High-Throughput Biochemical Fingerprinting of Oleaginous Aurantiochytrium sp. Strains by Fourier Transform Infrared Spectroscopy (FT-IR) for Lipid and Carbohydrate Productions
Abstract
:1. Introduction
2. Results and Discussion
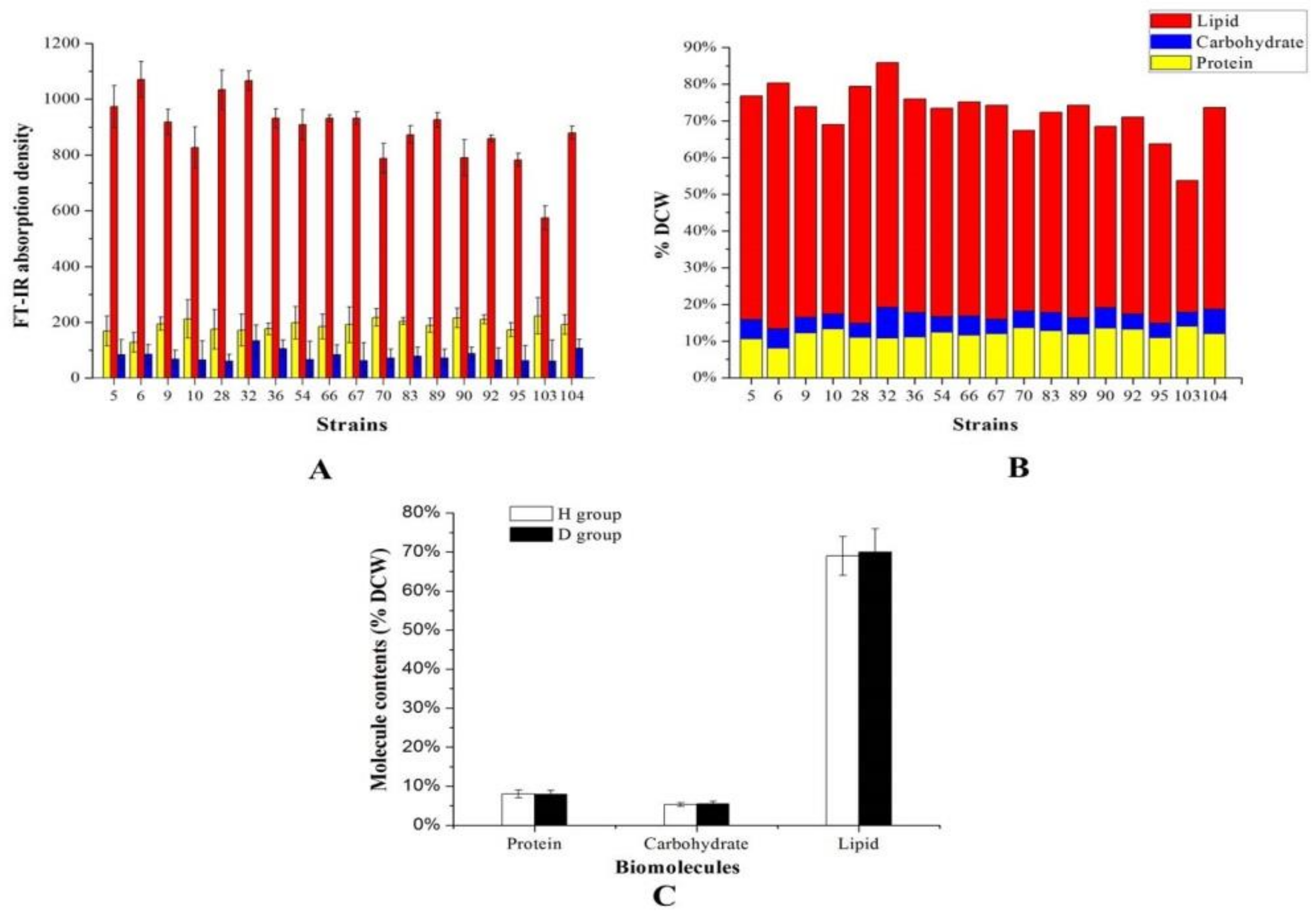
2.1. FT-IR in Aurantiochytrium sp. Strains Screening
2.2. Optimizations of Media for Lipid and Carbohydrate Productions
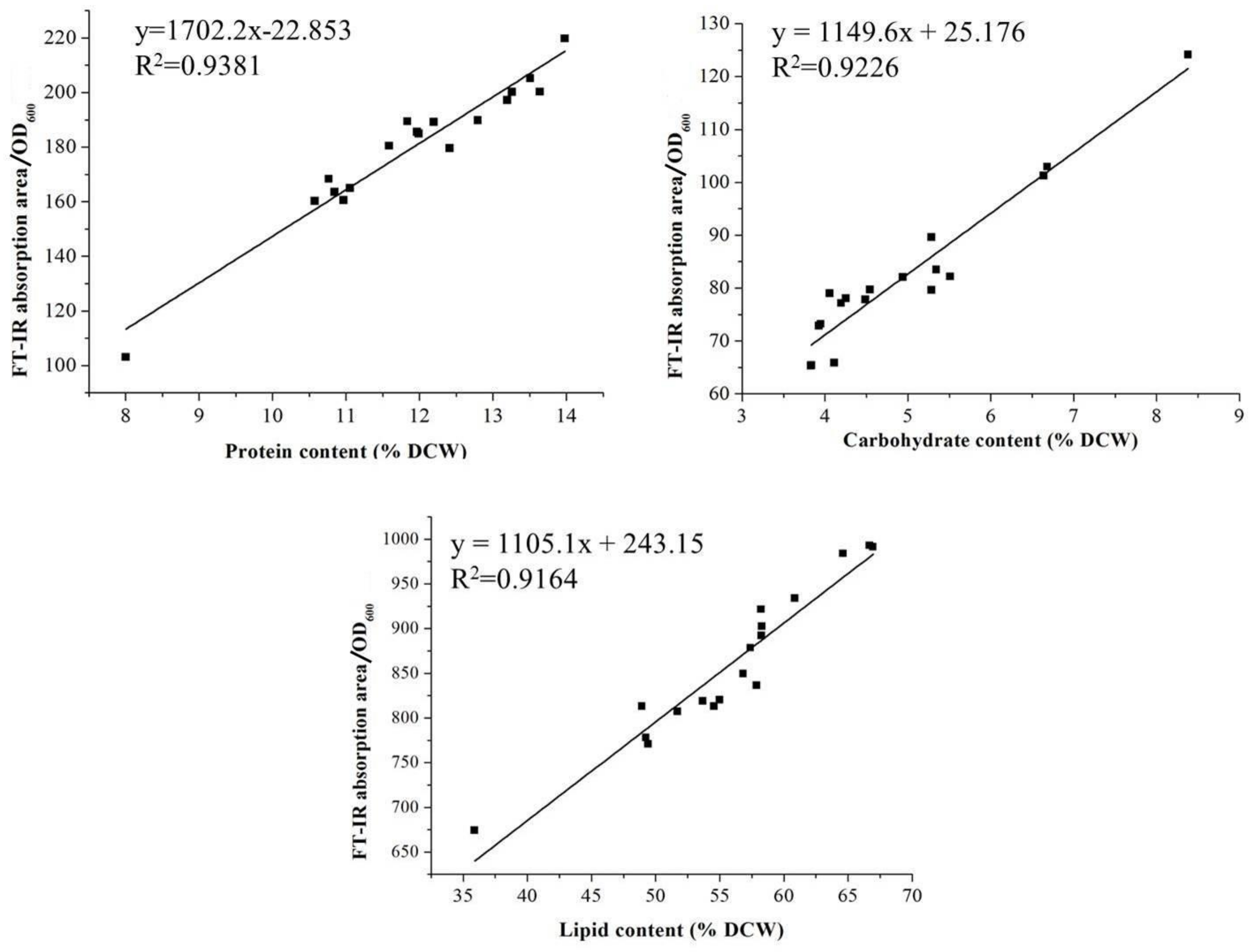
2.3. Assessments of the FT-IR and Traditional Methods
2.4. FT-IR for Monitoring Fermentative Parameters during Lipid and Carbohydrate Productions
3. Materials and Methods
3.1. Materials
3.2. Strains, Medium and Culture Conditions
3.3. FT-IR Spectroscopy Analysis
3.4. Determination of Biomass
3.5. Traditional Biochemical Analysis of Cellular Composition
3.6. Media Optimization for Lipid or Carbohydrate Productions
3.7. Fermentation in 5 L Bioreactor
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Horrocks, L.A.; Yeo, Y.K. Health benefits of docosahexaenoic acid (DHA). Pharmacol. Res. 1999, 40, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Madeira, M.S.; Cardoso, C.; Lopes, P.A.; Coelho, D.; Afonso, C.; Bandarra, N.M.; Prates, J.A.M. Microalgae as feed ingredients for livestock production and meat quality: A review. Livest. Sci. 2017, 205, 111–121. [Google Scholar] [CrossRef]
- Yokoyama, R.; Salleh, B.; Honda, D. Taxonomic rearrangement of the genus Ulkenia sensu lato based on morphology, chemotaxonomical characteristics, and 18S rRNA gene phylogeny (Thraustochytriaceae, Labyrinthulomycetes): Emendation for Ulkenia and erection of Botryochytrium, Parietichytrium, and Sicyoidochytrium gen. nov. Mycoscience 2007, 48, 329–341. [Google Scholar]
- Barclay, W.; Weaver, C.; Metz, J.; Hansen, J. 4-Development of a docosahexaenoic acid production technology using Schizochytrium: Historical perspective and update. In Single Cell Oils, 2nd ed.; Cohen, Z., Ratledge, C., Eds.; AOCS Press: Urbana, IL, USA, 2010; pp. 75–96. [Google Scholar]
- Xie, Y.X.; Sen, B.; Wang, G.Y. Mining terpenoids production and biosynthetic pathway in thraustochytrids. Bioresour. Technol. 2017, 244, 1269–1280. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.W.; Jiang, Y.; Ho, L.T.; Chen, F. Differentiation in fatty acid profiles of pigmented and nonpigmented Aurantiochytrium isolated from Hong Kong mangroves. J. Agric. Food Chem. 2009, 57, 6334–6341. