Detecting Zn(II) Ions in Live Cells with Near-Infrared Fluorescent Probes
Abstract
:1. Introduction
2. Experimental Section
2.1. Instrumentation
2.2. Cell Culture and Fluorescence Imaging
2.3. Materials
3. Results and Discussion
3.1. Probe Design and Synthesis
3.2. Optical Properties of Fluorescent Probes A and B in Different Solvents
3.3. Absorbance Responses of Intermediates and the Probes to Zn(II) Ions
3.4. Fluorescence Response of the Intermediates and Probes to Zn(II) Ions
3.5. Selectivity Studies
3.6. Photostability of the Probes
3.7. Theoretical Modeling Results
3.8. Cytotoxicity of the Probes
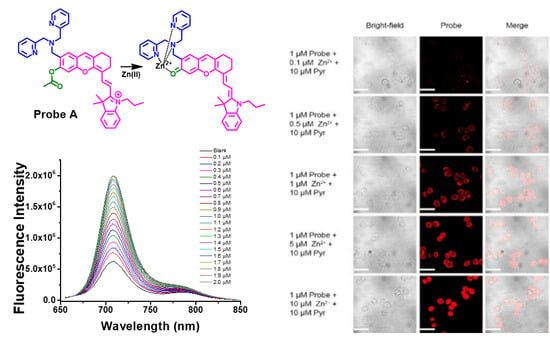
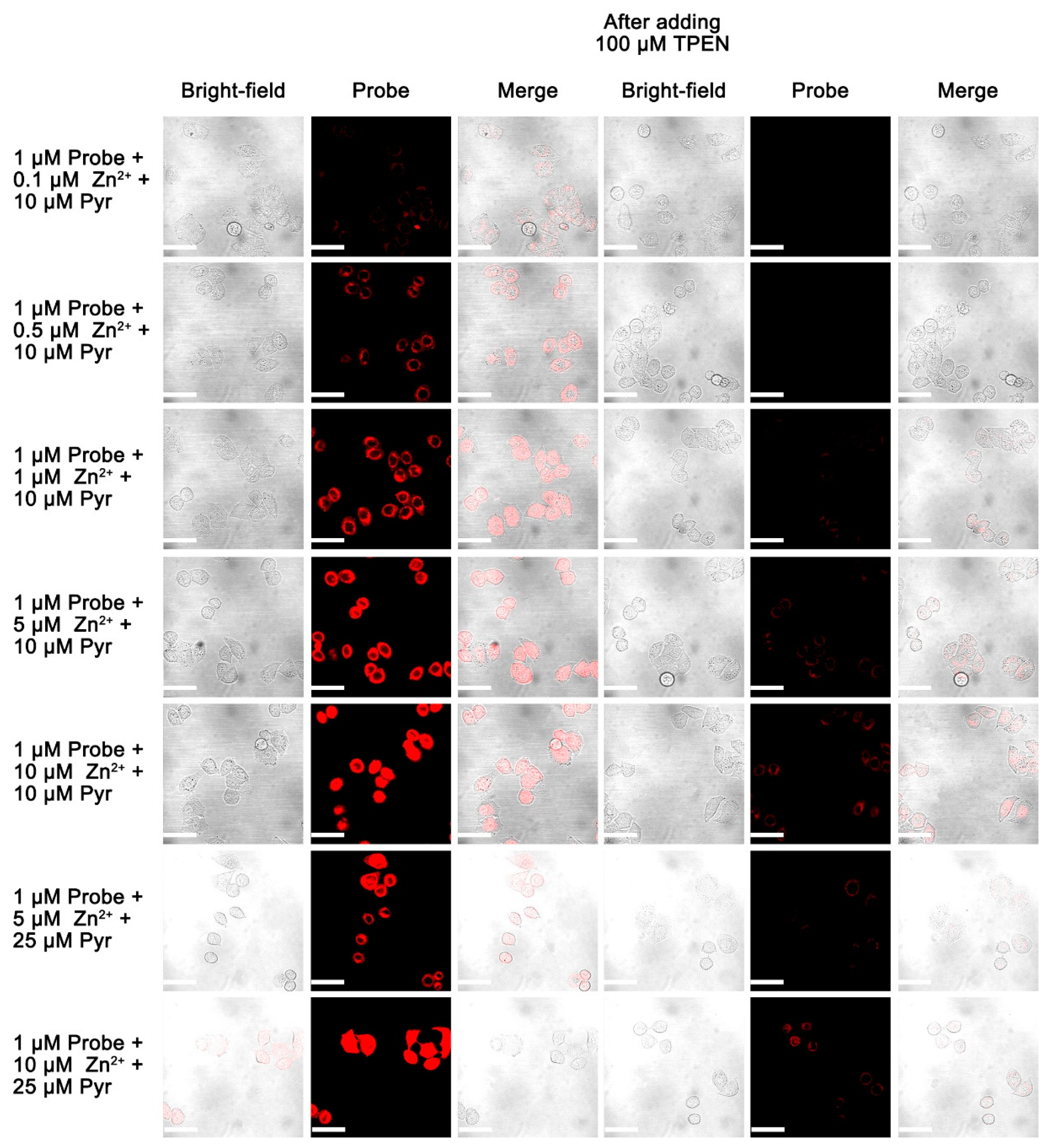
3.9. Live Cell Imaging of Fluorescent Probes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pluth, M.D.; Tomat, E.; Lippard, S.J. Biochemistry of Mobile Zinc and Nitric Oxide Revealed by Fluorescent Sensors. Annu. Rev. Biochem. 2011, 80, 333–355. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K. Design, synthesis and biological application of chemical probes for bio-imaging. Chem. Soc. Rev. 2010, 39, 2048–2053. [Google Scholar] [CrossRef] [PubMed]
- Truong-Tran, A.Q.; Ho, L.H.; Chai, F.; Zalewskiet, P.D. Cellular zinc fluxes and the regulation of apoptosis/gene-directed cell death. J. Nutr. 2000, 130, 1459S–1466S. [Google Scholar] [CrossRef] [PubMed]
- Truong-Tran, A.Q.; Carter, J.; Ruffin, R.; Zalewski, P.D. New insights into the role of zinc in the respiratory epithelium. Imunol. Cell Biol. 2001, 79, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Chai, F.G.; Truong-Tran, A.Q.; Ho, L.H.; Zalewskiet, P.D. Regulation of caspase activation and apoptosis by cellular zinc fluxes and zinc deprivation: A review. Imunol. Cell Biol. 1999, 77, 272–278. [Google Scholar] [CrossRef]
- Sensi, S.L.; Paoletti, P.; Bush, A.I.; Sekler, I. Zinc in the physiology and pathology of the CNS. Nat. Rev. Neurosci. 2009, 10, 780. [Google Scholar] [CrossRef]
