Evaluation of Yeast Derivative Products Developed as an Alternative to Lees: The Effect on the Polysaccharide, Phenolic and Volatile Content, and Colour and Astringency of Red Wines
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of YDs on the Polysaccharide Content
2.2. Effect of YDs Application on the Phenolic Content
2.3. Effect of YDs Application on the Colour of Wines
2.4. Effect of YDs on the Volatile Compounds
2.5. Effect of YDs on the Wine Astringency
3. Materials and Methods
3.1. Winemaking and Experimental Design
3.2. Reagents and Standards
3.3. Analytical Methods
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pozo-Bayón, M.A.; Andújar-Ortiz, I.; Moreno-Arribas, M.V. Scientific evidences beyond the application of inactive dry yeast preparations in winemaking. Food Res. Int. 2009, 42, 754–761. [Google Scholar] [CrossRef]
- Del Barrio-Galán, R.; Pérez-Magariño, S.; Ortega-Heras, M.; Guadalupe, Z.; Ayestarán, B. Polysaccharide characterization of commercial dry yeast preparations and their effect on white and red wine composition. LWT—Food Sci. Technol. 2012, 48, 215–223. [Google Scholar]
- Del Barrio-Galán, R.; Ortega-Heras, M.; Sánchez-Iglesias, M.; Pérez-Magariño, S. Interactions of phenolic and volatile compounds with yeast lees, commercial yeast derivatives and non toasted chips in model solutions and young red wines. Eur. Food Res. Technol. 2012, 234, 231–244. [Google Scholar] [CrossRef]
- Guadalupe, Z.; Martínez, L.; Ayestarán, B. Yeast mannoproteins in red winemaking: Effect on polysaccharide, polyphenolic, and colour composition. Am. J. Enol. Viticult. 2010, 61, 191–200. [Google Scholar]
- Del Barrio-Galán, R.; Pérez-Magariño, S.; Ortega-Heras, M.; Williams, P.; Doco, T. Effect of ageing on lees and of three different dry yeast derivative products on Verdejo white wine composition and sensorial characteristics. J. Agric. Food Chem. 2011, 59, 12433–12442. [Google Scholar] [CrossRef]
- Soubeyrand, V.; Luparia, V.; Williams, P.; Doco, T.; Vernhet, A.; Ortiz-Julien, A. Formation of micelle containing solubilized sterols during rehydration of active dry yeasts improves their fermentation capacity. J. Agric. Food Chem. 2005, 53, 8025–8032. [Google Scholar]
- Feuillat, M.; Gerreau, J. Les nouveaux activateurs de la fermentation alcoolique. Bull. OIV 1996, 789–790, 987–998. [Google Scholar]
- Díez, L.; Guadalupe, Z.; Ayestarán, B.; Ruiz-Larrea, F. Effect of yeast mannoproteins and grape polysaccharides on the growth of wine lactic acid and acetic acid bacteria. J. Agric. Food Chem. 2010, 58, 7731–7739. [Google Scholar]
- Del Barrio-Galán, R.; Medel-Marabolí, M.; Peña-Neira, A. Effect of different ageing techniques on the polysaccharide and phenolic composition and sensory characteristics of Syrah red wines fermented using different yeast strains. Food Chem. 2015, 179, 116–126. [Google Scholar] [PubMed]
- Del Barrio-Galán, R.; Pérez-Magariño, S.; Ortega-Heras, M. Techniques for improving or replacing ageing on lees of oak aged red wines: The effects on polysaccharides and the phenolic composition. Food Chem. 2011, 127, 528–540. [Google Scholar] [CrossRef]
- González-Royo, E.; Urtasun, A.; Gil, M.; Kontoudakis, N.; Esteruelas, M.; Fort, F.; Zamora, F. Effect of yeast strain and supplementation with inactive yeast during alcoholic fermentation on wine polysaccharides. Am. J. Enol. Viticult. 2013, 64, 268–273. [Google Scholar] [CrossRef]
- Li, S.; Bindon, K.; Bastian, S.; Wilkinson, K. Impact of Commercial Oenotannin and Mannoprotein Products on the Chemical and Sensory Properties of Shiraz Wines Made from Sequentially Harvested Fruit. Foods 2018, 7, 204. [Google Scholar] [CrossRef]
- Li, S.; Bindon, K.; Putnam Bastian, S.; Jiranek, V.; Wilkinson, K. Use of Winemaking Supplements to Modify the Composition and Sensory Properties of Shiraz Wine. J. Agric. Food Chem. 2017, 65, 1353–1364. [Google Scholar] [CrossRef]
- Mekoue-Nguela, J.; Sieczkowski, N.; Roi, S.; Vernhet, A. Sorption of Grape Proanthocyanidins and wine polyphenols by yeasts, inactivated yeasts, and yeast cell walls. J. Agric. Food Chem. 2015, 63, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Mekoue-Nguela, J.; Vernhet, A.; Sieczkowski, N.; Brillouet, J.M. Interactions of Condensed Tannins with Saccharomyces cerevisiae Yeast Cells and Cell Walls: Tannin Location by Microscopy. J. Agric. Food Chem. 2015, 63, 7539–7545. [Google Scholar] [CrossRef] [PubMed]
- Escot, S.; Feuillat, M.; Dulau, L.; Charpentier, C. Release of polysaccharides by yeast and the influence of released polysaccharides on colour stability and wine astringency. Aust. J. Grape Wine Res. 2001, 7, 153–159. [Google Scholar] [CrossRef]
- Francois, J.M.; Alexandre, H.; Granes, D.; Feuillat, M. Vers une meilleure connaissance des produits dérivés de levures. Rev. Oenolog. 2007, 122, 9–12. [Google Scholar]
- Guadalupe, Z.; Ayestarán, B. Effect of commercial mannoprotein addition on polysaccharide, polyphenolic, and colour composition in red wines. J. Agric. Food Chem. 2008, 56, 9022–9029. [Google Scholar] [CrossRef]
- Loira, I.; Vejarano, R.; Morata, A.; Ricardo-da-Silva, J.M.; Laureano, O.; González, M.C.; Suárez-Lepe, J.A. Effect of Saccharomyces strains on the quality of red wines aged on lees. Food Chem. 2013, 139, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.; Ricardo-Da-Silva, J.M.; Lucas, C.; Laureano, O. Effect of commercial mannoproteins on wine colour and tannins stability. Food Chem. 2012, 131, 907–914. [Google Scholar] [CrossRef]
- Chalier, P.; Angot, B.; Delteil, D.; Doco, T.; Gunata, Z. Interactions between aroma compounds and whole mannoprotein isolated from Saccharomyces cerevisiae strains. Food Chem. 2007, 100, 22–30. [Google Scholar] [CrossRef]
- Mahadevan, K.; Farmer, L. Key odor impact compounds in three yeast extracts pastes. J. Agric. Food Chem. 2006, 54, 7242–7250. [Google Scholar] [CrossRef]
