect of five common essential oil components on Listeria monocytogenes biofilms. Food Control 2008, 19, 1070–1075. [Google Scholar] [CrossRef]
- Nuryastuti, T.; Van der Mei, H.C.; Busscher, H.J.; Iravati, S.; Aman, A.T.; Krom, B.P. Effect of cinnamon oil on icaA expression and biofilm formation by Staphylococcus epidermidis. Appl. Environ. Microbiol. 2009, 75, 6850–6855. [Google Scholar] [CrossRef] [PubMed]
- Hendry, E.R.; Worthington, T.; Conway, B.R.; Lambert, P.A. Antimicrobial efficacy of eucalyptus oil and 1,8-cineole alone and in combination with chlorhexidine digluconate against microorganisms grown in planktonic and biofilm cultures. J. Antimicrob. Chemother. 2009, 64, 1219–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karpanen, T.J.; Worthington, T.; Hendry, E.R.; Conway, B.R.; Lambert, P.A. Antimicrobial efficacy of chlorhexidine digluconate alone and in combination with eucalyptus oil, tea tree oil and thymol against planktonic and biofilm cultures of Staphylococcus epidermidis. J. Antimicrob. Chemother. 2008, 62, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, O.; Uğur, A.; Saraç, N.; Ozcan, F.; Baygar, T. The in vitro antibiofilm activity of Rosmarinus officinalis L. essential oil against multiple antibiotic resistant Pseudomonas sp. and Staphylococcus sp. J. Food. Agric. Environ. 2014, 12, 82–86. [Google Scholar]
- Nostro, A.; Roccaro, A.S.; Bisignano, G.; Marino, A.; Cannatelli, M.A.; Pizzimenti, F.C.; Cioni, P.L.; Procopio, F.; Blanco, A.R. Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Med. Microbiol. 2007, 56, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Gooréa, S.G.; Ouattara, Z.A.; Yapi, A.T.; Békro, Y.A.; Bighelli, A.; Paoli, M.; Tomi, F. Chemical composition of the leaf oil of Artabotrys jollyanus from Côte d’Ivoire. Rev. Bras. Farmacogn. 2017, 27, 414–418. [Google Scholar]
- Bagamboula, C.; Uyttendaele, M.; Debevere, J. Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S. flexneri. J. Food microbiol. 2004, 21, 33–42. [Google Scholar] [CrossRef]
- El-Deeb, B.; Elhariry, H.; Mostafa, N.Y. Antimicrobial Activity of Silver and Gold Nanoparticles Biosynthesized Using Ginger Extract. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 1085. [Google Scholar]
- Gulluce, M.; Sahin, F.; Sokmen, M. Antimicrobial and antioxidant properties of the essential oils and methanol extract from Mentha longifolia L. Food Chem. 2007, 103, 1449–1456. [Google Scholar] [CrossRef]
- Oulkheir, S.; Aghrouch, M.; El Mourabit, F.; Dalha, F.; Graich, H.; Amouch, F.; Ouzaid, K.; Moukale, A.; Chadli, S. Antibacterial Activity of Essential Oils Extracts from Cinnamon, Thyme, Clove and Geranium Against a Gram Negative and Gram Positive Pathogenic Bacteria. JDMP 2017, 3, 1–5. [Google Scholar]
- Ben Abdallah, F.; Chaieb, K.; Zmantar, T.; Kallel, H.; Bakhrouf, A. Adherence assays and Slime production of Vibrio alginolyticus and Vibrio parahaemolyticus. Braz. J. Microbiol. 2009, 40, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S.; Shah, R.; Bhave, M.; Palombo, E.A. Inhibitory activity of yarrow essential oil on Listeria planktonic cells and biofilms. J. Food Control. 2013, 29, 125–130. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