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.J.; Liu, J.H.; Sun, J.; Zheng, J.Y.; Zhang, Y.J.; Wang, Z. Docosahexaenoic acid production from the acidic hydrolysate of Jerusalem artichoke by an efficient sugar-utilizing Aurantiochytrium sp. YLH70. Ind. Crop. Prod. 2016, 83, 372–378. [Google Scholar] [CrossRef]
- Yu, X.J.; Sun, J.; Sun, Y.Q.; Zheng, J.Y.; Wang, Z. Metabolomics analysis of phytohormone gibberellin improving lipid and DHA accumulation in Aurantiochytrium sp. Biochem. Eng. J. 2016, 112, 258–268. [Google Scholar] [CrossRef]
- Manikan, V.; Nazir, M.Y.M.; Kalil, M.S.; Isa, M.H.M.; Kader, A.J.A.; Yusoff, W.M.W.; Hamid, A.A. A new strain of docosahexaenoic acid producing microalga from Malaysian coastal waters. Algal. Res. 2015, 9, 40–47. [Google Scholar] [CrossRef]
- Montalbo-Lomboy, M.; Kantekin, M.N.; Wang, T. Lipid Estimation of surfactant-extracted microalgae oil using Nile Red. J. Am. Oil. Chem. Soc. 2014, 91, 665–680. [Google Scholar] [CrossRef]
- Kwak, M.; Kang, S.G.; Hong, W.K.; Han, J.I.; Chang, Y.K. Simultaneous cell disruption and lipid extraction of wet Aurantiochytrium sp. KRS101 using a high shear mixer. Bioprocess Biosyst. Eng. 2018, 41, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.; Han, J.I. Ultrasound-assisted in-situ transesterification of wet Aurantiochytrium sp. KRS 101 using potassium carbonate. Bioresour. Technol. 2018, 261, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Honda, K.; Okano, K.; Aki, T.; Omasa, T.; Ohtake, H. Thermal analysis for differentiating between oleaginous and non-oleaginous microorganisms. Biochem. Eng. J. 2011, 57, 23–29. [Google Scholar] [CrossRef]
- Arney, B.; Liu, W.S.; Forster, I.P.; McKinley, R.S.; Pearce, C.M. Feasibility of dietary substitution of live microalgae with spray-dried Schizochytrium sp. or Spirulina in the hatchery culture of juveniles of the Pacific geoduck clam (Panopea generosa). Aquaculture 2015, 444, 117–133. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Somani, B.L.; Khanade, J.; Sinha, R. A modified anthrone-sulfuric acid method for the determination of fructose in the presence of certain proteins. Anal. Biochem. 1987, 167, 327–330. [Google Scholar] [CrossRef]