- Rossetti, D.; Bongaerts, J.H.H.; Wantling, E.; Stokes, J.R.; Williamson, A.M. Astringency of tea catechins: More than an oral lubrication tactile percept. Food Hydrocoll. 2009, 23, 1984–1992. [Google Scholar] [CrossRef]
- Rossetti, D.; Yakubov, G.E.; Stokes, J.R.; Williamson, A.M.; Fuller, G.G. Interaction of human whole saliva and astringent dietary compounds investigated by interfacial shear rheology. Food Hydrocoll. 2008, 22, 1068–1078. [Google Scholar] [CrossRef]
- Bennick, A. Interaction of plant polyphenols with salivary proteins. Crit. Rev. Oral Biol. Med. 2002, 13, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Laguna, L.; Bartolomé, B.; Moreno-Arribas, M.V. Mouthfeel perception of wine: Oral physiology, components and instrumental characterization. Trends Food Sci. Technol. 2017, 59, 49–59. [Google Scholar] [CrossRef]
- Quijada-Morín, N.; Williams, P.; Rivas-Gonzalo, J.C.; Doco, T.; Escribano-Bailón, M.T. Polyphenolic, polysaccharide and oligosaccharide composition of Tempranillo red wines and their relationship with the perceived astringency. Food Chem. 2014, 154, 44–51. [Google Scholar] [CrossRef]
- Robichaud, J.L.; Noble, A.C. Astringency and bitterness of selected phenolics in wine. J. Sci. Food Agric. 1990, 53, 343–353. [Google Scholar] [CrossRef]
- Ishikawa, T.; Noble, A.C. Temporal perception of astringency and sweetness in red wine. Food Qual. Prefer. 1995, 6, 27–33. [Google Scholar] [CrossRef]
- Vidal, L.; Antúnez, L.; Giménez, A.; Medina, K.; Boido, E.; Ares, G. Sensory characterization of the astringency of commercial Tannat wines Uruguayan. Food Res. Int. 2017, 102, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Del Barrio-Galán, R.; Cáceres-Mella, A.; Medel-Marabolí, M.; Peña-Neira, A. Effect of selected Saccharomyces cerevisiae yeast strains and different ageing techniques on the polysaccharide and polyphenolic composition and sensorial characteristics of Cabernet Sauvignon red wines. J. Sci. Food Agric. 2015, 95, 2132–2144. [Google Scholar] [CrossRef]
- González-Royo, E.; Esteruelas, M.; Kontoudakis, N.; Fort, F.; Canals, J.M.; Zamora, F. The effect of supplementation with three commercial inactive dry yeasts on the colour, phenolic compounds, polysaccharides and astringency of a model wine solution and red wine. J. Sci. Food Agric. 2016, 97, 172–181. [Google Scholar] [CrossRef]
- Alcalde-Eon, C.; García-Estévez, I.; Puente, V.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Colour stabilization of red wines. A chemical and colloidal approach. J. Agric. Food Chem. 2014, 62, 6984–6994. [Google Scholar] [CrossRef]
- Watrelot, A.A.; Schulz, D.L.; Kennedy, J.A. Wine polysaccharides influence tannin-protein interactions. Food Chem. 2017, 63, 571–579. [Google Scholar] [CrossRef]
- Guzmán-Alfeo, M. Manual de Espectrofotometría para Enología, 1st ed.; AMV Editions: Madrid, Spain, 2010; pp. 110–129. ISBN 978-84-96709-54-6. [Google Scholar]
- Etievant, P.X. Volatile Compounds in Foods and Beverages, 1st ed.; Henk Maarse: New York, NY, USA, 1991; pp. 483–533. [Google Scholar]
- Pineau, N.; Schlich, P.; Cordelle, S.; Mathonnière, C.; Issanchou, S.; Imbert, A.; Rogeaux, M.P.; Etiévant, E.; Köster, E. Temporal Dominance of Sensations: Construction of the TDS curves and comparison with time-intensity. Food Qual. Prefer. 2009, 20, 450–455. [Google Scholar] [CrossRef]
- Pueyo, E.; Martínez-Rodríguez, A.; Polo, M.C.; Santa-Marín, G.; Bartolomé, B. Release of lipids during yeast autolysis in a model wine system. J. Agric. Food Chem. 2000, 48, 116–122. [Google Scholar] [CrossRef]
- Comuzzo, P.; Tat, L.; Fenzi, D.; Brotto, L.; Battistutta, F.; Zironi, R. Interactions between yeast autolysates and volatile compounds in wine and model solution. Food Chem. 2011, 127, 473–480. [Google Scholar] [CrossRef]
- Rodríguez-Bencomo, J.J.; Ortega-Heras, M.; Pérez-Magariño, S. Effect of alternative techniques to ageing on lees and use of non-toasted oak chips in alcoholic fermentation on the aromatic composition of red wine. Eur. Food Res. Technol. 2010, 230, 485–496. [Google Scholar]
- Pozo-Bayón, M.Á.; Andújar-Ortiz, I.; Moreno-Arribas, M.V. Volatile profile and potential of inactive dry yeast-based winemaking additives to modify the volatile composition of wines. J. Sci. Food Agric. 2009, 89, 1665–1673. [Google Scholar] [CrossRef]
- Mekoue-Nguela, J.; Poncet-Legrand, C.; Sieczkowski, N.; Vernhet, A. Interactions of grape tannins and wine polyphenols with a yeast protein extract, mannoproteins and β-glucan. Food Chem. 2016, 210, 671–682. [Google Scholar] [CrossRef]
- Poncet-Legrand, C.; Doco, T.; Williams, P.; Vernhet, A. Inhibition of grape seed tannin aggregation by wine mannoproteins: Effect of polysaccharide molecular weight. Am. J. Enol. Viticult. 2007, 58, 87–91. [Google Scholar]
- OIV (International Organisation of Vine and Wine). Compendium of International Methods of Wine and Must Analysis; OIV: París, France, 2015; Volumes 1 and 2. [Google Scholar]
- Ayestarán, B.; Guadalupe, Z.; León, D. Quantification of major grape polysaccharides (Tempranillo v.) released by maceration enzymes during the fermentation process. Anal. Chim. Acta 2004, 513, 29–39. [Google Scholar]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubordieu, D. The Chemistry of Wine Stabilization and Treatments. In Handbook of Enology; John Wiley & Sons: Chichester, UK, 2006; pp. 1–230. [Google Scholar]
- Peña-Neira, A.; Cáceres, A.; Pastenes, A. Low molecular weight phenolic and anthocyanin composition of grape skins from cv. Syrah (Vitis vinifera L.) in the Maipo Valley (Chile): Effect of clusters thinning and vineyard yield. Food Sci. Technol. Int. 2007, 13, 153–158. [Google Scholar] [CrossRef]
- Glories, Y. La couleur des vins rouges. 2éme partie. Mesure, origine et interprétation. Connaissance Vigne Vin 1984, 18, 253–271. [Google Scholar]
- Ayala, F.; Echávarri, J.F.; Negueruela, A.I. MSCV®. 2014. Available online: http://www.unirioja.es/colour/descargas.shtml (accessed on 29 March 2019).
