The Study of the Aggregated Pattern of TX100 Micelle by Using Solvent Paramagnetic Relaxation Enhancements
Abstract
:1. Introduction
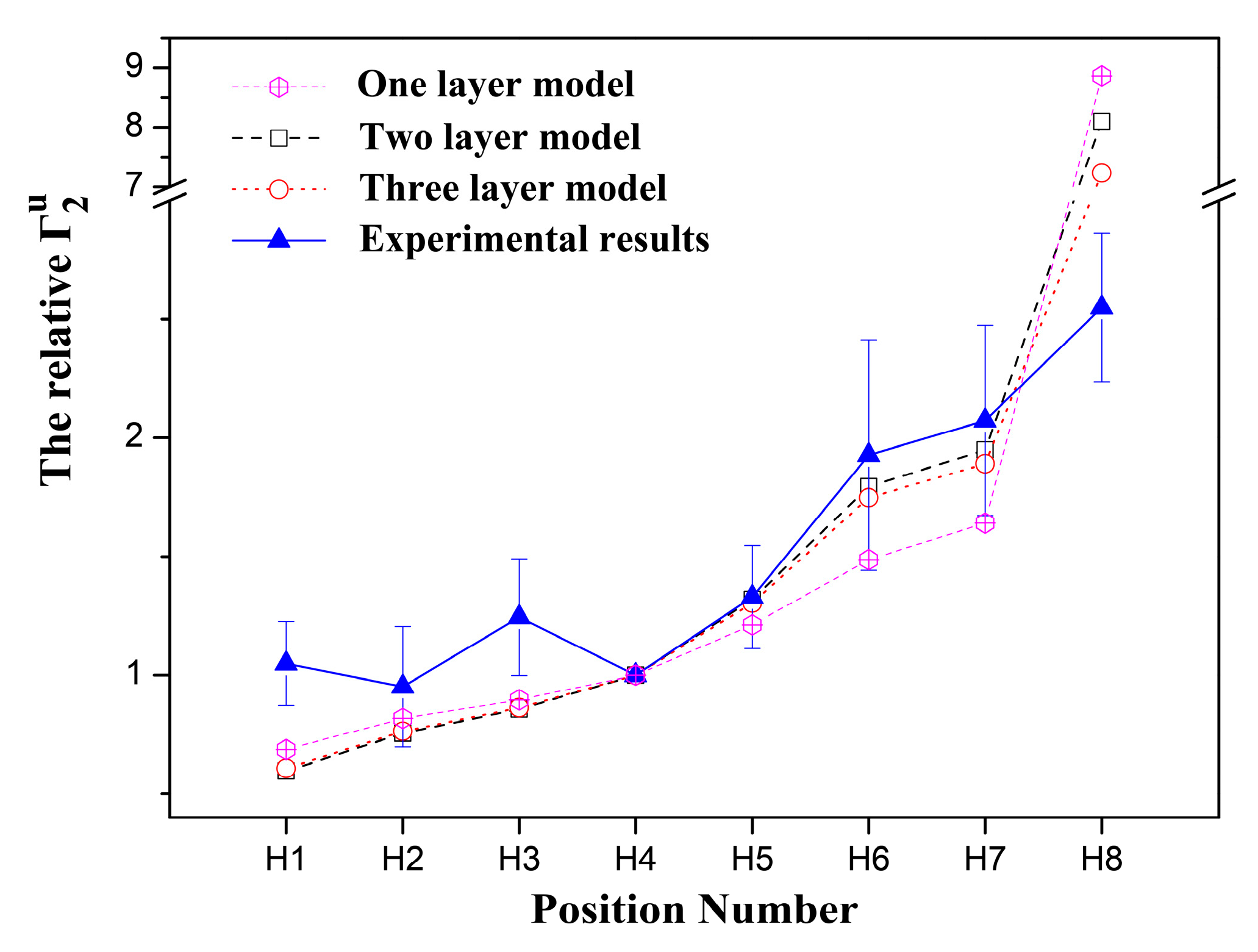
2. Results and Discussion
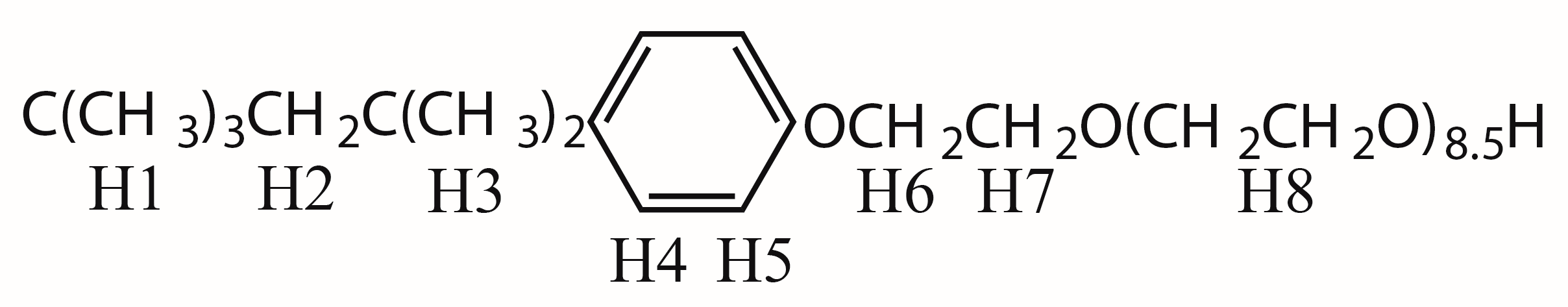
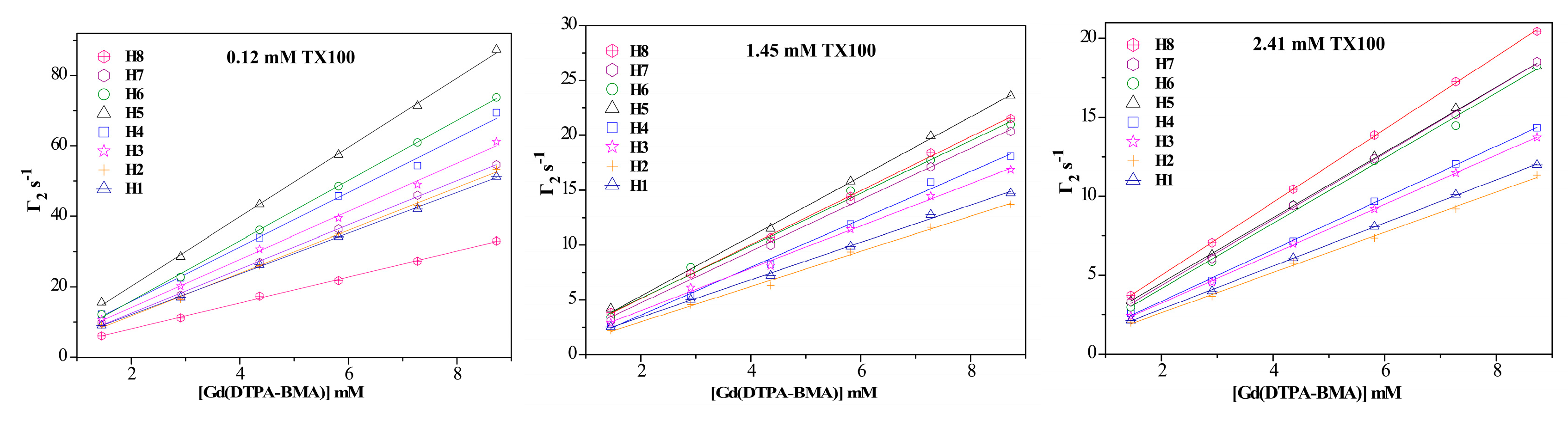
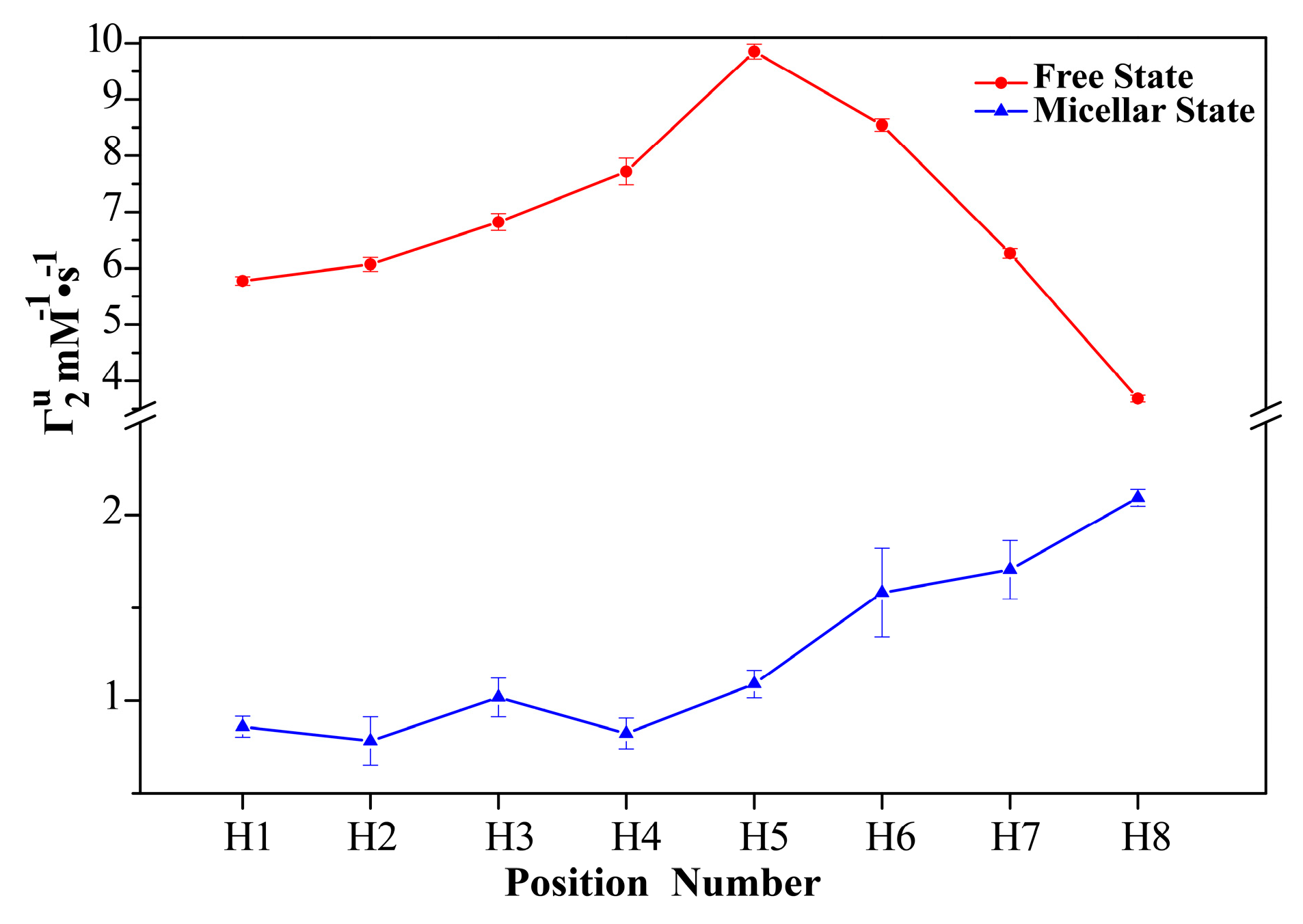
2.1. 1H sPREs of TX100
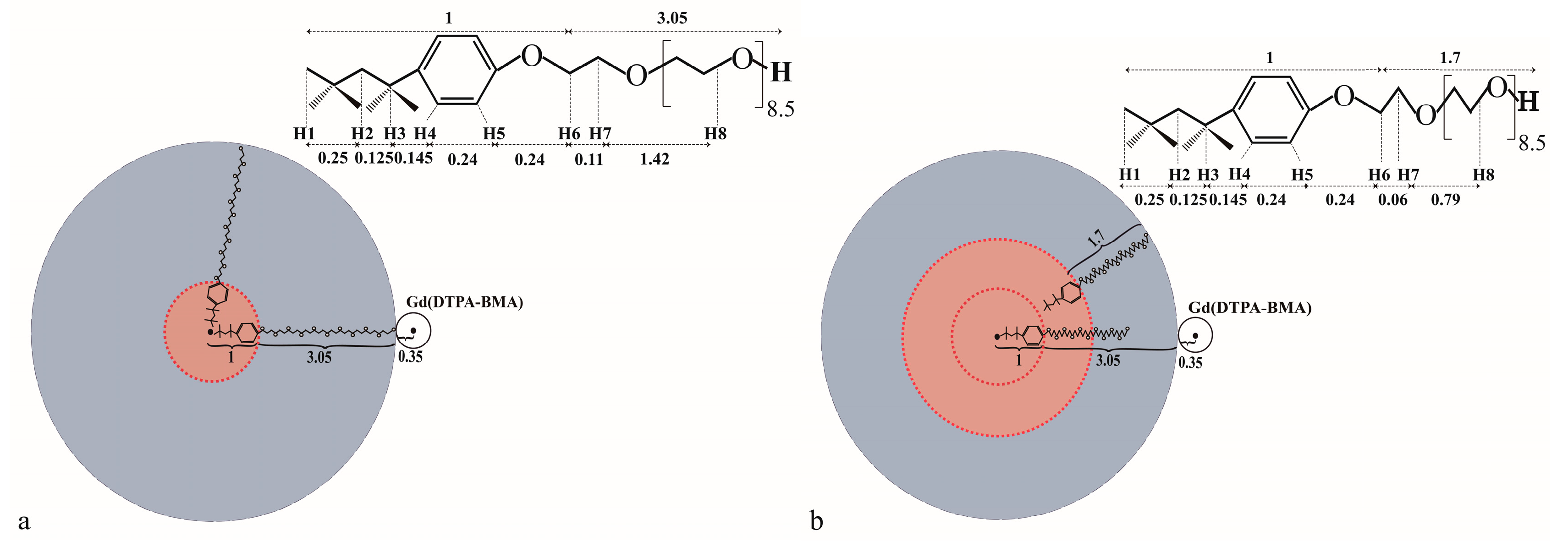
2.2. 1D Selective ROESY of TX100
3. Materials and Methods
3.1. Instruments and Reagents
3.2. Sample Preparation
3.3. NMR Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Denkova, P.S.; Van Lokeren, L.; Verbruggen, I.; Willem, R. Self-aggregation and supramolecular structure investigations of triton X-100 and SDP2S by NOESY and diffusion ordered NMR spectroscopy. J. Phys. Chem. B 2008, 112, 10935–10941. [Google Scholar] [CrossRef]
- Anand, U.; Jash, C.; Mukherjee, S. Spectroscopic determination of Critical Micelle Concentration in aqueous and non-aqueous media using a non-invasive method. J. Colloid Interf. Sci. 2011, 364, 400–406. [Google Scholar] [CrossRef]
- Cui, X.H.; Mao, S.Z.; Liu, M.L.; Yuan, H.Z.; Du, Y.R. Mechanism of surfactant micelle formation. Langmuir 2008, 24, 10771–10775. [Google Scholar] [CrossRef]
- Koley, D.; Bard, A.J. Triton X-100 concentration effects on membrane permeability of a single HeLa cell by scanning electrochemical microscopy (SECM). Proc. Natl. Acad. Sci. USA 2010, 107, 16783–16787. [Google Scholar] [CrossRef] [Green Version]
- Mattei, B.; Lira, R.B.; Perez, K.R.; Riske, K.A. Membrane permeabilization induced by Triton X-100: The role of membrane phase state and edge tension. Chem. Phys. Lipids 2017, 202, 28–37. [Google Scholar] [CrossRef]