- Giordano, M.; Kansiz, M.; Heraud, P.; Beardall, J.; Wood, B.; McNaughton, D. Fourier transform infrared spectroscopy as a novel tool to investigate changes in intracellular macromolecular pools in the marine microalga Chaetoceros muellerii (Bacillariophyceae). J. Phycol. 2001, 37, 271–279. [Google Scholar] [CrossRef]
- Meng, Y.; Yao, C.; Xue, S.; Yang, H. Application of Fourier transform infrared (FT-IR) spectroscopy in determination of microalgal compositions. Bioresour. Technol. 2014, 151, 347–354. [Google Scholar] [CrossRef]
- Vongsvivut, J.; Heraud, P.; Gupta, A.; Puri, M.; McNaughton, D.; Barrow, C.J. FTIR microspectroscopy for rapid screening and monitoring of polyunsaturated fatty acid production in commercially valuable marine yeasts and protists. Analyst 2013, 138, 6016–6031. [Google Scholar] [CrossRef]
- Gautam, R.; Varma, A.K.; Vinu, R. Apparent kinetics of fast pyrolysis of four different microalgae and product analyses using pyrolysis-FTIR and pyrolysis-GC/MS. Energ. Fuel 2017, 31, 12339–12349. [Google Scholar] [CrossRef]
- Vo, T.K.; Lee, O.K.; Lee, E.Y.; Kim, C.H.; Seo, J.W.; Kim, J.; Kim, S.S. Kinetics study of the hydrothermal liquefaction of the microalga Aurantiochytrium sp. KRS101. Chem. Eng. J. 2016, 306, 763–771. [Google Scholar] [CrossRef]
- Sun, L.; Ren, L.; Zhuang, X.; Ji, X.; Yan, J.; Huang, H. Differential effects of nutrient limitations on biochemical constituents and docosahexaenoic acid production of Schizochytrium sp. Bioresour. Technol. 2014, 159, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Marcotte, L.; Kegelaer, G.; Sandt, C.; Barbeau, J.; Lafleur, M. An alternative infrared spectroscopy assay for the quantification of polysaccharides in bacterial samples. Anal. Biochem. 2007, 361, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Palmucci, M.; Ratti, S.; Giordano, M. Ecological and evolutionary implications of carbon allocation in marine phytoplankton as a function of nitrogen availability: A fourier transform infrared spectroscopy approach. J. Phycol. 2011, 47, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Pistorius, A.M.A.; DeGrip, W.J.; Egorova-Zachernyuk, T.A. Monitoring of biomass composition from microbiological sources by means of FT-IR spectroscopy. Biotechnol. Bioeng. 2009, 103, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.J.; Yu, Z.Q.; Liu, Y.L.; Sun, J.; Zheng, J.Y.; Wang, Z. Utilization of high-fructose corn syrup for biomass production containing high levels of docosahexaenoic acid by a newly isolated Aurantiochytrium sp. YLH70. Appl. Biochem. Biotechnol. 