- Úbeda, C.; Del Barrio-Galán, R.; Peña-Neira, A.; Medel-Marabolí, M.; Durán-Guerrero, E. Location effects on the aromatic composition of monovarietal cv. Carignan wines. Am. J. Enol. Vitic. 2017, 68, 390–399. [Google Scholar]
- Medel-Marabolí, M.; Romero, J.L.; Obreque-Slier, E.; Contreras, A.; Peña-Neira, Á. Effect of a commercial tannin on the sensorial temporality of astringency. Food Res. Int. 2017, 102, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Foods, Principles and Practices; Springer: New York, NY, USA, 2010. [Google Scholar]
- Pineau, B.; Barbe, J.C.; Van Leeuwen, C.; Dubourdieu, D. Examples of perceptive interactions involved in specific “Red” and “Black-berry” aromas in red wines. J. Agric. Food Chem. 2009, 57, 3702–3708. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


 Control after malolactic fermentation;
Control after malolactic fermentation;  Control;
Control;  SIDY;
SIDY;  CW;
CW;  YA. MT: Months of treatment, MB: Months in bottle. Different letters indicate statistically significant differences (p < 0.05) between values.
YA. MT: Months of treatment, MB: Months in bottle. Different letters indicate statistically significant differences (p < 0.05) between values.
 Control after malolactic fermentation;
Control after malolactic fermentation;  Control;
Control;  SIDY;
SIDY;  CW;
CW;  YA. MT: Months of treatment, MB: Months in bottle. Different letters indicate statistically significant differences (p < 0.05) between values.
YA. MT: Months of treatment, MB: Months in bottle. Different letters indicate statistically significant differences (p < 0.05) between values.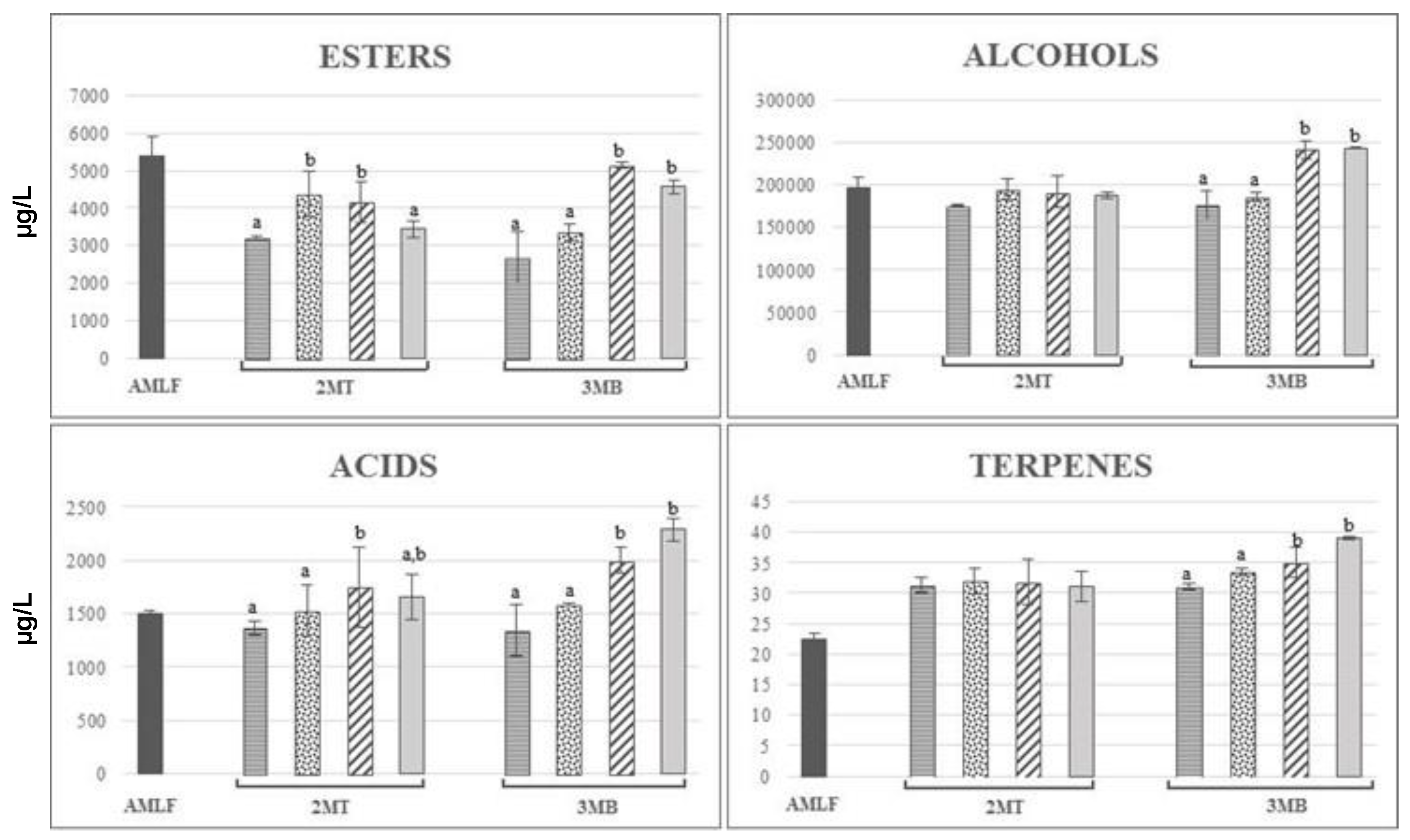
 Control after malolactic fermentation;
Control after malolactic fermentation;  Control;
Control;  SIDY,
SIDY,  CW,
CW,  YA. Columns with different letters indicate statistically significant differences (p < 0.05) between the different treatments. 2MT: two months of treatment, 3MB: three months in bottle.