- Kay, A.R.; Toth, K. Is Zinc a Neuromodulator? Sci. Signal. 2008, 1, 3. [Google Scholar] [CrossRef]
- Frederickson, C.J.; Koh, J.Y.; Bush, A.I. The neurobiology of zinc in health and diseas. Nat. Rev. Neurosci. 2005, 6, 449–462. [Google Scholar] [CrossRef]
- Takeda, A.; Tamano, H. Insight into zinc signaling from dietary zinc deficienc. Brain Res. Rev. 2009, 62, 33–44. [Google Scholar] [CrossRef]
- Wong, B.A.; Friedle, S.; Lippard, S.J. Solution and Fluorescence Properties of Symmetric Dipicolylamine-Containing Dichlorofluorescein-Based Zn2+ Sensors. JACS 2009, 131, 7142–7152. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.A.; Friedle, S.; Lippard, S.J. Subtle Modification of 2,2-Dipicolylamine Lowers the Affinity and Improves the Turn-On of Zn(II)-Selective Fluorescent Sensors. Inorg. Chem. 2009, 48, 7009–7011. [Google Scholar] [CrossRef]
- Nolan, E.M.; Jaworski, J.; Racine, M.E.; Sheng, M.; Lippard, S.J. Midrange affinity fluorescent Zn(II) sensors of the Zinpyr family: Syntheses, characterization, and biological imaging applications. Inorg. Chem. 2006, 45, 9748–9757. [Google Scholar] [CrossRef]
- Nolan, E.M.; Lippard, S.J. The zinspy family of fluorescent zinc sensors: Syntheses and spectroscopic investigations. Inorg. Chem. 2004, 43, 8310–8317. [Google Scholar] [CrossRef] [PubMed]
- Nolan, E.M.; Ryu, J.W.; Jaworski, J.; Feazell, R.P.; Sheng, M.; Lippard, S.J. Zinspy sensors with enhanced dynamic range for imaging neuronal cell zinc uptake and mobilization. JACS 2006, 128, 15517–15528. [Google Scholar] [CrossRef]
- Zhang, S.W.; Adhikari, R.; Fang, M.; Dorh, N.; Li, C.; Jaishi, M.; Zhang, J.; Tiwari, A.; Pati, R.; Luo, F.-T.; et al. Near-Infrared Fluorescent Probes with Large Stokes Shifts for Sensing Zn(II) Ions in Living Cells. ACS Sens. 2016, 1, 1408–1415. [Google Scholar] [CrossRef]
