Bone Regeneration Induced by Strontium Folate Loaded Biohybrid Scaffolds
Abstract
:1. Introduction
2. Factors Involved in the Repair of Bone Tissue
3. Dental Pulp Stem Cells and Osteogenic Differentiation
4. The Use of Strontium in Bone Repair
5. Role of Folic Acid and Vitamins B6 and B12 in Bone Regeneration
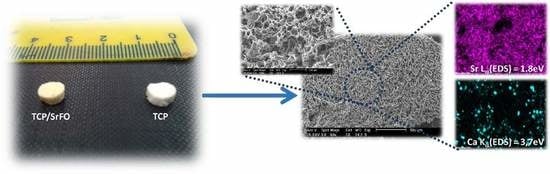
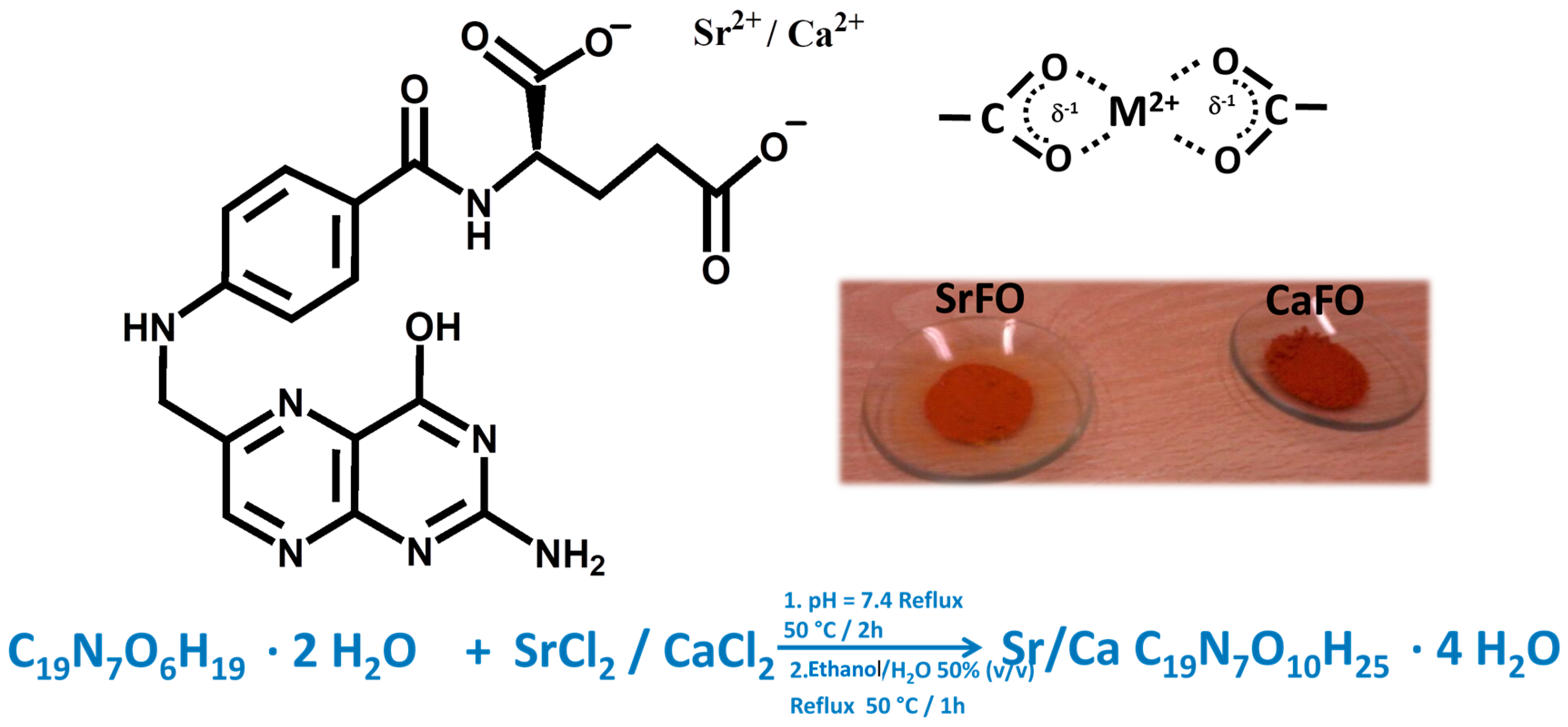
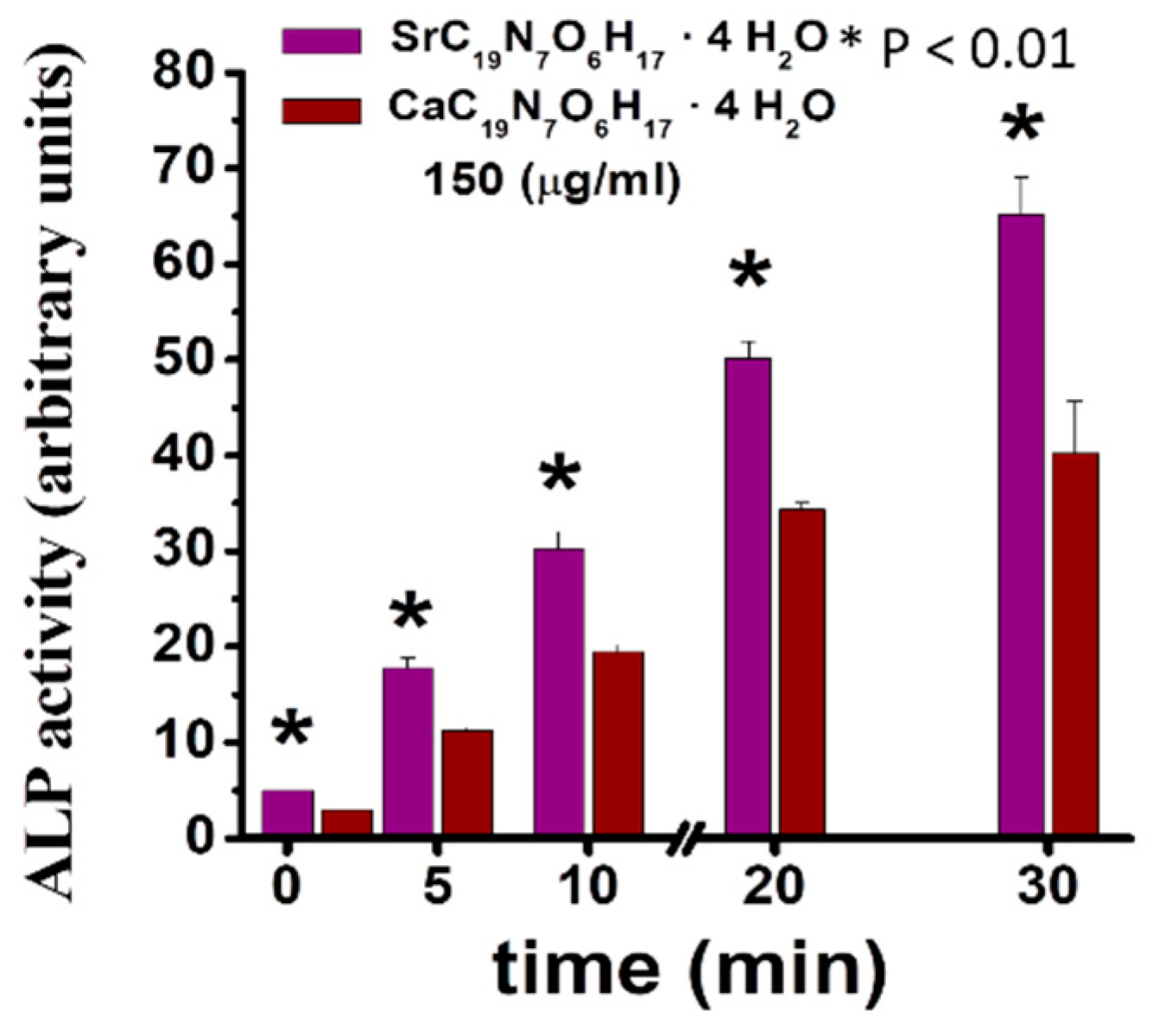
6. Synthesis and Properties of SrFO
7. SrFO Loaded Biohybrid Scaffold Seeded with Dental Pulp Stem Cells as a New Composite System
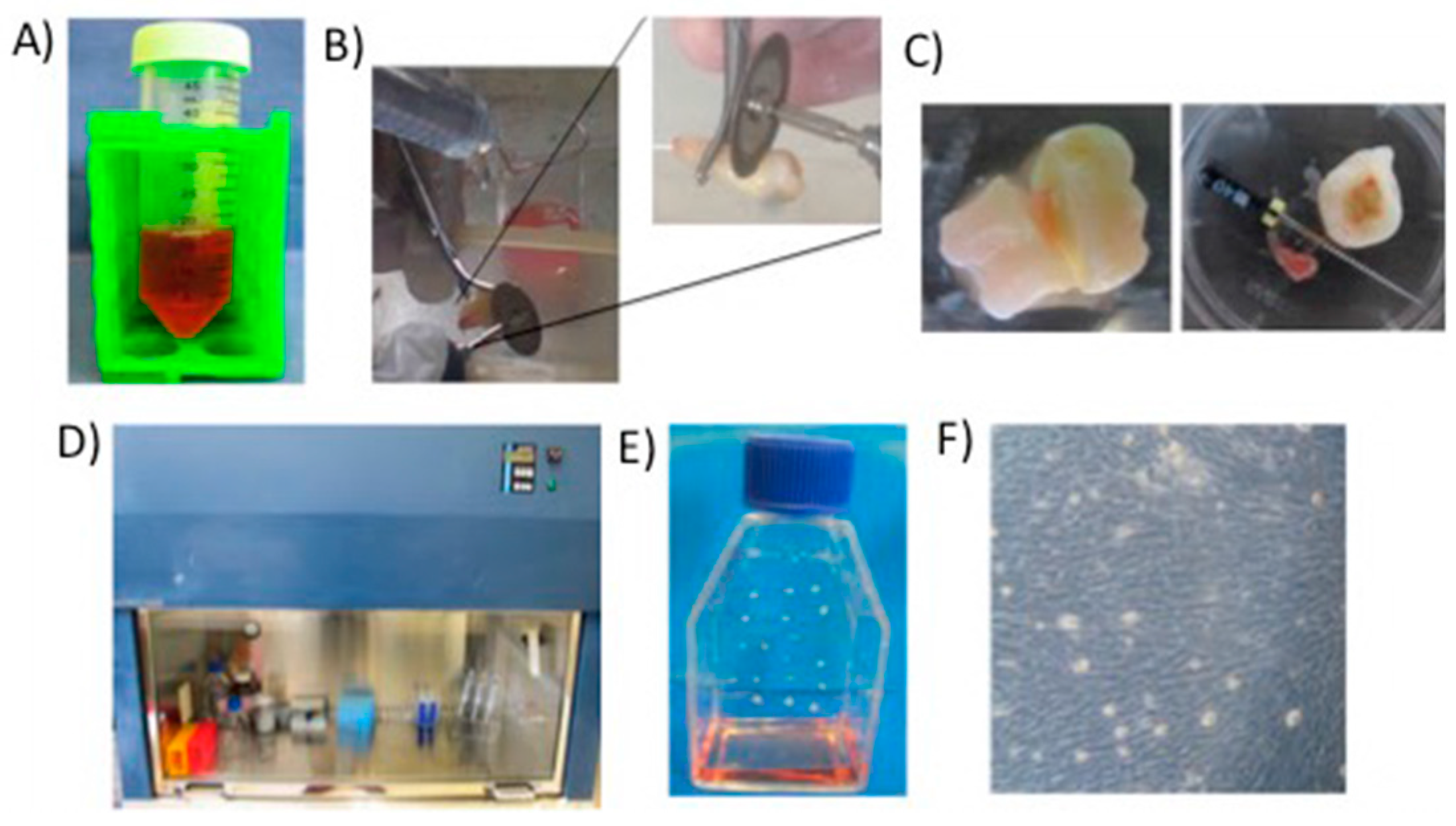
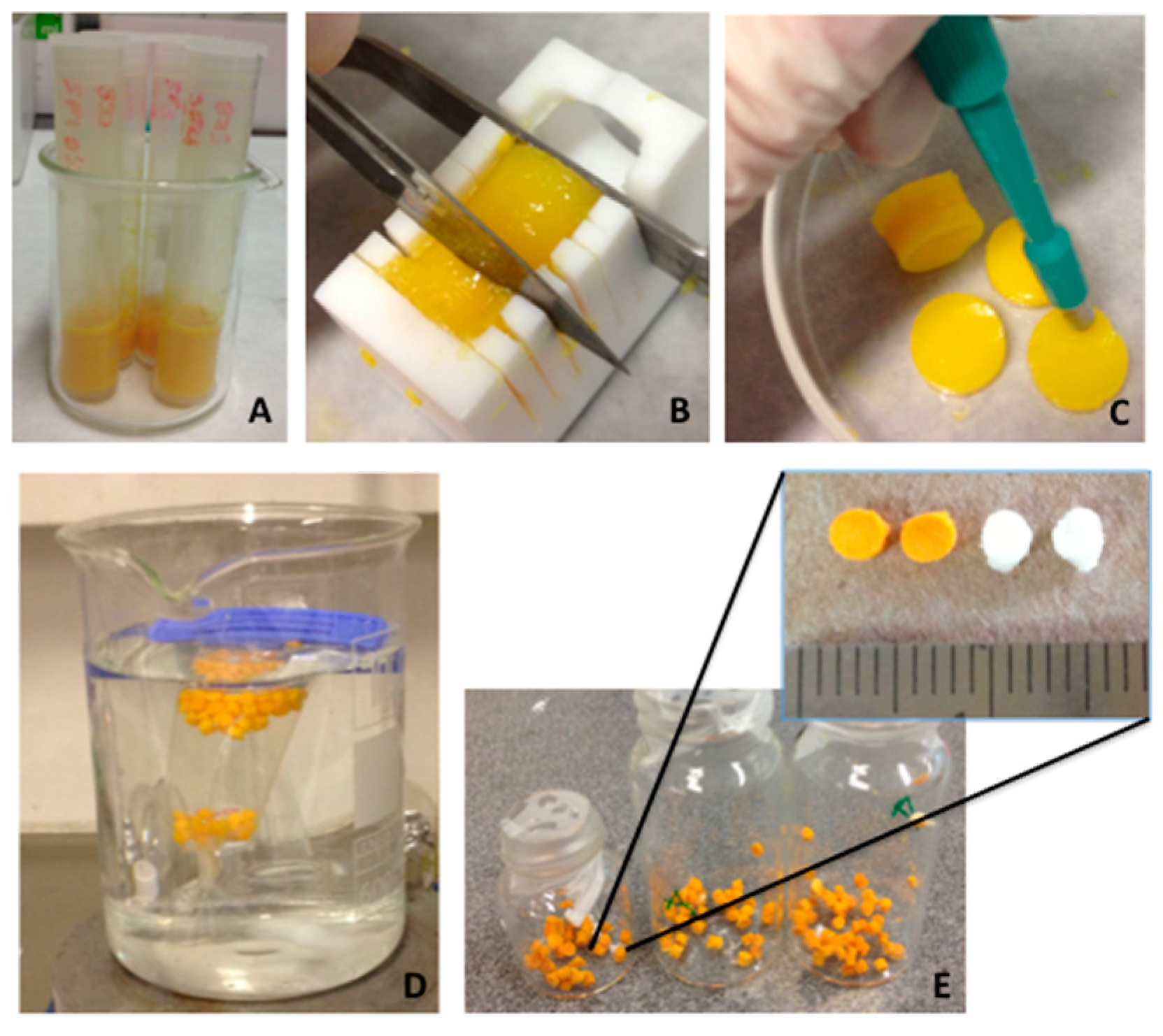
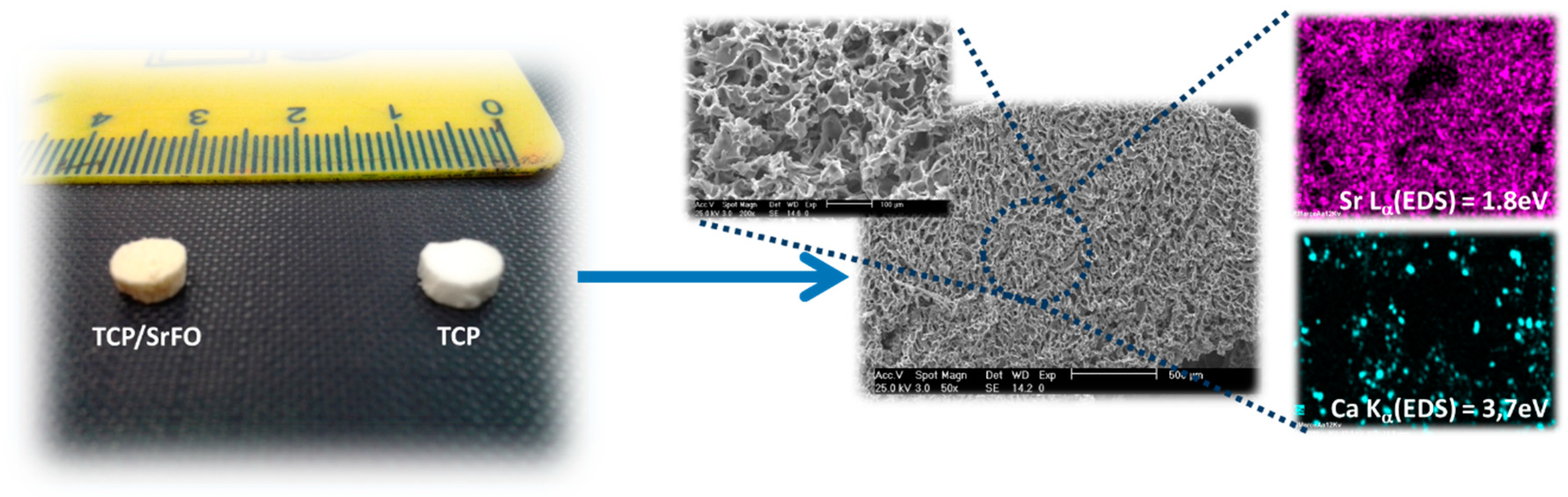
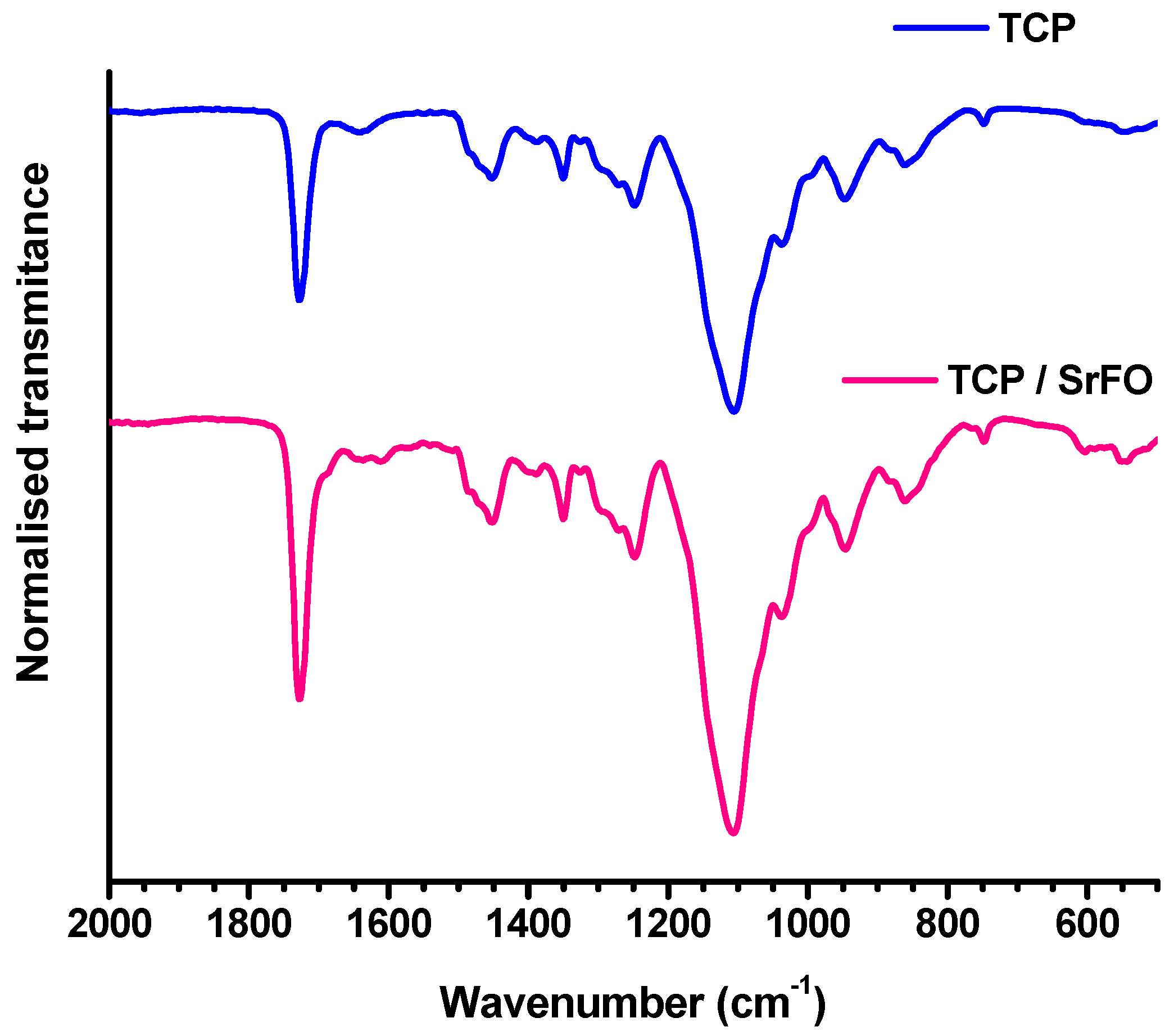
7.1. Scaffolds Preparation Procedures and Characterization Techniques
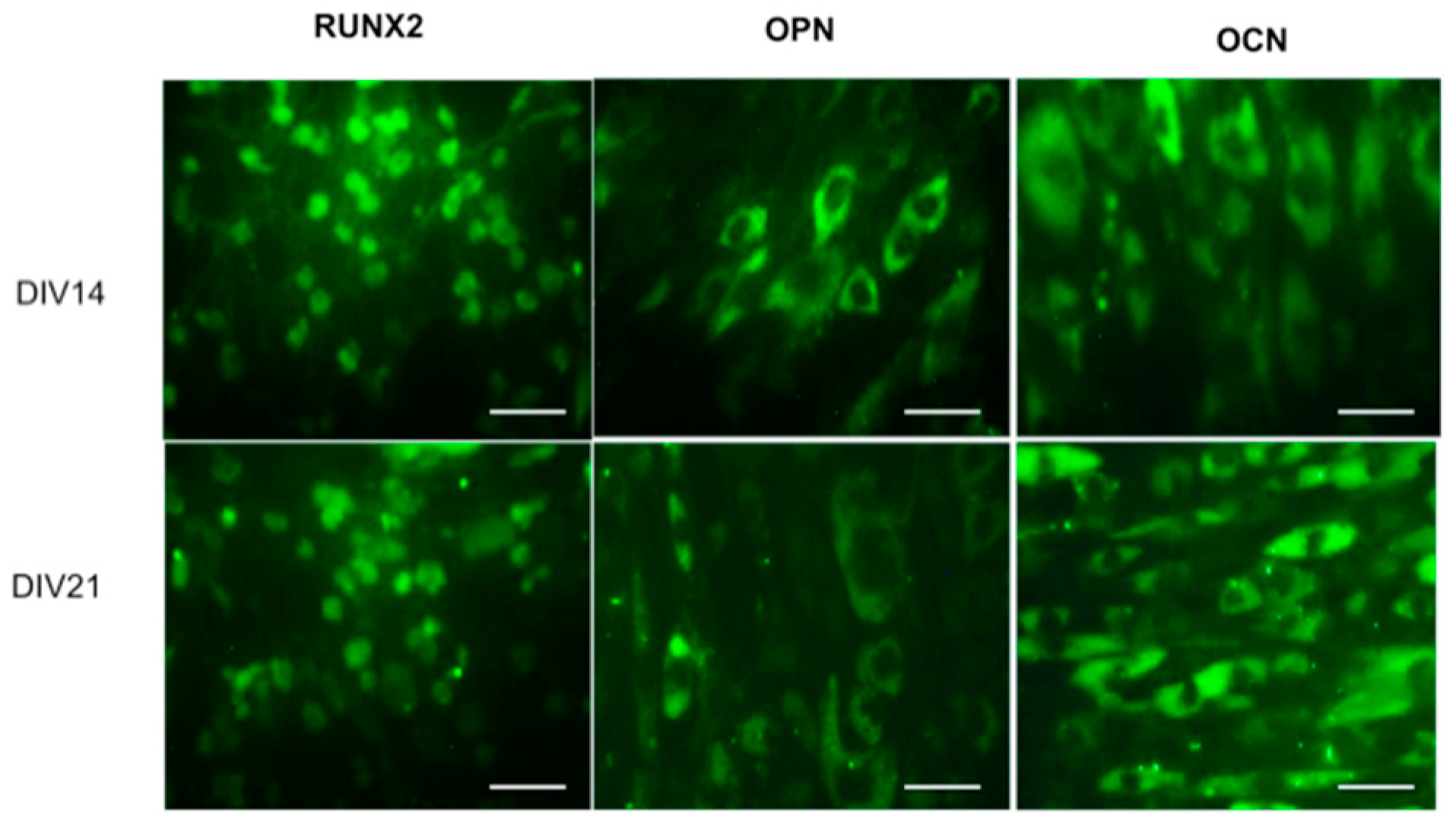
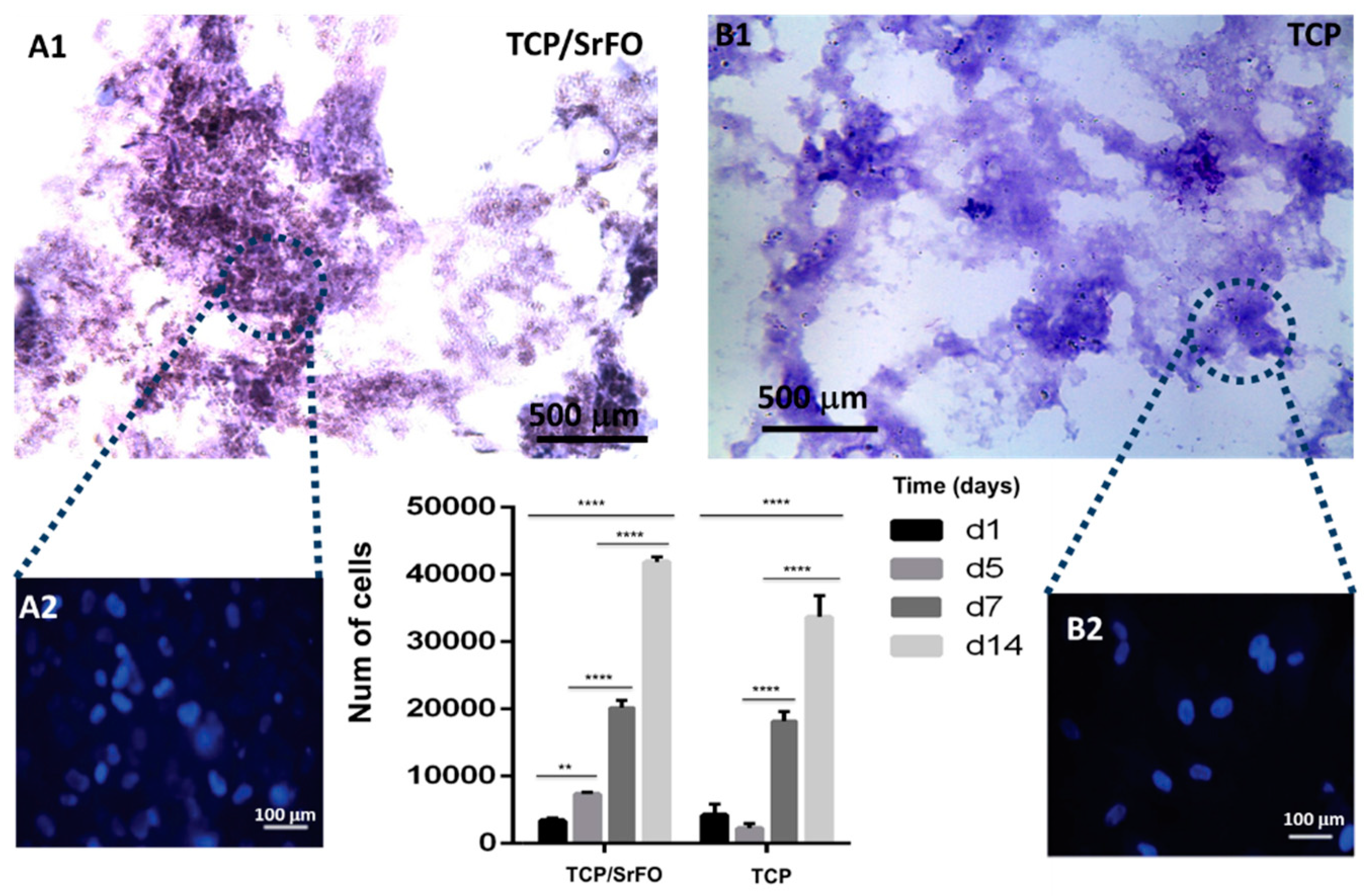
7.2. DPSC Viability on Scaffolds
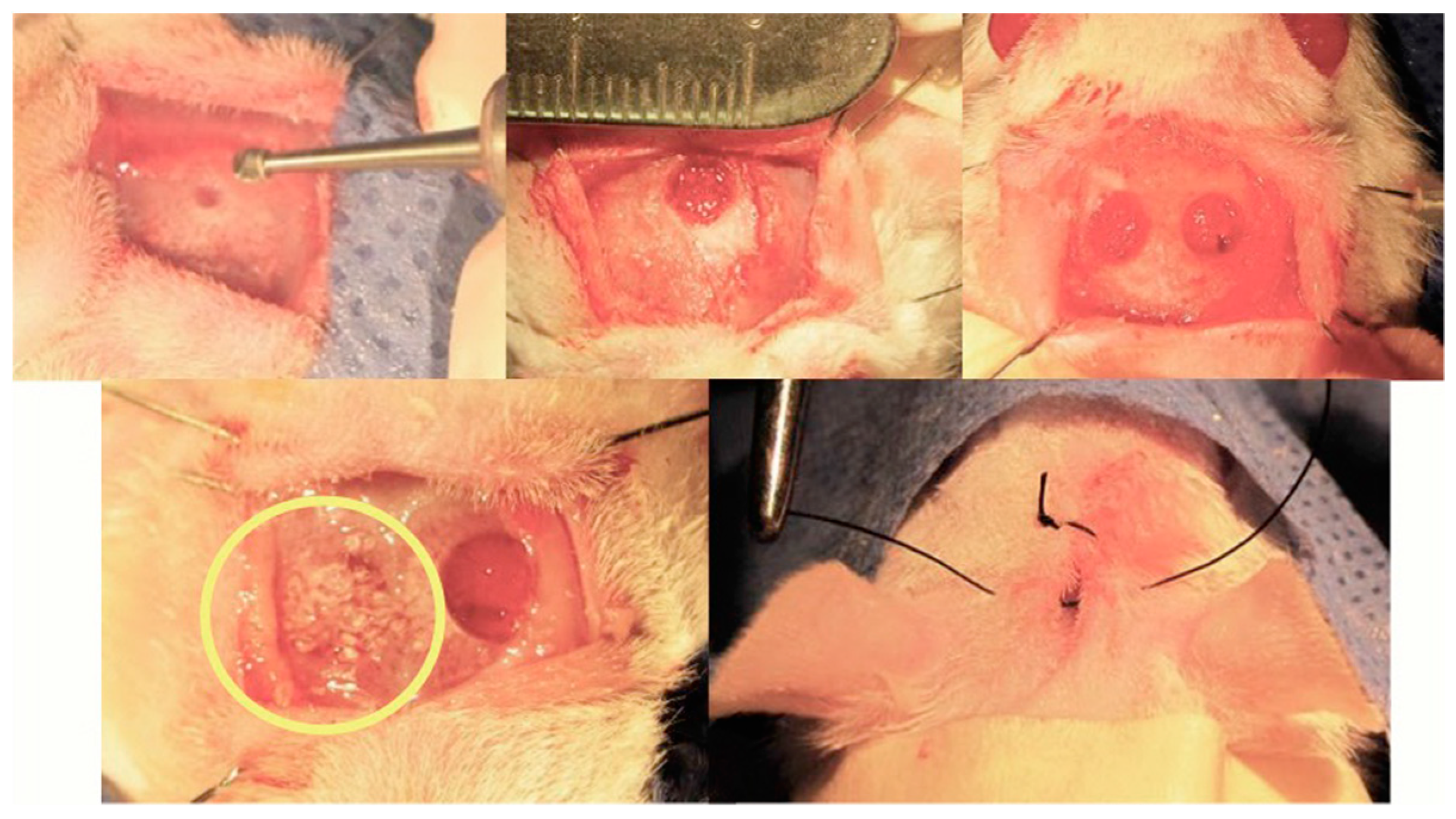
7.3. Rat Calvarial Defect Model and Implantation of the Scaffold
8. Perspectives and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rojo, L. Combination of Polymeric Supports and Drug Delivery Systems for Osteochondral Regeneration. In Osteochondral Tissue Engineering; Oliveira, J.M., Pina, S., Reis, R.L., San Roman, J., Eds.; Springer International Publishing: Cham, Switzerland, 2018; Volume 1059, pp. 301–313. ISBN 978-3-319-76734-5. [Google Scholar]
- Black, C.R.M.; Goriainov, V.; Gibbs, D.; Kanczler, J.; Tare, R.S.; Oreffo, R.O.C. Bone Tissue Engineering. Curr. Mol. Biol. Rep. 2015, 1, 132–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutmacher, D.W.; Schantz, J.T.; Lam, C.X.F.; Tan, K.C.; Lim, T.C. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J. Tissue Eng. Regen. Med. 2007, 1, 245–260. [Google Scholar] [CrossRef]
- Ciraldo, F.E.; Boccardi, E.; Melli, V.; Westhauser, F.; Boccaccini, A.R. Tackling bioactive glass excessive in vitro bioreactivity: Preconditioning approaches for cell culture tests. Acta Biomater. 2018, 75, 3–10. [Google Scholar] [CrossRef]
- Westhauser, F.; Höllig, M.; Reible, B.; Xiao, K.; Schmidmaier, G.; Moghaddam, A. Bone formation of human mesenchymal stem cells harvested from reaming debris is stimulated by low-dose bone morphogenetic protein-7 application in vivo. J. Orthop. 2016, 13, 404–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa, A.L.; de Oliveira, P.T.; Beloti, M.M. Macroporous scaffolds associated with cells to construct a hybrid biomaterial for bone tissue engineering. Expert Rev. Med. Devices 2008, 5, 719–728. [Google Scholar] [CrossRef]
- Sachlos, E.; Czernuszka, J.T. Making tissue engineering scaffolds work. Review: The application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Eur. Cell. Mater. 2003, 5, 29–39; discussion 39–40. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Yin, Z.; Li, C.; Yang, Z.; Ning, C.; Zhou, D.; Wang, R.; Xu, Y.; Qiu, J. Efficient and toxicity-free surface immobilization of nano-hydroxyapatite for bone-regenerative composite scaffolds by grafting polyvinyl pyrrolidone. Mater. Sci. Eng. C 2012, 32, 1032–1036. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Denry, I.; Goudouri, O.