YA. Columns with different letters indicate statistically significant differences (p < 0.05) between the different treatments. 2MT: two months of treatment, 3MB: three months in bottle.
 Control after malolactic fermentation;
Control after malolactic fermentation;  Control;
Control;  SIDY,
SIDY,  CW,
CW,  YA. Columns with different letters indicate statistically significant differences (p < 0.05) between the different treatments. 2MT: two months of treatment, 3MB: three months in bottle.
YA. Columns with different letters indicate statistically significant differences (p < 0.05) between the different treatments. 2MT: two months of treatment, 3MB: three months in bottle.


| Lalvin EC1118® | Uvaferm HPS® | |||||||
|---|---|---|---|---|---|---|---|---|
| 2MT | CONT | SIDY | CW | YA | CONT | SIDY | CW | YA |
| CI | 11.3 ± 0.07 b | 9.52 ± 1.07 ab | 9.15 ± 1.11 a | 10.6 ± 0.06 ab | 5.84 ± 0.01 a | 5.70 ± 0.02 a | 9.41 ± 0.90 c | 7.49 ± 0.41 b |
| L* | 49.3 ± 0.23 | 55.3 ± 4.29 | 56.8 ± 4.48 | 54.3 ± 4.21 | 71.0 ± 0.29 c | 71.7 ± 0.17c | 56.0 ± 2.71 a | 63.3 ± 0.81 b |
| a* | 44.0 ± 0.55 | 38.9 ± 3.60 | 37.7 ± 2.13 | 44.1 ± 2.00 | 31.6 ± 0.55 a | 31.3 ± 0.27 a | 47.3 ± 2.15 c | 40.1 ± 0.49 b |
| b* | 9.18 ± 0.24 | 10.0 ± 0.62 | 10.5 ± 1.68 | 8.32 ± 0.38 | 13.3 ± 2.15 b | 11.2 ± 0.23 ab | 8.4 ± a0.63 | 8.2 ± 1.54 a |
| HBA | 52.2 ± 0.42 | 51.4 ± 0.22 | 53.1 ± 0.54 | 54.5 ± 2.78 | 44.8 ± 0.07a | 45.5 ± 1.08 ab | 45.9 ± 0.82 ab | 47.2 ± 0.86 b |
| HCA | 14.8 ± 0.10 | 14.6 ± 0.20 | 14.9 ± 0.07 | 15.2 ± 0.72 | 14.1 ± 0.29 | 14.0 ± 1.21 | 14.3 ± 0.44 | 14.9 ± 0.60 |
| HCATE | 1.89 ± 0.04 | 1.84 ± 0.07 | 1.93 ± 0.01 | 2.09 ± 0.19 | 4.42 ± 0.06 | 4.48 ± 0.02 | 4.42 ± 0.22 | 4.64 ± 0.12 |
| TFL | 43.2 ± 0.20 a | 44.3 ± 0.59 ab | 47.4 ± 0.04 b | 45.1 ± 2.51 ab | 40.8 ± 1.11 b | 42.3 ± 1.02 b | 37.5 ± 0.57 a | 41.9 ± 1.22 b |
| TPRO | 32.9 ± 1.35 | 33.3 ± 0.61 | 35.1 ± 0.35 | 33.3 ± 2.79 | 29.7 ± 1.69 a | 32.2 ± 1.87 ab | 32.1 ± 0.50 ab | 34.8 ± 0.33 b |
| TFLAV | 38.5 ± 1.34 | 36.4 ± 0.84 | 38.4 ± 0.14 | 38.2 ± 2.89 | 29.7 ± 0.38 a | 30.6 ± 1.46 a | 33.2 ± 1.72 b | 32.8 ± 0.71 b |
| TSTILB | 5.13 ± 0.09 | 4.86 ± 0.16 | 5.50 ± 0.15 | 5.38 ± 0.33 | 5.07 ± 0.05 | 5.09 ± 0.08 | 5.73 ± 0.30 | 5.54 ± 0.20 |
| TALC | 16.6 ± 0.02 | 16.4 ± 0.13 | 17.1 ± 0.15 | 17.7 ± 0.88 | 22.2 ± 1.24 | 21.9 ± 0.76 | 22.5 ± 1.11 | 23.2 ± 0.01 |
| 3MB | CONT | SIDY | CW | YA | CONT | SIDY | CW | YA |
| CI | 11.1 ± 0.34 ab | 9.90 ± 0.41 a | 9.83 ± 1.08 a | 13.1 ± 0.95 b | 6.45 ± 0.34 a | 6.06 ± 0.02 a | 11.2 ± 0.51 c | 8.73 ± 0.66 b |
| L | 51.4 ± 0.73 ab | 54.7 ± 1.47 b | 55.6 ± 4.30 b | 46.0 ± 2.58 a | 69.1 ± 1.02 c | 71.0 ± 0.24 c | 51.7 ± 1.22 a | 60.4 ± 2.84 b |
| a | 43.1 ± 0.84 ab | 40.3 ± 1.06 ab | 39.2 ± 4.19 a | 45.7 ± 1.31 b | 34.4 ± 1.48 a | 32.2 ± 0.35 a | 46.4 ± 0.56 c | 41.8 ± 0.20 b |
| b | 13.7 ± 0.31 b | 12.6 ± 0.04 a | 13.0 ± 0.18 ab | 13.2 ± 0.57 ab | 11.8 ± 0.22 | 13.0 ± 1.51 | 14.3 ± 0.54 | 13.8 ± 3.21 |
| HBA | 46.1 ± 1.56 b | 45.7 ± 0.57 b | 45.7 ± 0.02b | 41.2 ± 2.45 a | 40.6 ± 0.83 | 43.0 ± 2.87 | 39.3 ± 2.40 | 42.8 ± 0.04 |
| HCA | 14.0 ± 0.74 b | 13.7 ± 0.05 b | 13.2 ± 0.62 ab | 11.9 ± 0.41 a | 14.1 ± 0.47 a | 15.7 ± 0.27 b | 14.4 ± 0.26 a | 15.2 ± 0.31 ab |
| HCATE | 2.49 ± 0.07 | 2.29 ± 0.13 | 2.25 ± 0.01 | 2.16 ± 0.08 | 3.95 ± 0.20 ab | 4.46 ± 0.13 b | 2.83 ± 0.02 a | 4.48 ± 0.16 b |
| TFL | 40.1 ± 2.10 b | 40.8 ± 2.00 b | 40.2 ± 1.83 b | 30.5 ± 1.73 a | 30.8 ± 1.81 ab | 39.4 ± 2.03 c | 28.0 ± 0.96 a | 35.6 ± 2.08 b |