- Gennuso, F.; Fernetti, C.; Tirolo, C.; Testa, N.; L’Episcopo, F.; Caniglia, S.; Morale, M.C.; Ostrow, J.D.; Pascolo, L.; Tiribelli, C.; et al. Bilirubin protects astrocytes from its own toxicity by inducing up-regulation and translocation of multidrug resistance-associated protein 1 (Mrp1). Proc. Natl. Acad. Sci. USA 2004, 101, 2470–2475. [Google Scholar] [CrossRef] [Green Version]
- Casadei, B.R.; Domingues, C.C.; de Paula, E.; Riske, K.A. Direct Visualization of the Action of Triton X-100 on Giant Vesicles of Erythrocyte Membrane Lipids. Biophys. J. 2014, 106, 2417–2425. [Google Scholar] [CrossRef] [Green Version]
- Carita, A.C.; Mattei, B.; Domingues, C.C.; de Paula, E.; Riske, K.A. Effect of Triton X-100 on Raft-Like Lipid Mixtures: Phase Separation and Selective Solubilization. Langmuir 2017, 33, 7312–7321. [Google Scholar] [CrossRef]
- Partearroyo, M.A.; Alonso, A.; Goni, F.M.; Tribout, M.; Paredes, S. Solubilization of phospholipid bilayers by surfactants belonging to the Triton X series: Effect of polar group size. J. Colloid Interf. Sci. 1996, 178, 156–159. [Google Scholar] [CrossRef]
- Mesa, M.; Pereanez, J.A.; Preciado, L.M.; Bernal, C. How the Triton X-100 modulates the activity/stability of the Thermomyces lanuginose lipase: Insights from experimental and molecular docking approaches. Int. J. Biol. Macromol. 2018, 120, 2410–2417. [Google Scholar] [CrossRef]
- Robson, R.J.; Dennis, E.A. The Size, Shape, and Hydration of Nonionic Surfactant Micelles. Triton X-100. J. Phys. Chem. 1977, 81, 11. [Google Scholar] [CrossRef]