- Tian, X.H.; Zhang, Q.; Zhang, M.; Uvdal, K.; Wang, Q.; Chen, J.; Du, W.; Huang, B.; Wu, J.; Tian, Y. Probe for simultaneous membrane and nucleus labeling in living cells and in vivo bioimaging using a two-photon absorption water-soluble Zn(II) terpyridine complex with a reduced pi-conjugation system. Chem. Sci. 2017, 8, 142–149. [Google Scholar] [CrossRef]
- Huang, Y.W.; Lin, Q.; Wu, J.; Fu, N. Design and synthesis of a squaraine based near-infrared fluorescent probe for the ratiometric detection of Zn2+ ions. Dyes Pigm. 2013, 99, 699–704. [Google Scholar] [CrossRef]
- Zhang, J.T.; Li, C.; Dutta, C.; Fang, M.; Zhang, S.; Tiwari, A.; Werner, T.; Luo, F.-T.; Liu, H. A novel near-infrared fluorescent probe for sensitive detection of beta-galactosidase in living cells. Anal. Chim. Acta. 2017, 968, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Lin, W.; Zhao, S.; Gao, W.; Chen, B.; He, L.; Zhu, S. A Unique Approach to Development of Near-Infrared Fluorescent Sensors for in Vivo Imaging. JACS 2012, 134, 13510–13523. [Google Scholar] [CrossRef] [PubMed]
- Zastrow, M.L.; Radford, R.J.; Chyan, W.; Anderson, C.T.; Zhang, D.Y.; Laos, A.; Tzounopoulos, T.; Lippard, S.J. Reaction-Based Probes for Imaging Mobile Zinc in Live Cells and Tissues. ACS Sens. 2016, 1, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.W.; Chen, T.-H.; Lee, H.-M.; Bi, J.; Ghosh, A.; Fang, M.; Gian, Z.; Xie, F.; Ainsley, J.; Christov, C.; et al. Luminescent Probes for Sensitive Detection of pH Changes in Live Cells through Two Near-Infrared Luminescence Channels. ACS Sens. 2017, 2, 924–931. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.M.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision B. 01. 2009; Gaussian, Inc.: Wallingford, CT, USA, 2009.
- Austin, A.; Petersson, G.A.; Frisch, M.J.; Dobek, F.J.; Scalmani, G.; Throssell, K. A Density Functional with Spherical Atom Dispersion Terms. JCTC 2012, 8, 4989–5007. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03. 2016; Gaussian, Inc.: Wallingford, CT, USA, 2016.