| TPRO | 31.2 ± 1.85 b | 31.2 ± 1.58 b | 28.2 ± 1.05 ab | 26.0 ± 0.69 a | 26.4 ± 1.62 b | 29.6 ± 0.35 c | 20.1 ± 0.12 a | 26.8 ± 1.23 b |
| TFLAV | 34.7 ± 1.07 d | 32.7 ± 0.57 c | 30.9 ± 0.21 b | 26.5 ± 0.20 a | 23.5 ± 0.22 | 26.6 ± 1.23 | 23.6 ± 1.74 | 25.4 ± 0.48 |
| TSTILB | 3.64 ± 0.14 | 3.66 ± 0.01 | 3.56 ± 0.22 | 3.21 ± 0.07 | 5.21 ± 0.08 | 5.57 ± 0.29 | 5.05 ± 0.34 | 5.50 ± 0.09 |
| TALC | 16.4 ± 0.64 b | 16.6 ± 0.20 b | 16.8 ± 0.19 b | 14.5 ± 0.01 b | 19.7 ± 0.10 a | 21.5 ± 1.55 ab | 20.3 ± 1.03 a | 22.6 ± 0.37 b |
| LRI | ID | AMLF | 2MT | 3MB | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | C | SIDY1 | CW | YA | C | SIDY1 | CW | YA | |||
| Ethyl esters | |||||||||||
| Ethyl butanoate | 1076 | A | 178 ± 13 | 144 ± 9 | 171 ± 10 | 159 ± 22 | 156 ± 7 | 138 ± 19a | 158 ± 90a | 204 ± 2b | 201 ± 2b |
| Ethyl hexanoate | 1246 | A | 291 ± 43 | 214 ± 4 | 254 ± 27 | 230 ± 64 | 199 ± 3 | 198 ± 34a | 225 ± 32a | 359b ± 3 | 375 ± 24b |
| Ethyl heptanoate | 1334 | B | 10.9 ± 0.90 | 10.5 ± 1.20 | 12.7 ± 0.80 | 10.3 ± 1.20 | 11.3 ± 0.20 | 7.87 ± 0.62a | 8.92a ± 0.69 | 10.7b ± 0.30 | 11.3b ± 0.60 |
| Ethyl lactate | 1413 | A | 19.9 ± 1.60 | 29.9 ± 8.40 | 38.2 ± 7.30 | 42.1 ± 1.20 | 38.9 ± 4.2 | 19.2 ± 1.70a | 20.8 ± 3.50ab | 31.0 ± 6.30b | 28.4 ± 1.50b |
| Ethyl octanoate | 1460 | A | 888 ± 83 | 739 ± 12 | 782 ± 59 | 728 ± 95 | 794 ± 69 | 629 ± 111a | 720 ± 7a | 998 ± 15b | 1096 ± 152b |
| Ethyl nonanoate | 1558 | A | 45.6 ± 2.7 | 27.0 ± 3.50 | 36.0 ± 0.10 | 32.5 ± 12.70 | 28.1 ± 6.50 | 17.9 ± 0.60a | 21.7 ± 0.30ab | 22.9 ± 1.10b | 24.1 ± 3.60b |
| Ethyl succinate | 1701 | A | 92 ± 5 | 108 ± 2 | 111 ± 9 | 113 ± 23 | 114 ± 11 | 134 ± 9a | 142 ± 2a | 176 ± 10b | 195 ± 7b |
| Ethyl decanoate | 1715 | A | 127 ± 7 | 51.0 ± 3.80a | 52.6 ± 5.30ab | 47.7 ± 2.0a | 60.7 ± 5.60b | 34.5 ± 7.80a | 47.1 ± 0.30ab | 61.8 ± 4.10b | 70.9 ± 21b |
| Ethyl isovalerate | 1806 | A | 4.09 ± 0.08 | 2.64 ± 1.36 | 2.82 ± 1.90 | 5.55 ± 2.19 | 6.27 ± 2.94 | 1.50 ± 0.210 | 3.36 ± 1.02 | 2.09 ± 0.34 | 3.18 ± 3.02 |
| Ethyl undecanoate | 1824 | A | 1.20 ± 0.10 | 0.876 ± 0.06 | 1.07 ± 0.09 | 1.07 ± 0.27 | 1.07 ± 0.08 | 0.680 ± 0.014a | 0.745 ± 0.05a | 0.810 ± 0.03a | 1.01 ± 0.07b |
| Ethyl dodecanoate | 1869 | B | 116 ± 8 | 31.5 ± 1.0 | 38.3 ± 3.30 | 32.3 ± 2.50 | 39.5 ± 12.40 | 14.5 ± 2.10ab | 10.5 ± 2.10a | 20.2 ± 0.20b | 20.4 ± 5.60b |
| Ethyl tetradecanoate | 2068 | B | 16.0 ± 2.10 | 9.90 ± 0.53 | 11.6 ± 1.10 | 11.1 ± 0.20 | 11.1 ± 1.30 | 8.26 ± 0.01a | 7.02 ± 1.62a | 12.1 ± 0.60b | 9.37 ± 0.87a |
| Ethyl hexadecanoate | < 2100 | B | 21.6 ± 1.80 | 14.5 ± 0.80 | 15.8 ± 0.10 | 17.2 ± 2.0 | 14.1 ± 1.10 | 11.5 ± 0.30a | 11.1 ± 2.0a | 14.7 ± 3.80b | 16.6 ± 0.70b |
| Methyl esters | |||||||||||
| Methyl hexanoate | 1183 | A | 1.63 ± 0.23 | 0.99 ± 0.28 | 1.45 ± 0.25 | 1.45 ± 0.16 | 1.36 ± 0.02 | 0.81a ± 0.36 | 1.27ab ± 0.07 | 2.00b ± 0.25 | 2.09b ± 0.50 |
| Methyl octanoate | 1420 | A | 6.80 ± 0.66 | 5.10 ± 0.12 | 5.65 ± 0.32 | 5.26 ± 0.86 | 6.40 ± 0.59 | 4.69 ± 0.50a | 4.55 ± 0.69a | 6.52 ± 0.06ab | 7.82 ± 1.52b |
| Methyl decanoate | 1632 | A | 2.06 ± 0.25 | nd | nd | nd | nd | nd | nd | nd | nd |
| Acetate esters | |||||||||||
| Isoamyl acetate | 1163 | A | 2117 ± 253 | 584 ± 30a | 1472 ± 306b | 1572 ± 110b | 884 ± 170a | 706 ± 291a | 939 ± 61a | 1872 ± 90b | 1084 ± 163a |
| Hexyl acetate | 1306 | A | 2.39 ± 0.07 | 1.73 ± 0.10 | 1.92 ± 0.02 | 1.78 ± 0.00 | 1.92 ± 0.16 | 2.01 ± 0.40 | 1.68 ± 0.26 | 2.30 ± 0.05 | 2.82 ± 0.76 |