-M.; Fredericks, D.C.; Akkouch, A.; Acevedo, M.R.; Holloway, J.A. Strontium-releasing fluorapatite glass-ceramic scaffolds: Structural characterization and in vivo performance. Acta Biomater. 2018, 75, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Rojo, L.; Vázquez, B.; San Román, J. Biomaterials for scaffolds: Synthetic polymers. In Scaffolds for Tissue Engineering: Biological Design, Materials, and Fabrication, 1st ed.; Pan Stanford Publishing Pte. Ltd.: Singapore, 10 June 2014; pp. 263–300. [Google Scholar]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [Green Version]
- LeGeros, R.Z. Properties of osteoconductive biomaterials: Calcium phosphates. Clin. Orthop. 2002, 81–98. [Google Scholar] [CrossRef]
- Jiménez, M.; Abradelo, C.; Román, J.S.; Rojo, L. Bibliographic review on the state of the art of strontium and zinc based regenerative therapies. Recent developments and clinical applications. J. Mater. Chem. B 2019, 7, 1974–1985. [Google Scholar] [CrossRef]
- Place, E.S.; Rojo, L.; Gentleman, E.; Sardinha, J.P.; Stevens, M.M. Strontium- and zinc-alginate hydrogels for bone tissue engineering. Tissue Eng. Part A 2011, 17, 2713–2722. [Google Scholar] [CrossRef]
- Martin-del-Campo, M.; Rosales-Ibañez, R.; Alvarado, K.; Sampedro, J.G.; Garcia-Sepulveda, C.A.; Deb, S.; San Román, J.; Rojo, L. Strontium folate loaded biohybrid scaffolds seeded with dental pulp stem cells induce in vivo bone regeneration in critical sized defects. Biomater. Sci. 2016, 4, 1596–1604. [Google Scholar] [CrossRef]
- Xu, K.; Chen, W.; Mu, C.; Yu, Y.; Cai, K. Strontium folic acid derivative functionalized titanium surfaces for enhanced osteogenic differentiation of mesenchymal stem cells in vitro and bone formation in vivo. J. Mater. Chem. B 2017, 5, 6811–6826. [Google Scholar] [CrossRef]
- Rojo, L.; Radley-Searle, S.; Fernandez-Gutierrez, M.; Rodriguez-Lorenzo, L.M.; Abradelo, C.; Deb, S.; Roman, J.S. The synthesis and characterisation of strontium and calcium folates with potential osteogenic activity. J. Mater. Chem. B 2015, 3, 2708–2713. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhao, S.; Zhu, Y.; Huang, Y.; Zhu, M.; Tao, C.; Zhang, C. Three-dimensional printing of strontium-containing mesoporous bioactive glass scaffolds for bone regeneration. Acta Biomater. 2014, 10, 2269–2281. [Google Scholar] [CrossRef]
- Liao, W.; Zhong, J.; Yu, J.; Xie, J.; Liu, Y.; Du, L.; Yang, S.; Liu, P.; Xu, J.; Wang, J.; et al. Therapeutic benefit of human umbilical cord derived mesenchymal stromal cells in intracerebral hemorrhage rat: Implications of anti-inflammation and angiogenesis. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2009, 24, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Caverzasio, J. Strontium ranelate promotes osteoblastic cell replication through at least two different mechanisms. Bone 2008, 42, 1131–1136. [Google Scholar] [CrossRef]
- Marquez, L.; de Abreu, F.A.M.; Ferreira, C.L.; Alves, G.D.; Miziara, M.N.; Alves, J.B. Enhanced bone healing of rat tooth sockets after administration of epidermal growth factor (EGF) carried by liposome. Injury 2013, 44, 558–564. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Veis, A. Phosphorylated Proteins and Control over Apatite Nucleation, Crystal Growth, and Inhibition. Chem. Rev. 2008, 108, 4670–4693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zurick, K.M.; Qin, C.; Bernards, M.T. Adhesion of MC3T3-E1 cells bound to dentin phosphoprotein specifically bound to collagen type I. J. Biomed. Mater. Res. A 2012, 100, 2492–2498. [Google Scholar] [CrossRef] [Green Version]