- Streletzky, K.; Phillies, G.D.J. Temperature-Dependence of Triton X-100 Micelle Size and Hydration. Langmuir 1995, 11, 42–47. [Google Scholar] [CrossRef]
- Phillies, G.D.J.; Yambert, J.E. Solvent and solute effects on hydration and aggregation numbers of triton X-100 micelles. Langmuir 1996, 12, 3431–3436. [Google Scholar] [CrossRef]
- Valaulikar, B.S.; Manohar, C. The Mechanism of Clouding in Triton X-100 - the Effect of Additives. J. Colloid Interf. Sci. 1985, 108, 403–406. [Google Scholar] [CrossRef]
- Molina-Bolivar, J.A.; Aguiar, J.; Ruiz, C.C. Growth and hydration of Triton X-100 micelles in monovalent alkali salts: A light scattering study. J. Phys. Chem. B 2002, 106, 870–877. [Google Scholar] [CrossRef]
- Yuan, H.Z.; Cheng, G.Z.; Zhao, S.; Miao, X.J.; Yu, J.Y.; Shen, L.F.; Du, Y.R. Conformational dependence of triton X-100 on environment studied by 2D NOESY and H-1 NMR relaxation. Langmuir 2000, 16, 3030–3035. [Google Scholar] [CrossRef]
- De Nicola, A.; Kawakatsu, T.; Rosano, C.; Celino, M.; Rocco, M.; Milano, G. Self-Assembly of Triton X-100 in Water Solutions: A Multiscale Simulation Study Linking Mesoscale to Atomistic Models. J. Chem. Theory Comput. 2015, 11, 4959–4971. [Google Scholar] [CrossRef] [PubMed]
- Cabane, B. Structure of the water/surfactant interface in micelles: An NMR study of SDS micelles labelled with paramagnetic ions. J. Phys. 1981, 42, 847–859. [Google Scholar] [CrossRef]
- Zemb, T.; Chachaty, C. Alkyl Chain Conformations in a Micellar System from the Nuclear-Spin Relaxation Enhanced by Paramagnetic-Ions. Chem. Phys. Lett. 1982, 88, 68–73. [Google Scholar] [CrossRef]