- Xia, S.; Wang, J.; Bi, J.; Wang, X.; Fang, M.; Phillips, T.; May, A.; Conner, N.; Tanasova, M.; Luo, F.-T.; et al. Fluorescent probes based on pi-conjugation modulation between hemicyanine and coumarin moieties for ratiometric detection of pH changes in live cells with visible and near-infrared channels. Sens. Actuator B-Chem. 2018, 265, 699–708. [Google Scholar] [CrossRef]
- Wang, J.B.; Xia, S.; Bi, J.; Fang, M.; Mazi, W.; Zhang, Y.; Conner, N.; Luo, F.-T.; Lu, H.P.; Liu, H. Ratiometric Near-Infrared Fluorescent Probes Based On Through Bond Energy Transfer and pi-Conjugation Modulation between Tetraphenylethene and Hemicyanine Moieties for Sensitive Detection of pH Changes in Live Cells. Bioconjug. Chem. 2018, 29, 1406–1418. [Google Scholar] [CrossRef]
- Zhu, S.L.; Zhang, J.; Janjanam, J.; Vegesna, G.; Luo, F.T.; Tiwari, A.; Liu, H. Highly water-soluble BODIPY-based fluorescent probes for sensitive fluorescent sensing of zinc(II). J. Mater. Chem. B 2013, 1, 1722–1728. [Google Scholar] [CrossRef]
Sample Availability: Probes A and B are available from the authors. |









| Solvent | λabs (nm) | λem (nm) | εmax (104 M−1cm−1) | Φf (%) | |
|---|---|---|---|---|---|
| Compound 4 | Buffer (pH7.0) | 687 | 703 | 3.2 × 104 | 1.4 |
| Ethanol | 705 | 718 | 5.5 × 104 | 6.7 | |
| THF | 708 | 725 | 5.6 × 104 | 7.0 | |
| Probe A | Buffer (pH7.0) | 695 | 701 | 3.0 × 104 | 0.6 |
| Ethanol | 707 | 717 | 4.6 × 104 | 6.0 | |
| THF | 710 | 723 | 3.1 × 104 | 6.8 | |
| Compound 9 | Buffer (pH7.0) | 685 | 712 | 3.5 × 104 | 1.6 |
| Ethanol | 705 | 717 | 6.1 × 104 | 5.2 | |
| THF | 706 | 721 | 4.2 × 104 | 7.5 | |
| Probe B | Buffer (pH7.0) | 688 | 709 | 4.1 × 104 | 0.8 |
| Ethanol | 705 | 714 | 6.0 × 104 | 4.1 | |
| THF | 717 | 719 | 5.1 × 104 | 5.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, M.; Xia, S.; Bi, J.; Wigstrom, T.P.; Valenzano, L.; Wang, J.; Tanasova, M.; Luck, R.L.; Liu, H. Detecting Zn(II) Ions in Live Cells with Near-Infrared Fluorescent Probes. Molecules 2019, 24, 1592. https://doi.org/10.3390/molecules24081592
Fang M, Xia S, Bi J, Wigstrom TP, Valenzano L, Wang J, Tanasova M, Luck RL, Liu H. Detecting Zn(II) Ions in Live Cells with Near-Infrared Fluorescent Probes. Molecules. 2019; 24(8):1592. https://doi.org/10.3390/molecules24081592
Chicago/Turabian StyleFang, Mingxi, Shuai Xia, Jianheng Bi, Travis P. Wigstrom, Loredana Valenzano, Jianbo Wang, Marina Tanasova, Rudy L. Luck, and Haiying Liu. 2019. "Detecting Zn(II) Ions in Live Cells with Near-Infrared Fluorescent Probes" Molecules 24, no. 8: 1592. https://doi.org/10.3390/molecules24081592