- Zurick, K.M.; Qin, C.; Bernards, M.T. Mineralization induction effects of osteopontin, bone sialoprotein, and dentin phosphoprotein on a biomimetic collagen substrate. J. Biomed. Mater. Res. A 2013, 101, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.; Takeshita, S.; Barker, J.E.; Kanagawa, O.; Ross, F.P.; Teitelbaum, S.L. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J. Clin. Investig. 2000, 106, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Maroufi, N.F.; Ghorbanihaghjo, A.; Melli, M.S.; Vaezi, M.; MEHRABANI, Z.H.A.; Amirkhiz, M.B.; Rashtchizadeh, N. Effects of High and Low Doses of Folic Acid on the Soluble Receptor Activator of Nuclear Factor-kappa B Ligand/Osteoprotegerin Ratio during Pregnancy. Iran. J. Public Health 2017, 46, 517. [Google Scholar]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, J.-S.; Santolaya-Forgas, J.; Romero, R.; Espinoza, J.; Gonçalves, L.F.; Kim, Y.M.; Edwin, S.; Yoon, B.H.; Nien, J.K.; Hassan, S.; et al. Maternal plasma osteoprotegerin concentration in normal pregnancy. Am. J. Obstet. Gynecol. 2005, 193, 1011–1015. [Google Scholar] [CrossRef]
- Klejna, K.; Naumnik, B.; Gasowska, K.; Myśliwiec, M. OPG/RANK/RANKL signaling system and its significance in nephrology. Folia Histochem. Cytobiol. 2009, 47, 199–206. [Google Scholar] [CrossRef]
- Naylor, K.E.; Rogers, A.; Fraser, R.B.; Hall, V.; Eastell, R.; Blumsohn, A. Serum osteoprotegerin as a determinant of bone metabolism in a longitudinal study of human pregnancy and lactation. J. Clin. Endocrinol. Metab. 2003, 88, 5361–5365. [Google Scholar] [CrossRef]
- Uemura, H.; Yasui, T.; Kiyokawa, M.; Kuwahara, A.; Ikawa, H.; Matsuzaki, T.; Maegawa, M.; Furumoto, H.; Irahara, M. Serum osteoprotegerin/osteoclastogenesis-inhibitory factor during pregnancy and lactation and the relationship with calcium-regulating hormones and bone turnover markers. J. Endocrinol. 2002, 174, 353–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yano, K.; Shibata, O.; Mizuno, A.; Kobayashi, F.; Higashio, K.; Morinaga, T.; Tsuda, E. Immunological study on circulating murine osteoprotegerin/osteoclastogenesis inhibitory factor (OPG/OCIF): Possible role of OPG/OCIF in the prevention of osteoporosis in pregnancy. Biochem. Biophys. Res. Commun. 2001, 288, 217–224. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [Green Version]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.W.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem cell properties of human dental pulp stem cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef]
- Gronthos, S.; Arthur, A.; Bartold, P.M.; Shi, S. A method to isolate and culture expand human dental pulp stem cells. Methods Mol. Biol. 2011, 698, 107–121. [Google Scholar]