| 2-phenylethyl acetate | 1851 | A | 139 ± 34 | 64.9 ± 2.8 | 68.5 ± 3.20 | 61.4 ± 2.70 | 73.5 ± 10.80 | 121 ± 42ab | 80.6 ± 14.40a | 91.4 ± 16.70a | 174 ± 15b |
| Isoamyl esters | |||||||||||
| Isopentyl hexanoate | 1478 | A | 0.740 ± 0.01 | 0.581 ± 0.03 | 0.605 ± 0.09 | 0.471 ± 0.195 | 0.597 ± 0.05 | 0.423 ± 0.185a | 0.526 ± 0.09a | 0.843 ± 0.045b | 0.898 ± 0.06b |
| Isoamyl octanoate | 1748 | A | 1172 ± 110 | 1152 ± 1 | 1252 ± 171 | 1055 ± 168 | 955 ± 64 | 662a ± 147 | 958b ± 17 | 1238c ± 45 | 1205c ± 97 |
| Isoamyl decanoate | 1909 | A | 138 ± 10 | 44.5 ± 2.0 | 48.2 ± 3.80 | 40.0 ± 2.70 | 51.8 ± 3.30 | 16.4 ± 5.10 | 12.7 ± 0.80 | 20.9 ± 2.0 | 28.2 ± 8.2 |
| Alcohols | |||||||||||
| Isobutanol | 1108 | A | 70577 ± 4434 | 60577 ± 1383 | 65910 ± 4970 | 63910 ± 11482 | 62910 ± 2805 | 64243 ± 6881a | 68910 ± 1391a | 89243 ± 6641b | 90243 ± 2579b |
| 3-Methyl-1-butanol | 1197 | A | 107593 ± 6486 | 98038 ± 396 | 106260 ± 5796 | 107593 ± 3115 | 99704 ± 2800 | 97816 ± 7230a | 104593 ± 4162a | 134927 ± 3090b | 133816 ± 3935b |
| Hexanol | 1391 | A | 2377 ± 177 | 2227 ± 10 | 2477 ± 193 | 2377 ± 134 | 2327 ± 24 | 2402 ± 154a | 2577 ± 170a | 3452 ± 247b | 3502 ± 76b |
| Heptanol | 1478 | B | 29.4 ± 1.30 | 30.4 ± 1.0 | 32.9 ± 1.90 | 31.9 ± 2.60 | 31.4 ± 1.50 | 31.9 ± 0.40a | 34.4 ± 2.0a | 43.4 ± 3.10b | 43.9 ± 1.90b |
| Octanol | 1578 | A | 7.24 ± 0.43 | 8.61 ± 0.01 | 9.72 ± 1.20 | 9.26 ± 1.30 | 9.17 ± 0.45 | 9.44 ± 0.15a | 10.3 ± 0.40a | 14.3 ± 0.60b | 14.5 ± 0.10b |
| Decanol | 1773 | A | 3.09 ± 0.29 | 3.32 ± 0.02 | 3.56 ± 0.08 | 3.50 ± 0.29 | 3.44 ± 0.06 | 3.50 ± 0.00 | 3.74 ± 0.13 | 4.68 ± 0.02 | 4.74 ± 0.13 |
| Benzyl alcohol | 1978 | B | 360 ± 18 | 618 ± 16 | 675 ± 79 | 650 ± 164 | 643 ± 82 | 658 ± 114 | 705 ± 171 | 910 ± 334 | 925 ± 102 |
| 2-Phenylethanol | 2020 | A | 15689 ± 731 | 14329 ± 173a | 15829 ± 2002b | 15209 ± 3789ab | 14989 ± 1973a | 15349 ± 2347a | 16609 ± 176a | 22109 ± 421b | 22509 ± 2191b |
| Volatile fatty acids | |||||||||||
| Hexanoic acid | 1894 | A | 1170 ± 20 | 1200 ± 15 | 1268 ± 135.60 | 1385 ± 250 | 1288 ± 118 | 1168 ± 125a | 1290 ± 28ab | 1568 ± 88bc | 1660 ± 151c |
| Octanoic acid | < 2100 | A | 209 ± 19 | 113 ± 45 | 203 ± 98.90 | 304 ± 125 | 284 ± 80 | 124 ± 109a | 243 ± 37ab | 364 ± 22b | 534 ± 26c |
| Decanoic acid | < 2100 | A | 102 ± 1 | 42.0 ± 0.5 | 42.8 ± 3.0 | 46.8 ± 4.80 | 66.8 ± 15.50 | 41.6 ± 3.90a | 45.6 ± 3.60ab | 52.4 ± 6.90ab | 75.6 ± 22.4b |
| Dodecanoic acid | < 2100 | B | 14.04 ± 0.84 | 14.84 ± 1.46 | 16.1 ± 0.10 | 16.8 ± 1.0 | 14.84 ± 0.48 | 15.24 ± 0.12 | 14.84 ± 0.62 | 16.4 ± 1.40 | 16.04 ± 0.31 |
| Terpenes | |||||||||||
| Citronellol | 1785 | A | 3.44 ± 0.09 | 3.60 ± 0.01 | 3.76 ± 0.50 | 3.76 ± 0.44 | 3.60 ± 0.10 | 3.76 ± 0.12a | 3.92 ± 0.10a | 4.87 ± 0.00b | 5.03 ± 0.13b |
| Nerol | 1887 | A | 16.6 ± 1.0 | 24.3 ± 1.10 | 25.7 ± 1.40 | 25.2 ± 3.10 | 24.8 ± 2.50 | 25.2 ± 0.70a | 26.6 ± 0.70a | 32.5 ± 2.40b | 33.0 ± 0.30b |
| trans-nerolidol | 2056 | A | 2.61 ± 0.09 | 3.66 ± 0.18 | 3.95 ± 0.14 | 3.86 ± 0.16 | 3.86 ± 0.08 | 3.86 ± 0.07a | 4.05 ± 0.08a | 4.91 ± 0.06b | 5.01 ± 0.15b |
| LRI | ID | AMLF | 2MT | 3BS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | C | SIDY1 | CW | YA | C | SIDY1 | CW | YA | |||
| Ethyl esters | |||||||||||
| Ethyl butanoate | 1076 | A | 234 ± 10 | 203 ± 8a | 214 ± 7ab | 203 ± 12a | 232 ± 2b | 192 ± 16 | 213 ± 1 | 207 ± 5 | 199 ± 1 |
| Ethyl hexanoate | 1246 | A | 396 ± 29 | 310 ± 32ab | 325 ± 7ab | 292 ± 49a | 392 ± 17b | 304 ± 28a | 346 ± 13b | 327 ± 19ab | 303 ± 13a |