- Chevalier, Y.; Chachaty, C. Hydrocarbon Chain Conformations in Micelles - a Nuclear Magnetic-Relaxation Study. J. Phys. Chem. 1985, 89, 875–880. [Google Scholar] [CrossRef]
- Wasylishen, R.E.; Kwak, J.C.T.; Gao, Z.S.; Verpoorte, E.; Macdonald, J.B.; Dickson, R.M. Nmr-Studies of Hydrocarbons Solubilized in Aqueous Micellar Solutions. Can. J. Chem. 1991, 69, 822–833. [Google Scholar] [CrossRef]
- Gao, Z.S.; Wasylishen, R.E.; Kwak, J.C.T. An Nmr Paramagnetic Relaxation Method to Determine Distribution Coefficients of Solubilizates in Micellar Systems. J. Phys. Chem. 1989, 93, 2190–2192. [Google Scholar] [CrossRef]
- Gao, Z.; Kwak, J.C.T.; Labonte, R.; Marangoni, D.G.; Wasylishen, R.E. Solubilization Equilibria of Alcohols and Polymers in Micellar Solutions - Nmr Paramagnetic Relaxation Studies. Colloid Surf. 1990, 45, 269–281. [Google Scholar] [CrossRef]
- Beswick, V.; Guerois, R.; Cordier-Ochsenbein, F.; Coic, Y.M.; Huynh-Dinh, T.; Tostain, J.; Noel, J.P.; Sanson, A.; Neumann, J.M. Dodecylphosphocholine micelles as a membrane like environment: New results from NMR relaxation and paramagnetic relaxation enhancement analysis. Eur. Biophys. J. Biophy. 1998, 28, 48–58. [Google Scholar] [CrossRef]
- Hocking, H.G.; Zangger, K.; Madl, T. Studying the structure and dynamics of biomolecules by using soluble paramagnetic probes. Chemphyschem 2013, 14, 3082–3094. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Schwieters, C.D.; Tang, C. Theory and practice of using solvent paramagnetic relaxation enhancement to characterize protein conformational dynamics. Methods 2018, 148, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Madl, T.; Guttler, T.; Gorlich, D.; Sattler, M. Structural Analysis of Large Protein Complexes Using Solvent Paramagnetic Relaxation Enhancements. Angew Chem. Int. Edit. 2011, 50, 3993–3997. [Google Scholar] [CrossRef]