- Morito, A.; Kida, Y.; Suzuki, K.; Inoue, K.; Kuroda, N.; Gomi, K.; Arai, T.; Sato, T. Effects of basic fibroblast growth factor on the development of the stem cell properties of human dental pulp cells. Arch. Histol. Cytol. 2009, 72, 51–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-H.; Kim, Y.-S.; Lee, S.-Y.; Kim, K.-H.; Lee, Y.-M.; Kim, W.-K.; Lee, Y.-K. Gene expression profile in mesenchymal stem cells derived from dental tissues and bone marrow. J. Periodontal Implant Sci. 2011, 41, 192–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laino, G.; d’Aquino, R.; Graziano, A.; Lanza, V.; Carinci, F.; Naro, F.; Pirozzi, G.; Papaccio, G. A new population of human adult dental pulp stem cells: A useful source of living autologous fibrous bone tissue (LAB). J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2005, 20, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Darley, R.L.; Hallett, M.; Evans, W.H. Low connexin channel-dependent intercellular communication in human adult hematopoietic progenitor/stem cells: Probing mechanisms of autologous stem cell therapy. Cell Commun. Adhes. 2009, 16, 138–145. [Google Scholar] [CrossRef] [PubMed]
- El-Backly, R.M.; Massoud, A.G.; El-Badry, A.M.; Sherif, R.A.; Marei, M.K. Regeneration of dentine/pulp-like tissue using a dental pulp stem cell/poly(lactic-co-glycolic) acid scaffold construct in New Zealand white rabbits. Aust. Endod. J. 2008, 34, 52–67. [Google Scholar] [CrossRef]
- Choudhary, S.; Halbout, P.; Alander, C.; Raisz, L.; Pilbeam, C. Strontium ranelate promotes osteoblastic differentiation and mineralization of murine bone marrow stromal cells: Involvement of prostaglandins. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2007, 22, 1002–1010. [Google Scholar] [CrossRef]
- Peng, S.; Liu, X.S.; Wang, T.; Li, Z.; Zhou, G.; Luk, K.D.K.; Guo, X.E.; Lu, W.W. In vivo anabolic effect of strontium on trabecular bone was associated with increased osteoblastogenesis of bone marrow stromal cells. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2010, 28, 1208–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Yang, D.; Tu, J.; Zheng, Q.; Cai, L.; Wang, L. Strontium enhances osteogenic differentiation of mesenchymal stem cells and in vivo bone formation by activating Wnt/catenin signaling. Stem Cells Dayt. Ohio 2011, 29, 981–991. [Google Scholar] [CrossRef]
- Bruyere, O.; Roux, C.; Detilleux, J.; Slosman, D.O.; Spector, T.D.; Fardellone, P.; Brixen, K.; Devogelaer, J.-P.; Diaz-Curiel, M.; Albanese, C.; et al. Relationship between bone mineral density changes and fracture risk reduction in patients treated with strontium ranelate. J. Clin. Endocrinol. Metab. 2007, 92, 3076–3081. [Google Scholar] [CrossRef]
- Fernández-Murga, M.L.; Serna, E.; Sanz-Salvador, L.; Hervás-Lorente, A.; Portero, J.; Cano, A. Response of osteoblasts to compounds of strontium or calcium: Proliferation, differentiation, mineralisation and whole genome response. Rev. Osteoporos. Metab. Miner. 2013, 5, 133–140. [Google Scholar] [CrossRef]
- Fromigué, O.; Haÿ, E.; Barbara, A.; Marie, P.J. Essential role of nuclear factor of activated T cells (NFAT)-mediated Wnt signaling in osteoblast differentiation induced by strontium ranelate. J. Biol. Chem. 2010, 285, 25251–25258. [Google Scholar] [CrossRef]
- Rybchyn, M.S.; Slater, M.; Conigrave, A.D.; Mason, R.S. An Akt-dependent increase in canonical Wnt signaling and a decrease in sclerostin protein levels are involved in strontium ranelate-induced osteogenic effects in human osteoblasts. J. Biol. Chem. 2011, 286, 23771–23779. [Google Scholar] [CrossRef]