| Ethyl heptanoate | 1334 | B | 9.57 ± 0.21 | 9.24 ± 1.89 | 8.33 ± 1.08 | 9.37 ± 1.78 | 9.96 ± 0.42 | 7.41 ± 0.45 | 7.80 ± 0.43 | 8.26 ± 0.32 | 7.61 ± 0.04 |
| Ethyl lactate | 1413 | A | 3.14 ± 1.17 | 2.86 ± 0.21 | 2.82 ± 1.04 | 1.75 ± 0.73 | 3.12 ± 0.55 | 10.5 ± 2.8c | 6.64 ± 2.73ab | 14.5 ± 1.7c | 3.40 ± 0.04a |
| Ethyl octanoate | 1460 | A | 1143 ± 47 | 928 ± 117a | 1049 ± 17ab | 826 ± 107a | 1229 ± 120b | 786 ± 61a | 1069 ± 96b | 853 ± 70ab | 924 ± 79ab |
| Ethyl nonanoate | 1558 | A | 56.7 ± 0.9 | 31.7 ± 5.3 | 26.6 ± 4.5 | 23.8 ± 0.7 | 28.8 ± 9.8 | 14.3 ± 2.0 | 15.4 ± 2.0 | 17.7 ± 1.7 | 17.9 ± 1.0 |
| Ethyl succinate | 1701 | A | 333 ± 6 | 172 ± 5a | 345 ± 1b | 132 ± 11a | 297 ± 2b | 159 ± 7a | 278 ± 7b | 170 ± 6a | 267 ± 4b |
| Ethyl decanoate | 1715 | A | 22.1 ± 2.4 | 36.4 ± 2.2b | 29.7 ± 3.8a | 36.1 ± 2.4b | 38.1 ± 29.3b | 54.8 ± 3.8ab | 54.0 ± 14.2ab | 59.7 ± 0.6b | 48.9 ± 7.7a |
| Ethyl undecanoate | 1824 | A | 0.680 ± 0.030 | 0.549 ± 0.016b | 0.614 ± 0.042c | 0.418 ± 0.021a | 0.484 ± 0.133a | 2.18 ± 0.04 | 3.16 ± 0.05 | 2.44 ± 0.01 | 2.05 ± 0.07 |
| Ethyl dodecanoate | 1869 | B | 14.3 ± 1.0 | 6.95 ± 0.17a | 20.5 ± 0.4c | 5.25 ± 0.22a | 12.9 ± 2.6b | 22.6 ± 1.4a | 34.1 ± 1.5b | 23.9 ± 0.9a | 24.9 ± 1.8a |
| Ethyl tetradecanoate | 2068 | B | 16.0 ± 1.1 | 7.54 ± 0.01a | 9.83 ± 0.27bc | 9.05 ± 0.52ab | 11.1 ± 1.1c | 16.7 ± 2.5b | 14.8 ± 1.5b | 18.3 ± 1.8b | 7.35 ± 0.52a |
| Ethyl hexadecanoate | < 2100 | B | 28.3 ± 4.2 | 14.6 ± 0.8a | 21.3 ± 0.4c | 18.1 ± 1.2b | 23.4 ± 0.2c | 16.4 ± 1.3a | 29.7 ± 1.8b | 16.7 ± 0.2a | 13.4 ± 1.0a |
| Methyl esters | |||||||||||
| Methyl hexanoate | 1183 | A | 1.63 ± 0.27 | 1.17 ± 0.11a | 1.27 ± 0.02a | 1.08 ± 0.21a | 1.72b ± 0.00 | 1.08 ± 0.20 | 1.17 ± 0.47 | 1.08 ± 0.32 | 0.807 ± 0.167 |
| Methyl octanoate | 1420 | A | 6.64 ± 0.29 | 4.33 ± 0.86a | 4.98 ± 0.37ab | 4.09 ± 0.83a | 6.54 ± 1.35b | 3.64 ± 0.51a | 5.42 ± 0.41b | 4.02 ± 0.41a | 4.31 ± 0.55ab |
| Methyl decanoate | 1632 | A | 2.72 ± 0.03 | nd | 1.46 ± 0.17 | nd | 1.26 ± 1.07 | nd | 1.39 ± 0.44 | nd | nd |
| Acetate esters | |||||||||||
| Isoamyl acetate | 1163 | A | 5928 ± 400 | 4350 ± 560a | 5328 ± 123b | 4261 ± 121a | 5717 ± 149b | 3572 ± 111a | 4695 ± 412b | 3350 ± 292a | 3750 ± 189a |
| Hexyl acetate | 1306 | A | 8.22 ± 0.43 | 4.38 ± 1.83a | 21.6 ± 0.4b | 4.86 ± 0.51a | 14.6 ± 8.1ab | 3.63 ± 0.01a | 17.3 ± 2.0b | 3.01 ± 0.05a | 8.13 ± 1.02ab |
| 2-Phenylethyl acetate | 1851 | A | 1069 ± 37 | 285 ± 21a | 869 ± 47c | 246 ± 11a | 604 ± 56b | 188 ± 48a | 309 ± 8b | 138 ± 15a | 201 ± 36a |
| Isoamyl esters | |||||||||||
| Isoamyl hexanoate | 1478 | A | 1.53 ± 0.07 | 1.31 ± 0.25 | 1.07 ± 0.07 | 1.14 ± 0.22 | 1.51 ± 0.19 | 0.922 ± 0.104 | 1.19 ± 0.10 | 1.10 ± 0.16 | 0.977 ± 0.079 |
| Isoamyl octanoate | 1748 | A | 1898 ± 79 | 2008 ± 21b | 2422 ± 29c | 1362 ± 12a | 2155 ± 155b | 1338 ± 37a | 1802 ± 44b | 1385 ± 87a | 1685 ± 163b |
| Isoamyl decanoate | 1909 | A | 533 ± 65 | 595 ± 24a | 571 ± 6a | 673 ± 77ab | 743 ± 19b | 44.5 ± 5.3a | 96.4 ± 1.0c | 38.2 ± 4.5a | 74.5 ± 5.7b |
| Alcohols | |||||||||||
| Isobutanol | 1108 | A | 64910 ± 4893 | 61577 ± 1415 | 60910 ± 6598 | 54243 ± 2798 | 62910 ± 94 | 59243 ± 4028 | 58910 ± 4529 | 63910 ± 3131 | 58910 ± 3525 |
| 3-Methyl-1-butanol | 1197 | A | 136038 ± 6490 | 128260 ± 953a | 129371 ± 5136a | 123816 ± 6513a | 142704 ± 3344b | 134927 ± 1213 | 140482 ± 9572 | 134927 ± 3572 | 128260 ± 1694 |
| Hexanol | 1391 | A | 2402 ± 141 | 2252 ± 4ab | 2252 ± 131ab | 2152 ± 136a | 2502 ± 102b | 2402 ± 46 | 2477 ± 218 | 2377 ± 91 | 2252 ± 54 |