2015, 177, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Yokochi, T.; Honda, D.; Higashihara, T.; Nakahara, T. Optimization of docosahexaenoic acid production by Schizochytrium limacinum SR21. Appl. Microbiol. Biotechnol. 1998, 49, 72–76. [Google Scholar] [CrossRef]
- Huang, T.Y.; Lu, W.C.; Chu, I.M. A fermentation strategy for producing docosahexaenoic acid in Aurantiochytrium limacinum SR21 and increasing C22:6 proportions in total fatty acid. Bioresour. Technol. 2012, 123, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Ryu, B.G.; Kim, K.; Kim, J.; Han, J.I.; Yang, J.W. Use of organic waste from the brewery industry for high-density cultivation of the docosahexaenoic acid-rich microalga, Aurantiochytrium sp. KRS101. Bioresour. Technol. 2013, 129, 351–359. [Google Scholar] [CrossRef]
- Pyle, D.J.; Garcia, R.A.; Wen, Z.Y. Producing docosahexaenoic acid (DHA)-rich algae from blodiesel-derived crude glycerol: Effects of impurities on DHA production and algal biomass composition. J. Agric. Food Chem. 2008, 56, 3933–3939. [Google Scholar] [CrossRef]
- Nagano, N.; Taoka, Y.; Honda, D.; Hayashi, M. Effect of trace elements on growth of marine eukaryotes, tharaustochytrids. J. Biosci. Bioeng. 2013, 116, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Liu, Z.X.; Langner, U.; Stehfest, K.; Wilhelm, C. The use of FTIR spectroscopy to assess quantitative changes in the biochemical composition of microalgae. J. Biophotonics 2010, 3, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.D.; Zhang, F.; Cheng, L.H.; Xu, X.H.; Zhang, L.; Chen, H.L. Evaluation of FT-IR and Nile Red methods for microalgal lipid characterization and biomass composition determination. Bioresour. Technol. 2013, 128, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Ethier, S.; Woisard, K.; Vaughan, D.; Wen, Z.Y. Continuous culture of the microalgae Schizochytrium limacinum on biodiesel-derived crude glycerol for producing docosahexaenoic acid. Bioresour. Technol. 2011, 102, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Dean, A.P.; Nicholson, J.M.; Sigee, D.C. Impact of phosphorus quota and growth phase on carbon allocation in Chlamydomonas reinhardtii: An FTIR microspectroscopy study. Eur. J. Phycol. 2008, 43, 345–354. [Google Scholar] [CrossRef]