- Pasqualetti, S.; Banfi, G.; Mariotti, M. The zebrafish scale as model to study the bone mineralization process. J. Mol. Histol. 2012, 43, 589–595. [Google Scholar] [CrossRef]
- Zarins, J.; Pilmane, M.; Sidhom, E.; Salma, I. Does Local Application of Strontium Increase Osteogenesis and Biomaterial Osteointegration in Osteoporotic and Other Bone Tissue Conditions: Review of Literature. Acta Chir. Latv. 2016, 16, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wei, L.; Chang, J.; Miron, R.J.; Shi, B.; Yi, S.; Wu, C. Strontium-incorporated mesoporous bioactive glass scaffolds stimulating in vitro proliferation and differentiation of bone marrow stromal cells and in vivo regeneration of osteoporotic bone defects. J. Mater. Chem. B 2013, 1, 5711–5722. [Google Scholar] [CrossRef]
- Atkins, G.J.; Welldon, K.J.; Halbout, P.; Findlay, D.M. Strontium ranelate treatment of human primary osteoblasts promotes an osteocyte-like phenotype while eliciting an osteoprotegerin response. Osteoporos. Int. 2009, 20, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Bakker, A.D.; Zandieh-Doulabi, B.; Klein-Nulend, J. Strontium Ranelate affects signaling from mechanically-stimulated osteocytes towards osteoclasts and osteoblasts. Bone 2013, 53, 112–119. [Google Scholar] [CrossRef]
- Cianferotti, L.; D’Asta, F.; Brandi, M.L. A review on strontium ranelate long-term antifracture efficacy in the treatment of postmenopausal osteoporosis. Ther. Adv. Musculoskelet. Dis. 2013, 5, 127–139. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.; Zhang, B.; Zhu, X.; Ao, P.; Guo, H.; Yi, W.; Zhou, G.-Q. Deregulation of bone forming cells in bone diseases and anabolic effects of strontium-containing agents and biomaterials. BioMed Res. Int. 2014, 2014, 814057. [Google Scholar] [CrossRef] [PubMed]
- Polak-Juszczak, L. Impact of strontium on skeletal deformities in Baltic cod (Gadus morhua callaris L.). Chemosphere 2011, 83, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Szostek, K.; Głab, H.; Pudło, A. The use of strontium and barium analyses for the reconstruction of the diet of the early medieval coastal population of Gdańsk (Poland): A preliminary study. Homo Int. Z. Vgl. Forsch. Am Menschen 2009, 60, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Qiu, K.; Zhao, X.J.; Wan, C.X.; Zhao, C.S.; Chen, Y.W. Effect of strontium ions on the growth of ROS17/2.8 cells on porous calcium polyphosphate scaffolds. Biomaterials 2006, 27, 1277–1286. [Google Scholar] [CrossRef]
- Chen, Y.W.; Shi, G.Q.; Ding, Y.L.; Yu, X.X.; Zhang, X.H.; Zhao, C.S.; Wan, C.X. In vitro study on the influence of strontium-doped calcium polyphosphate on the angiogenesis-related behaviors of HUVECs. J. Mater. Sci. Mater. Med. 2008, 19, 2655–2662. [Google Scholar] [CrossRef] [PubMed]
- Ni, G.X.; Chiu, K.Y.; Lu, W.W.; Wang, Y.; Zhang, Y.G.; Hao, L.B.; Li, Z.Y.; Lam, W.M.; Lu, S.B.; Luk, K.D.K. Strontium-containing hydroxyapatite bioactive bone cement in revision hip arthroplasty. Biomaterials 2006, 27, 4348–4355. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.W.; Leong, J.C.; Lu, W.W.; Luk, K.D.; Cheung, K.M.; Chiu, K.Y.; Chow, S.P. A novel injectable bioactive bone cement for spinal surgery: A developmental and preclinical study. J. Biomed. Mater. Res. 2000, 52, 164–170. [Google Scholar] [CrossRef]