| Components | J. communis % | Z. officinale % | O. majorana % | T. zygis % | R. officinalis % |
|---|---|---|---|---|---|
| α-Pinene | 47.1 | 2.6 | 0.46 | 3.6 | 11.7 |
| Sabinene | 3.6 | - | 8 | 0.84 | - |
| β-Pinene | 2.5 | - | 1.4 | 0.33 | 6.3 |
| β-Myrcene | 11.7 | - | 1.1 | 8.6 | 1.5 |
| α-phellandrene | 0.43 | - | 0.30 | 0.48 | - |
| Limonene | 6.2 | 5.7 | 3.5 | 2.6 | 2.2 |
| Terpinen-4-ol | 2.3 | - | 25.9 | 11.7 | - |
| Bornyl acetate | 0.22 | - | - | 0.07 | 0.4 |
| β-Caryophyllene | 2.8 | - | 2.3 | 1.6 | - |
| α-Thujene | 1.1 | - | 0.33 | 0.21 | - |
| Camphene | 0.43 | 7.4 | 0.03 | 0.74 | 3.2 |
| ∆3-Carene | 0.12 | - | - | - | - |
| α-Terpinene | 1.6 | - | 7.7 | 4.2 | - |
| p-Cymene | 0.63 | - | 3.4 | 2.2 | 1 |
| 1,8-Cineole | - | 2.6 | 0.15 | - | 47.7 |
| γ-Terpinene | 2.6 | - | 16.9 | 7.6 | - |
| Terpinolene | 1.6 | - | 1.7 | 2 | - |
| Linalool | 0.07 | - | 10.9 | 39.7 | 0.859 |
| Borneol | 0.1 | - | - | 1.9 | 2 |
| α-Terpineol | 0.47 | - | 2.5 | 1.7 | 2.5 |
| α-cubebene | - | - | - | - | - |
| α-Copaene | 0.48 | - | - | - | - |
| Camphor | - | - | - | 0.22 | 9.6 |
| β-Elemene | 0.84 | - | - | - | - |
| γ -Elemene | 0.67 | - | - | - | - |
| trans-β-pharnesene | 0.49 | - | - | - | - |
| α-Humulene | 2 | - | 0.05 | - | - |
| γ-Muurolene | 0.7 | - | - | - | - |
| Germacrene D | 1.2 | - | - | - | - |
| cis and trans-thujan-4-ol | - | - | 2.2–2.3 | 0.88–2.2 | - |
| cis and trans piperitol | - | - | 0.13–0.18 | 0.13–0.08 | - |
| Linalyl acetate | - | - | 7 | 0.5 | - |
| Carvacrol | - | - | 0.03 | 0.08 | - |
| Thymol | - | - | 0.05 | 0.52 | - |
| Bicyclogermacrene | - | - | 0.41 | 0.16 | - |
| Cis and trans-p-menth-2-en-1-ol | - | - | 0.59–0.32 | 0.37–0.25 | - |
| α-Selinene | Trace | - | - | - | - |
| β-Selinene | 0.27 | - | - | - | - |
| α-Muurolene | 1.1 | - | - | - | - |
| γ-Cadinene | 0.52 | - | - | - | - |
| δ-Cadinene | 2 | - | - | - | - |
| Germacrene B | 0.14 | - | - | - | - |
| T-Cadinol | 0.06 | - | - | - | - |
| α-Cadinol | 0.1 | - | - | - | - |
| T-Muurolol | 0.13 | - | - | - | - |
| Caryophyllene oxide | - | - | 0.04 | - | - |
| Ocimene | - | - | 0.07 | - | - |
| Spathulenol | - | - | 0.01 | - | - |
| cis-Dihydrocarvone | - | - | - | 0.17 | - |
| trans-Dihydrocarvone | - | - | - | 0.2 | - |
| Verbenone | - | - | - | - | 0.2 |
| ar-curcumene | - | 8 | - | - | - |
| α-Zingiberene | - | 33.1 | - | - | - |
| α-Farnesene | - | 3.4 | - | - | - |
| β-Bisabolene | - | 6.4 | - | - | - |
| β-Sesquiphellandrene | - | 13.5 | - | - | - |
| Essential Oils | E. coli Isolates | ||||
|---|---|---|---|---|---|
| (+ + + + ) | (+ + + ) | (+ + ) | (+ ) | (−) | |
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| J. communis | 50 (100%) | ||||
| Z. officinale | 50 (100%) | ||||
| O. majorana | 13 (26%) | 30 (60%) | 5 (10%) | 2 (4%) | 0% |
| T. zygis | 45 (90%) | 2 (4%) | 3 (6%) | 0% | |
| R. officinalis | 7 (14%) | 12 (24%) | 10 (20%) | 21 (42%) | 0% |
| Isolates | O. Majorana | T. Zygis | R. Officinalis | |||