| Heptanol | 1478 | B | 20.9 ± 0.5 | 19.9 ± 0.3 | 18.9 ± 1.1 | 19.9 ± 0.9 | 21.4 ± 0.8 | 21.4 ± 0.1 | 20.9 ± 1.7 | 21.9 ± 1.0 | 19.9 ± 0.1 |
| Octanol | 1578 | A | 9.17 ± 0.85 | 8.06 ± 0.33a | 8.71 ± 0.65a | 8.61 ± 0.45a | 10.2 ± 0.3b | 9.17 ± 0.17 | 9.62 ± 0.56 | 8.98 ± 0.68 | 8.89 ± 0.03 |
| Decanol | 1773 | A | 3.38 ± 0.22 | 2.79 ± 0.06 | 2.97 ± 0.11 | 2.91 ± 0.21 | 3.21 ± 0.22 | 2.97 ± 0.05 | 3.03 ± 0.14 | 2.74 ± 0.03 | 2.74 ± 0.03 |
| Benzyl alcohol | 1978 | B | 265 ± 47 | 220 ± 4 | 220 ± 18 | 215 ± 21 | 240 ± 19 | 260 ± 17 | 268 ± 24 | 283 ± 40 | 213 ± 2 |
| 2-Phenylethanol | 2020 | A | 24709 ± 4375 | 20509 ± 30 | 21109 ± 1625 | 20909 ± 2112 | 23709 ± 1537 | 21309 ± 452ab | 26109 ± 2628b | 23309 ± 2543ab | 20509 ± 38a |
| Volatile fatty acids | |||||||||||
| Hexanoic acid | 1894 | A | 1885 ± 262 | 1695 ± 16 | 1785 ± 132 | 1707 ± 179 | 1932 ± 120 | 1810 ± 7ab | 2070 ± 128b | 1852 ± 158ab | 1760 ± 23a |
| Octanoic acid | < 2100 | A | 1074 ± 246 | 584 ± 46a | 1054 ± 185b | 634 ± 116a | 1124 ± 15b | 754 ± 58a | 1354 ± 96b | 724 ± 87a | 864 ± 49a |
| Decanoic acid | < 2100 | A | 166 ± 12 | 56.8 ± 2.0a | 138 ± 5b | 60.0 ± 5.8a | 135 ± 10b | 69.2 ± 0.6a | 150 ± 11c | 74.0 ± 3.7a | 112 ± 9b |
| Dodecanoic acid | < 2100 | B | 18.1 ± 0.4 | 16.8 ± 0.3 | 16.4 ± 0.3 | 15.6 ± 0.8 | 16.4 ± 0.3 | 14.8b ± 0.2 | 18.1d ± 0.7 | 16.4c ± 0.4 | 12.4a ± 0.4 |
| Terpenes | |||||||||||
| Citronellol | 1785 | A | 4.56 ± 0.24 | 4.24 ± 0.05 | 4.40 ± 0.46 | 4.40 ± 0.32 | 4.87 ± 0.22 | 4.24 ± 0.24 | 4.56 ± 0.34 | 4.24 ± 0.12 | 4.08 ± 0.29 |
| Nerol | 1887 | A | 13.9 ± 1.5 | 12.5 ± 0.3 | 11.6 ± 0.5 | 13.4 ± 1.4 | 13.9 ± 0.5 | 12.0 ± 0.7 | 12.0 ± 0.6 | 11.6 ± 0.1 | 11.6 ± 0.2 |
| trans-Nerolidol | 2056 | A | 2.51 ± 0.08 | 2.80 ± 0.24ab | 2.32 ± 0.08a | 3.18 ± 0.03b | 2.22 ± 0.33a | 3.09 ± 0.49a | 2.61 ± 0.23a | 4.63 ± 0.01b | 2.41 ± 0.18a |
| Lalvin EC1118® | Uvaferm HPS® | |
|---|---|---|
| Alcoholic degree (vol %) | 13.8 | 13.7 |
| pH | 3.91 | 3.82 |
| AT (g/L) | 3.2 | 3.4 |
| AV (g/L) | 0.41 | 0.38 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Barrio-Galán, R.; Úbeda, C.; Gil, M.; Medel-Marabolí, M.; Sieczkowski, N.; Peña-Neira, Á. Evaluation of Yeast Derivative Products Developed as an Alternative to Lees: The Effect on the Polysaccharide, Phenolic and Volatile Content, and Colour and Astringency of Red Wines. Molecules 2019, 24, 1478. https://doi.org/10.3390/molecules24081478
Del Barrio-Galán R, Úbeda C, Gil M, Medel-Marabolí M, Sieczkowski N, Peña-Neira Á. Evaluation of Yeast Derivative Products Developed as an Alternative to Lees: The Effect on the Polysaccharide, Phenolic and Volatile Content, and Colour and Astringency of Red Wines. Molecules. 2019; 24(8):1478. https://doi.org/10.3390/molecules24081478
Chicago/Turabian StyleDel Barrio-Galán, Rubén, Cristina Úbeda, Mariona Gil, Marcela Medel-Marabolí, Nathalie Sieczkowski, and Álvaro Peña-Neira. 2019. "Evaluation of Yeast Derivative Products Developed as an Alternative to Lees: The Effect on the Polysaccharide, Phenolic and Volatile Content, and Colour and Astringency of Red Wines" Molecules 24, no. 8: 1478. https://doi.org/10.3390/molecules24081478
APA StyleDel Barrio-Galán, R., Úbeda, C., Gil, M., Medel-Marabolí, M., Sieczkowski, N., & Peña-Neira, Á. (2019). Evaluation of Yeast Derivative Products Developed as an Alternative to Lees: The Effect on the Polysaccharide, Phenolic and Volatile Content, and Colour and Astringency of Red Wines. Molecules, 24(8), 1478. https://doi.org/10.3390/molecules24081478