- Hernández, L.; Gurruchaga, M.; Goñi, I. Injectable acrylic bone cements for vertebroplasty based on a radiopaque hydroxyapatite. Formulation and rheological behaviour. J. Mater. Sci. Mater. Med. 2009, 20, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Reginster, J.-Y.; Badurski, J.; Bellamy, N.; Bensen, W.; Chapurlat, R.; Chevalier, X.; Christiansen, C.; Genant, H.; Navarro, F.; Nasonov, E.; et al. Efficacy and safety of strontium ranelate in the treatment of knee osteoarthritis: Results of a double-blind, randomised placebo-controlled trial. Ann. Rheum. Dis. 2013, 72, 179–186. [Google Scholar] [CrossRef]
- Reginster, J.-Y.; Lecart, M.-P.; Deroisy, R.; Lousberg, C. Strontium ranelate: A new paradigm in the treatment of osteoporosis. Expert Opin. Investig. Drugs 2004, 13, 857–864. [Google Scholar] [CrossRef]
- Bergamini, P.; Marchesi, E.; Pagnoni, A.; Lambertini, E.; Franceschetti, T.; Penolazzi, L.; Piva, R. Synthesis, characterization of strontium-bile acid salts and their bioactivity vs. the anti-osteoporosis drug strontium ranelate. J. Inorg. Biochem. 2009, 103, 891–897. [Google Scholar] [CrossRef]
- Shin, H.D.; Yang, K.J.; Park, B.R.; Son, C.W.; Jang, H.J.; Ku, S.K. Antiosteoporotic effect of Polycan, beta-glucan from Aureobasidium, in ovariectomized osteoporotic mice. Nutr. Burbank Los Angel. Cty. Calif 2007, 23, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Yodthong, T.; Kedjarune-Leggat, U.; Smythe, C.; Wititsuwannakul, R.; Pitakpornpreecha, T. l-Quebrachitol Promotes the Proliferation, Differentiation, and Mineralization of MC3T3-E1 Cells: Involvement of the BMP-2/Runx2/MAPK/Wnt/β-Catenin Signaling Pathway. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.B.; Gasparini, G.; Harris, A.L. Angiogenesis: Pathological, prognostic, and growth-factor pathways and their link to trial design and anticancer drugs. Lancet Oncol. 2001, 2, 278–289. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Rozen, N.; Bick, T.; Bajayo, A.; Shamian, B.; Schrift-Tzadok, M.; Gabet, Y.; Yayon, A.; Bab, I.; Soudry, M.; Lewinson, D. Transplanted blood-derived endothelial progenitor cells (EPC) enhance bridging of sheep tibia critical size defects. Bone 2009, 45, 918–924. [Google Scholar] [CrossRef]
- Salinas, A.J.; Blanco-Bécares, J.M.; Mersinlioglu, O.; Casarrubios, L.; Fernández-Villa, D.; Feito, M.J.; Portolés, T.; González, B.; Vallet-Regí, M. Synthesis, Characterization and Biocompatibility of Mesolamellar Calcium Phosphate Hybrids Prepared by Anionic Surfactant Templating. ChemistrySelect 2018, 3, 6880–6891. [Google Scholar] [CrossRef]
- Denry, I.; Holloway, J.A. Low temperature sintering of fluorapatite glass-ceramics. Dent. Mater. 2014, 30, 112–121. [Google Scholar] [CrossRef]
- Choi, H.; Kim, G.-J.; Yoo, H.-S.; Song, D.; Chung, K.-H.; Lee, K.-J.; Koo, Y.; An, J. Vitamin C Activates Osteoblastogenesis and Inhibits Osteoclastogenesis via Wnt/β-Catenin/ATF4 Signaling Pathways. Nutrients 2019, 11, 506. [Google Scholar] [CrossRef]