- Zhang, L.; Chai, X.; Sun, P.; Zhu, Q.J.; Zhang, X.; Liu, M.L. Characterization of the aggregated pattern of CHAPS using solvent paramagnetic relaxation enhancements. Colloid Surf. A 2018, 555, 332–338. [Google Scholar] [CrossRef]
- Solomon, I. Relaxation Processes in a System of 2 Spins. Phys. Rev. 1955, 99, 559–565. [Google Scholar] [CrossRef]
- Respondek, M.; Madl, T.; Gobl, C.; Golser, R.; Zangger, K. Mapping the orientation of helices in micelle-bound peptides by paramagnetic relaxation waves. J. Am. Chem. Soc. 2007, 129, 5228–5234. [Google Scholar] [CrossRef]
- Regev, O.; Zana, R. Aggregation behavior of Tyloxapol, a nonionic surfactant oligomer, in aqueous solution. J. Colloid Interf. Sci. 1999, 210, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Phillies, G.D.J.; Stott, J.; Ren, S.Z. Probe Diffusion in the Presence of Nonionic Amphiphiles - Triton X-100. J. Phys. Chem. 1993, 97, 11563–11568. [Google Scholar] [CrossRef]
- Giorgio, G.; Colafemmina, G.; Mavelli, F.; Murgia, S.; Palazzo, G. The impact of alkanes on the structure of Triton X100 micelles. RSC Adv. 2016, 6, 825–836. [Google Scholar] [CrossRef]
- Zangger, K.; Respondek, M.; Goebl, C.; Hohlweg, W.; Rasmussen, K.; Grampp, G.; Madl, T. Positioning of Micelle-Bound Peptides by Paramagnetic Relaxation Enhancements. J. Phys. Chem. B 2009, 113, 4400–4406. [Google Scholar] [CrossRef] [PubMed]