- Pacheco-Vega, J.M.; Cadena-Roa, M.A.; Ascencio, F.; Rangel-Davalos, C.; Rojas-Contreras, M. Assessment of endemic microalgae as potential food for Artemia franciscana culture. Lat. Am. J. Aquat. Res. 2015, 43, 23–32. [Google Scholar] [CrossRef]
- Brown, M.R.; McCausland, M.A.; Kowalski, K. The nutritional value of four Australian microalgal strains fed to Pacific oyster Crassostrea gigas spat. Aquaculture 1998, 165, 281–293. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds microalgal lipid and carbohydrate are available from the authors. |




| Evaluation Index | Traditional Biochemical Methods | FT-IR Method |
|---|---|---|
| Cultivation scale | 50 mL (in 250 mL flash) | 200 μL (in tube) |
| Sample condition | Lyophilized cells | Liquid sample |
| Time consumed | ~72 h | ~4 h |
| Chemical reagents | Liquid nitrogen, chloroform, and methanol for lipid; sulfuric acid and anthrone for carbohydrate | None |
| Equipment | Centrifuge, freeze dryer, mortar, pestle, evaporimeter, and balance | FT-IR spectrometer and spectrophotometer |
| Cost | Very high | Low |
| Accuracy | High | High |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.-J.; Huang, C.-Y.; Chen, H.; Wang, D.-S.; Chen, J.-L.; Li, H.-J.; Liu, X.-Y.; Wang, Z.; Sun, J.; Wang, Z.-P. High-Throughput Biochemical Fingerprinting of Oleaginous Aurantiochytrium sp. Strains by Fourier Transform Infrared Spectroscopy (FT-IR) for Lipid and Carbohydrate Productions. Molecules 2019, 24, 1593. https://doi.org/10.3390/molecules24081593
Yu X-J, Huang C-Y, Chen H, Wang D-S, Chen J-L, Li H-J, Liu X-Y, Wang Z, Sun J, Wang Z-P. High-Throughput Biochemical Fingerprinting of Oleaginous Aurantiochytrium sp. Strains by Fourier Transform Infrared Spectroscopy (FT-IR) for Lipid and Carbohydrate Productions. Molecules. 2019; 24(8):1593. https://doi.org/10.3390/molecules24081593
Chicago/Turabian StyleYu, Xin-Jun, Chang-Yi Huang, Hong Chen, Dong-Sheng Wang, Jing-Liang Chen, Hui-Juan Li, Xiao-Yan Liu, Zhao Wang, Jie Sun, and Zhi-Peng Wang. 2019. "High-Throughput Biochemical Fingerprinting of Oleaginous Aurantiochytrium sp. Strains by Fourier Transform Infrared Spectroscopy (FT-IR) for Lipid and Carbohydrate Productions" Molecules 24, no. 8: 1593. https://doi.org/10.3390/molecules24081593