- Park, J.-K.; Lee, E.-M.; Kim, A.-Y.; Lee, E.-J.; Min, C.-W.; Kang, K.-K.; Lee, M.-M.; Jeong, K.-S. Vitamin C deficiency accelerates bone loss inducing an increase in PPAR-γ expression in SMP30 knockout mice. Int. J. Exp. Pathol. 2012, 93, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Koide, M.; Kobayashi, Y.; Yamashita, T.; Uehara, S.; Nakamura, M.; Hiraoka, B.Y.; Ozaki, Y.; Iimura, T.; Yasuda, H.; Takahashi, N.; et al. Bone Formation Is Coupled to Resorption Via Suppression of Sclerostin Expression by Osteoclasts. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2017, 32, 2074–2086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemonnier, J.; Ghayor, C.; Guicheux, J.; Caverzasio, J. Protein kinase C-independent activation of protein kinase D is involved in BMP-2-induced activation of stress mitogen-activated protein kinases JNK and p38 and osteoblastic cell differentiation. J. Biol. Chem. 2004, 279, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, S.; Kim, K. Bone Tissue Engineering Strategies in Co-Delivery of Bone Morphogenetic Protein-2 and Biochemical Signaling Factors. In Cutting-Edge Enabling Technologies for Regenerative Medicine; Chun, H.J., Park, C.H., Kwon, I.K., Khang, G., Eds.; Springer: Singapore, 2018; pp. 233–244. ISBN 9789811309502. [Google Scholar]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; Crombrugghe, B. de The Novel Zinc Finger-Containing Transcription Factor Osterix Is Required for Osteoblast Differentiation and Bone Formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef]
- Blom, H.J.; Smulders, Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J. Inherit. Metab. Dis. 2011, 34, 75–81. [Google Scholar] [CrossRef]
- Ebara, S. Nutritional role of folate. Congenit. Anom. 2017, 57, 138–141. [Google Scholar] [CrossRef]
- Barua, S.; Kuizon, S.; Junaid, M.A. Folic acid supplementation in pregnancy and implications in health and disease. J. Biomed. Sci. 2014, 21, 77. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Amaretti, A.; Raimondi, S. Folate Production by Probiotic Bacteria. Nutrients 2011, 3, 118–134. [Google Scholar] [CrossRef] [Green Version]
- Morris, M.S.; Jacques, P.F.; Selhub, J. Relation between homocysteine and B-vitamin status indicators and bone mineral density in older Americans. Bone 2005, 37, 234–242. [Google Scholar] [CrossRef]
- Xian, C.J.; Cool, J.C.; Scherer, M.A.; Fan, C.; Foster, B.K. Folinic acid attenuates methotrexate chemotherapy-induced damages on bone growth mechanisms and pools of bone marrow stromal cells. J. Cell. Physiol. 2008, 214, 777–785. [Google Scholar] [CrossRef]
- Hossein-Nezhad, A.; Mirzaei, K.; Maghbooli, Z.; Najmafshar, A.; Larijani, B. The influence of folic acid supplementation on maternal and fetal bone turnover. J. Bone Miner. Metab. 2011, 29, 186–192. [Google Scholar] [CrossRef]
- Wei, S.; Kitaura, H.; Zhou, P.; Ross, F.P.; Teitelbaum, S.L. IL-1 mediates TNF-induced osteoclastogenesis. J. Clin. Investig. 2005, 115, 282–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weitzmann, M.N. The Role of Inflammatory Cytokines, the RANKL/OPG Axis, and the Immunoskeletal Interface in Physiological Bone Turnover and Osteoporosis. Available online: https://www.hindawi.com/journals/scientifica/2013/125705/ (accessed on 22 October 2018).