- Iwahara, J.; Schwieters, C.D.; Clore, G.M. Ensemble approach for NMR structure refinement against H-1 paramagnetic relaxation enhancement data arising from a flexible paramagnetic group attached to a macromolecule. J. Am. Chem. Soc. 2004, 126, 5879–5896. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.R.; Woolf, L.A. Temperature and volume dependence of the viscosity of water and heavy water at low temperatures. J. Chem. Eng. Data 2004, 49, 1064–1069. [Google Scholar] [CrossRef]
- Zwahlen, C.; Vincent, S.J.F.; Dibari, L.; Levitt, M.H.; Bodenhausen, G. Quenching Spin-Diffusion in Selective Measurements of Transient Overhauser Effects in Nuclear-Magnetic-Resonance- Applications to Oligonucleotides. J. Am. Chem. Soc. 1994, 116, 362–368. [Google Scholar] [CrossRef]
Sample Availability: Samples of the Tx100 and Gd(DTPA-BMA) are available from the authors. |

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Chai, X.; Sun, P.; Yuan, B.; Jiang, B.; Zhang, X.; Liu, M. The Study of the Aggregated Pattern of TX100 Micelle by Using Solvent Paramagnetic Relaxation Enhancements. Molecules 2019, 24, 1649. https://doi.org/10.3390/molecules24091649
Zhang L, Chai X, Sun P, Yuan B, Jiang B, Zhang X, Liu M. The Study of the Aggregated Pattern of TX100 Micelle by Using Solvent Paramagnetic Relaxation Enhancements. Molecules. 2019; 24(9):1649. https://doi.org/10.3390/molecules24091649
Chicago/Turabian StyleZhang, Liang, Xin Chai, Peng Sun, Bin Yuan, Bin Jiang, Xu Zhang, and Maili Liu. 2019. "The Study of the Aggregated Pattern of TX100 Micelle by Using Solvent Paramagnetic Relaxation Enhancements" Molecules 24, no. 9: 1649. https://doi.org/10.3390/molecules24091649