|---|---|---|---|---|---|---|
| MBC | MIC | MBC | MIC | MBC | MIC | |
| All samples | 1.56–12.5 | 0.19–0.78 | 1.56–6.25 | 0.19–0.78 | 12.5 | 1.56–3.125 |
| Males | 1.56–12.5 | 0.19–0.78 | 1.56–3.125 | 0.19–0.78 | 12.5 | 1.56–3.125 |
| Adult males | 1.56–12.5 | 0.19–0.78 | 1.56–3.125 | 0.19–0.78 | 12.5 | 1.56–3.125 |
| Children males | 1.56 | 0.19–0.78 | 1.56 | 0.19 | 12.5 | 1.56–3.125 |
| Females | 1.56–3.125 | 0.19–0.78 | 1.56–6.25 | 0.19–0.78 | 12.5 | 1.56–3.125 |
| Adult females | 1.56–3.125 | 0.19–0.78 | 1.56–6.25 | 0.19–0.78 | 12.5 | 1.56–3.125 |
| Children females | 1.56 | 0.19–0.39 | 1.56–6.25 | 0.19–0.39 | 12.5 | 1.56–3.125 |
| Isolates | OD570 ± SD | Biofilm Formation | Isolates | OD570 ± SD | Biofilm Formation |
|---|---|---|---|---|---|
| 1 | 0.025 ± 0.012 | Negative | 26 | 0.166 ± 0.038 | low-grade positive |
| 2 | 0.025 ± 0.008 | Negative | 27 | 0.139 ± 0.025 | low-grade positive |
| 3 | 0.041 ± 0.006 | Negative | 28 | 0.175 ± 0.013 | low-grade positive |
| 4 | 0.104 ± 0.039 | low-grade positive | 29 | 0.543 ± 0.02 | low-grade positive |
| 5 | 0.286 ± 0.019 | low-grade positive | 30 | 0.279 ± 0.041 | low-grade positive |
| 6 | 0.174 ± 0.058 | low-grade positive | 31 | 0.292 ± 0.03 | low-grade positive |
| 7 | 0.160 ± 0.045 | low-grade positive | 32 | 0.142 ± 0.018 | low-grade positive |
| 8 | 0.183 ± 0.078 | low-grade positive | 33 | 0.019 ± 0.008 | Negative |
| 9 | 0.015 ± 0.003 | Negative | 34 | 0.021 ± 0.015 | Negative |
| 10 | 0.030 ± 0.005 | Negative | 35 | 0.011 ± 0.022 | Negative |
| 11 | 0.093 ± 0.016 | Negative | 36 | 0.068 ± 0.038 | Negative |
| 12 | 0.046 ± 0.009 | Negative | 37 | 0.058 ± 0.049 | Negative |
| 13 | 0.145 ± 0.011 | low-grade positive | 38 | 0.063 ± 0.032 | Negative |
| 14 | 0.059 ± 0.018 | Negative | 39 | 0.031 ± 0.006 | Negative |
| 15 | 0.171 ± 0.087 | low-grade positive | 40 | 0.026 ± 0.008 | Negative |
| 16 | 0.355 ± 0.076 | low-grade positive | 41 | 0.042 ± 0.058 | Negative |
| 17 | 0.102 ± 0.036 | low-grade positive | 42 | 0.093 ± 0.035 | Negative |
| 18 | 0.426 ± 0.068 | low-grade positive | 43 | 0.104 ± 0.011 | low-grade positive |
| 19 | 0.110 ± 0.022 | low-grade positive | 44 | 0.096 ± 0.053 | Negative |
| 20 | 0.025 ± 0.006 | Negative | 45 | 0.02 ± 0.019 | Negative |
| 21 | 0.018 ± 0.016 | Negative | 46 | 0.021 ± 0.008 | Negative |
| 22 | 0.030 ± 0.013 | Negative | 47 | 0.166 ± 0.027 | low-grade positive |
| 23 | 0.018 ± 0.008 | Negative | 48 | 0.104 ± 0.041 | low-grade positive |
| 24 | 0.030 ± 0.004 | Negative | 49 | 0.024 ± 0.05 | Negative |
| 25 | 0.347 ± 0.012 | low-grade positive | 50 | 0.037 ± 0.009 | Negative |
| ATCC 25922 | 0.115 ± 0.028 | low-grade positive |
| Isolates | Control OD570 ± SD | O. majorana OD570 ± SD | Inhibition (%) | T. zygis OD570 ± SD | Inhibition (%) | R. officinalis OD570 ± SD | Inhibition (%) |
|---|---|---|---|---|---|---|---|
| 4 | 0.104 ± 0.039 | 0.103 ± 0.019 | 0 | 0.103 ± 0.044 | 0 | 0.105 ± 0.044 | 0 |
| 5 | 0.286 ± 0.019 | 0.285 ± 0.089 | 0 | 0.183 ± 0.029 | 36.01 | 0.015 ± 0.006 | 94.75 |