- Zhang, Y.H.; Heulsmann, A.; Tondravi, M.M.; Mukherjee, A.; Abu-Amer, Y. Tumor necrosis factor-alpha (TNF) stimulates RANKL-induced osteoclastogenesis via coupling of TNF type 1 receptor and RANK signaling pathways. J. Biol. Chem. 2001, 276, 563–568. [Google Scholar] [CrossRef]
- Lewicki, S.; Lewicka, A.; Kalicki, B.; Kłos, A.; Bertrandt, J.; Zdanowski, R. The influence of vitamin B12 supplementation on the level of white blood cells and lymphocytes phenotype in rats fed a low-protein diet. Cent. Eur. J. Immunol. 2014, 39, 419–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Villa, D.; Jiménez Gómez-Lavín, M.; Abradelo, C.; San Román, J.; Rojo, L. Tissue Engineering Therapies Based on Folic Acid and Other Vitamin B Derivatives. Functional Mechanisms and Current Applications in Regenerative Medicine. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef]
- Herrmann, M.; Schmidt, J.; Umanskaya, N.; Colaianni, G.; Al Marrawi, F.; Widmann, T.; Zallone, A.; Wildemann, B.; Herrmann, W. Stimulation of osteoclast activity by low B-vitamin concentrations. Bone 2007, 41, 584–591. [Google Scholar] [CrossRef]
- Hodgson, S.F.; Watts, N.B.; Bilezikian, J.P.; Clarke, B.L.; Gray, T.K.; Harris, D.W.; Johnston, C.C.; Kleerekoper, M.; Lindsay, R.; Luckey, M.M.; et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the prevention and treatment of postmenopausal osteoporosis: 2001 edition, with selected updates for 2003. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2003, 9, 544–564. [Google Scholar]
- Bainbridge, K.E.; Sowers, M.; Lin, X.; Harlow, S.D. Risk factors for low bone mineral density and the 6-year rate of bone loss among premenopausal and perimenopausal women. Osteoporos. Int. 2004, 15, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Widmann, T.; Colaianni, G.; Colucci, S.; Zallone, A.; Herrmann, W. Increased osteoclast activity in the presence of increased homocysteine concentrations. Clin. Chem. 2005, 51, 2348–2353. [Google Scholar] [CrossRef] [PubMed]
- Salari, P.; Abdollahi, M. Controversial effects of non-steroidal anti-inflammatory drugs on bone: A review. Inflamm. Allergy Drug Targets 2009, 8, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Salari, P.; Abdollahi, M.; Heshmat, R.; Meybodi, H.A.; Razi, F. Effect of folic acid on bone metabolism: A randomized double blind clinical trial in postmenopausal osteoporotic women. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2014, 22, 62. [Google Scholar] [CrossRef]
- Cashman, K.D. Homocysteine and osteoporotic fracture risk: A potential role for B vitamins. Nutr. Rev. 2005, 63, 29–36. [Google Scholar] [CrossRef] [PubMed]
- McLean, R.R.; Jacques, P.F.; Selhub, J.; Fredman, L.; Tucker, K.L.; Samelson, E.J.; Kiel, D.P.; Cupples, L.A.; Hannan, M.T. Plasma B Vitamins, Homocysteine, and Their Relation with Bone Loss and Hip Fracture in Elderly Men and Women. J. Clin. Endocrinol. Metab. 2008, 93, 2206–2212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fratoni, V.; Brandi, M.L. B Vitamins, Homocysteine and Bone Health. Nutrients 2015, 7, 2176–2192. [Google Scholar] [CrossRef] [Green Version]
- Riancho, J.A. Homocisteø ena, vitaminas y masa sea. Rev. Esp. Enfermedades Metabólicas Óseas 2006, 15, 85–87. [Google Scholar] [CrossRef]
- Van Meurs, J.B.J.; Dhonukshe-Rutten, R.A.M.; Pluijm, S.M.F.; van der Klift, M.; de Jonge, R.; Lindemans, J.; de Groot, L.C.P.G.M.; Hofman, A.; Witteman, J.C.M.; van Leeuwen, J.P.T.M.; et al. Homocysteine levels and the risk of osteoporotic fracture. N. Engl. J. Med. 2004, 350, 2033–2041. [Google Scholar] [CrossRef] [PubMed]
- Cagnacci, A.; Baldassari, F.; Rivolta, G.; Arangino, S.; Volpe, A. Relation of homocysteine, folate, and vitamin B12 to bone mineral density of postmenopausal women. Bone 2003, 33, 956–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golbahar, J.; Hamidi, A.; Aminzadeh, M.A.; Omrani, G.R. Association of plasma folate, plasma total homocysteine, but not methylenetetrahydrofolate reductase C667T polymorphism, with bone mineral density in postmenopausal Iranian women: A cross-sectional study. Bone 2004, 35, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Dhonukshe-Rutten, R.A.M.; Lips, M.; de Jong, N.; Chin, A.; Paw, M.J.M.; Hiddink, G.J.; van Dusseldorp, M.; De Groot, L.C.P.G.M.; van Staveren, W.A. Vitamin B-12 status is associated with bone mineral content and bone mineral density in frail elderly women but not in men. J. Nutr. 2003, 133, 801–807. [Google Scholar] [CrossRef]
- Asensio, G.; Ramirez, R.; González, J.; Abrelo, C.; San Roman, J.; Rojo, L. Evaluation of folic acid derivatives and biomimetic scaffolds for osteochondral tissue engineering. In Proceedings of the Actas del XLI Congreso de la Sociedad Ibérica de Biomecánica y Biomateriales, Madrid, Spain, 18–19 October 2018. [Google Scholar]
- Roy, M.; Bose, S. Osteoclastogenesis and osteoclastic resorption of tricalcium phosphate: Effect of strontium and magnesium doping. J. Biomed. Mater. Res. A 2012, 100, 2450–2461. [Google Scholar] [CrossRef] [PubMed]
- San Roman, J.; Martin, M.; Rojo, L.; Rosales, R.; Deb, S. Biohybrid Scaffolds based on Polymers and Strontiun Salts for the Regeneration of Bone Tissue. Tissue Eng. Part A 2015, 21, S59. [Google Scholar]
- Fong, E.L.S.; Chan, C.K.; Goodman, S.B. Stem cell homing in musculoskeletal injury. Biomaterials 2011, 32, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Weick, J.P.; Johnson, M.A.; Skroch, S.P.; Williams, J.C.; Deisseroth, K.; Zhang, S.-C. Functional control of transplantable human ESC-derived neurons via optogenetic targeting. Stem Cells Dayt. Ohio 2010, 28, 2008–2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-del-Campo, M.; Sampedro, J.G.; Flores-Cedillo, M.L.; Rosales-Ibañez, R.; Rojo, L. Bone Regeneration Induced by Strontium Folate Loaded Biohybrid Scaffolds. Molecules 2019, 24, 1660. https://doi.org/10.3390/molecules24091660
Martín-del-Campo M, Sampedro JG, Flores-Cedillo ML, Rosales-Ibañez R, Rojo L. Bone Regeneration Induced by Strontium Folate Loaded Biohybrid Scaffolds. Molecules. 2019; 24(9):1660. https://doi.org/10.3390/molecules24091660
Chicago/Turabian StyleMartín-del-Campo, Marcela, José G. Sampedro, María Lisseth Flores-Cedillo, Raul Rosales-Ibañez, and Luis Rojo. 2019. "Bone Regeneration Induced by Strontium Folate Loaded Biohybrid Scaffolds" Molecules 24, no. 9: 1660. https://doi.org/10.3390/molecules24091660
APA StyleMartín-del-Campo, M., Sampedro, J. G., Flores-Cedillo, M. L., Rosales-Ibañez, R., & Rojo, L. (2019). Bone Regeneration Induced by Strontium Folate Loaded Biohybrid Scaffolds. Molecules, 24(9), 1660. https://doi.org/10.3390/molecules24091660