| 6 | 0.174 ± 0.058 | 0.148 ± 0.022 | 14.94 | 0.143 ± 0.009 | 17.81 | 0.110 ± 0.013 | 36.78 |
| 7 | 0.160 ± 0.045 | 0.164 ± 0.043 | 0 | 0.079 ± 0.003 | 50.62 | 0.059 ± 0.004 | 63.12 |
| 8 | 0.183 ± 0.078 | 0.181 ± 0.037 | 0 | 0.083 ± 0.006 | 54.64 | 0.041 ± 0.008 | 77.59 |
| 13 | 0.145 ± 0.011 | 0.146 ± 0.025 | 0 | 0.144 ± 0.028 | 0 | 0.063 ± 0.006 | 56.55 |
| 15 | 0.171 ± 0.087 | 0.109 ± 0.014 | 36.25 | 0.108 ± 0.019 | 36.84 | 0.021 ± 0.004 | 87.71 |
| 16 * | 0.355 ± 0.076 | 0.099 ± 0.008 | 72.11 | 0.073 ± 0.004 | 79.43 | 0.032 ± 0.008 | 90.98 |
| 17 * | 0.102 ± 0.036 | 0.086 ± 0.003 | 28.33 | 0.103 ± 0.013 | 0 | 0.043 ± 0.002 | 64.16 |
| 18 * | 0.426 ± 0.068 | 0.089 ± 0.009 | 79.10 | 0.171 ± 0.032 | 59.85 | 0.080 ± 0.004 | 81.22 |
| 19 | 0.110 ± 0.022 | 0.109 ± 0.007 | 0 | 0.118 ± 0.015 | 0 | 0.113 ± 0.011 | 0 |
| 25 * | 0.347 ± 0.012 | 0.342 ± 0.079 | 0 | 0.084 ± 0.004 | 75.79 | 0.057 ± 0.005 | 83.57 |
| 26 * | 0.166 ± 0.038 | 0.041 ± 0.009 | 75.30 | 0.097 ± 0.029 | 41.56 | 0.021 ± 0.004 | 87.34 |
| 27 | 0.139 ± 0.025 | 0.137 ± 0.018 | 0 | 0.076 ± 0.017 | 45.32 | 0.015 ± 0.003 | 89.2 |
| 28 | 0.175 ± 0.013 | 0.172 ± 0.021 | 0 | 0.174 ± 0.024 | 0 | 0.176 ± 0.012 | 0 |
| 29 * | 0.543 ± 0.02 | 0.064 ± 0.005 | 88.21 | 0.077 ± 0.009 | 85.81 | 0.031 ± 0.007 | 94.29 |
| 30 * | 0.279 ± 0.041 | 0.075 ± 0.008 | 73.11 | 0.098 ± 0.006 | 64.87 | 0.058 ± 0.003 | 79.21 |
| 31 | 0.292 ± 0.03 | 0.084 ± 0.004 | 71.23 | 0.165 ± 0.004 | 43.49 | 0.045 ± 0.008 | 84.58 |
| 32 * | 0.142 ± 0.018 | 0.140 ± 0.029 | 0 | 0.089 ± 0.003 | 36.42 | 0.071 ± 0.005 | 49.28 |
| 43 * | 0.104 ± 0.011 | 0.044 ± 0.004 | 57.69 | 0.105 ± 0.012 | 0 | 0.074 ± 0.006 | 28.84 |
| 47 * | 0.166 ± 0.027 | 0.102 ± 0.016 | 38.55 | 0.162 ± 0.019 | 0 | 0.099 ± 0.004 | 40.36 |
| 48* | 0.104 ± 0.041 | 0.103 ± 0.023 | 0 | 0.103 ± 0.044 | 0 | 0.044 ± 0.009 | 57.69 |
| ATCC 25922 * | 0.115 ± 0.028 | 0.114 ± 0.017 | 0 | 0.071 ± 0.006 | 38.26 | 0.059 ± 0.006 | 48.69 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lagha, R.; Ben Abdallah, F.; AL-Sarhan, B.O.; Al-Sodany, Y. Antibacterial and Biofilm Inhibitory Activity of Medicinal Plant Essential Oils Against Escherichia coli Isolated from UTI Patients. Molecules 2019, 24, 1161. https://doi.org/10.3390/molecules24061161
Lagha R, Ben Abdallah F, AL-Sarhan BO, Al-Sodany Y. Antibacterial and Biofilm Inhibitory Activity of Medicinal Plant Essential Oils Against Escherichia coli Isolated from UTI Patients. Molecules. 2019; 24(6):1161. https://doi.org/10.3390/molecules24061161
Chicago/Turabian StyleLagha, Rihab, Fethi Ben Abdallah, Badriah Osama AL-Sarhan, and Yassin Al-Sodany. 2019. "Antibacterial and Biofilm Inhibitory Activity of Medicinal Plant Essential Oils Against Escherichia coli Isolated from UTI Patients" Molecules 24, no. 6: 1161. https://doi.org/10.3390/molecules24061161
APA StyleLagha, R., Ben Abdallah, F., AL-Sarhan, B. O., & Al-Sodany, Y. (2019). Antibacterial and Biofilm Inhibitory Activity of Medicinal Plant Essential Oils Against Escherichia coli Isolated from UTI Patients. Molecules, 24(6), 1161. https://doi.org/10.3390/molecules24061161






