Mulinum crassifolium Phil; Two New Mulinanes, Gastroprotective Activity and Metabolomic Analysis by UHPLC-Orbitrap Mass Spectrometry
Abstract
:1. Introduction
2. Results and Discussion
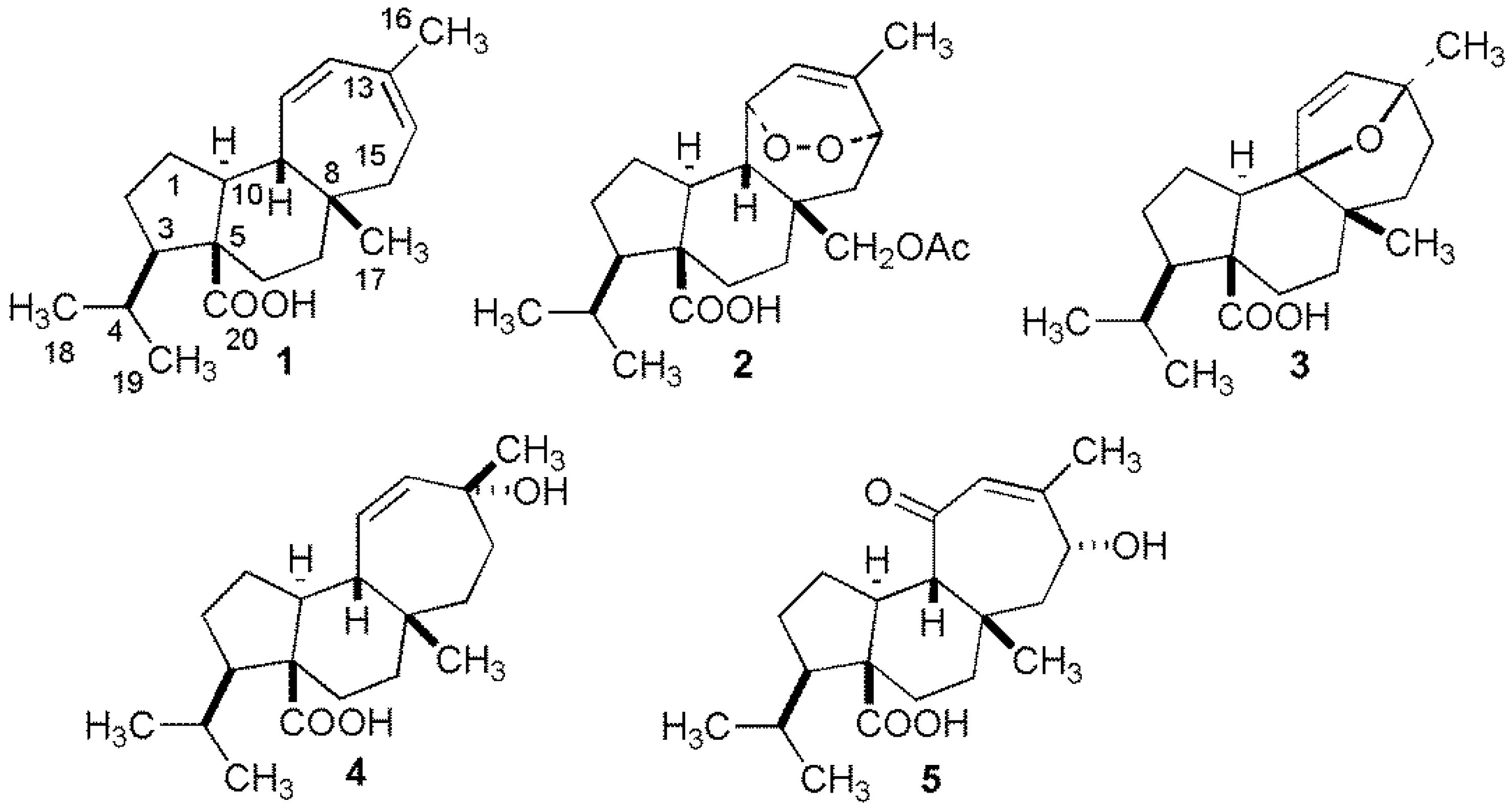
2.1. Isolation of Diterpenoids
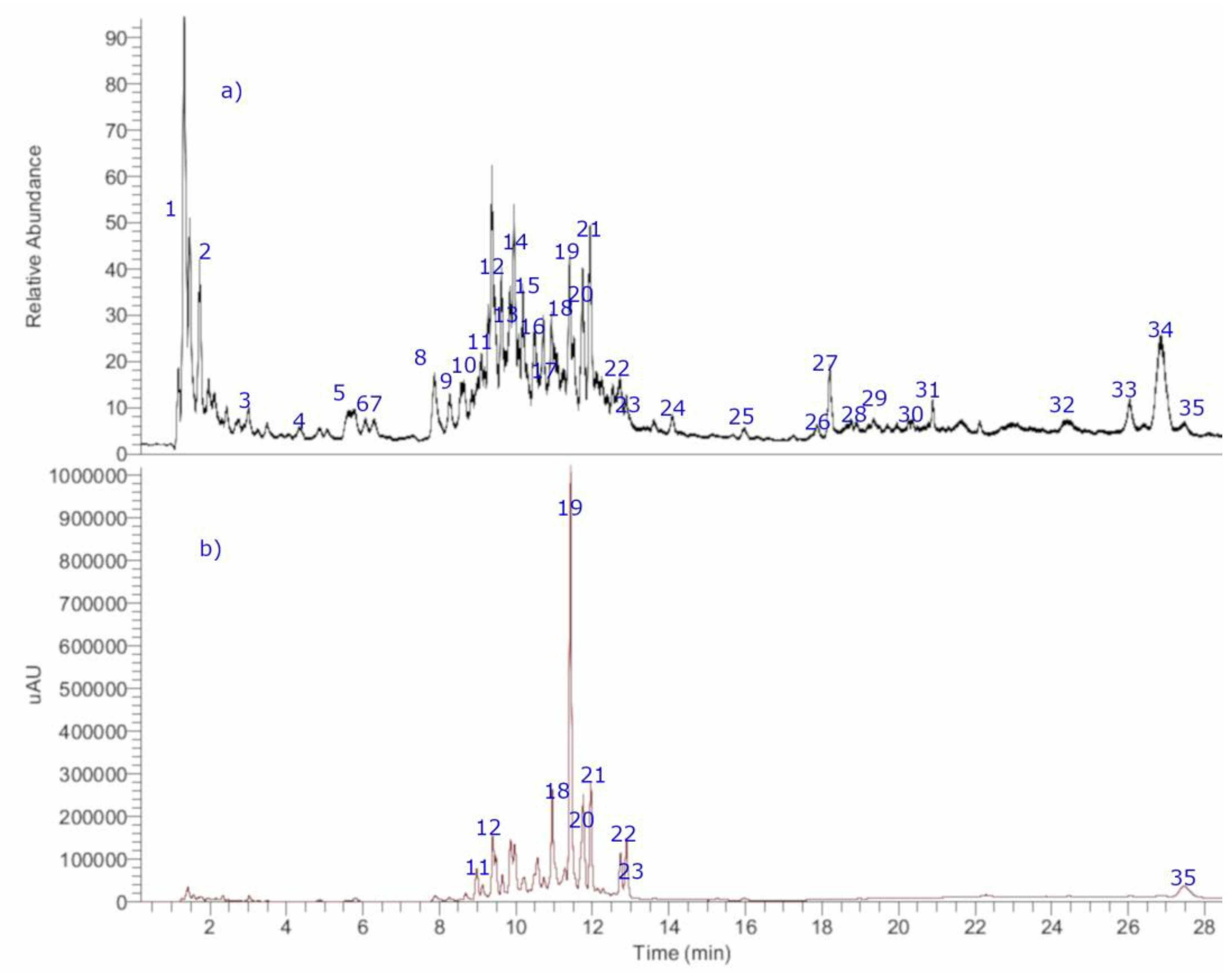
2.2. Metabolomic Profiling of the Infusion by Using UHPLC-ESI-MS/HRMS
2.2.1. Simple Organic Acids
2.2.2. Phenolic Acids
2.2.3. Flavonoids
2.2.4. Fatty Acids
2.2.5. Mulinane Diterpenoids
2.3. Gastroprotective Activity
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material
3.3. Extraction and Isolation
3.4. UHPLC-ESI-MS/HRMS Studies
3.5. Animals
3.6. HCl/EtOH-Induced Lesions in Mice
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wickens, G.E. Llareta (Azorella compacta, Umbelliferae): A review. Econ. Bot. 1995, 49, 207–212. [Google Scholar] [CrossRef]
- Riveros, R.; Morales, G.; Loyola, A.; Torres, R. Scopoletin and aromatic acids from mulinum crassifolium. Fitoterapia 1984, 55, 234–236. [Google Scholar]
- Loyola, L.A.; Morales, G.; De la Torre, M.C.; Pedreros, S.; Rodriguez, B. 17-acetoxymulinic acid, a rearranged diterpenoid from Mulinum crassifolium. Phytochemistry 1990, 29, 3950–3951. [Google Scholar] [CrossRef]
- Loyola, L.A.; Morales, G.; Rodriguez, B.; Jimenez-Barbero, J.; de la Torre, M.C.; Perales, A.; Torres, M. Mulinic and isomulinic acids. Rearranged diterpenes with a new carbon skeleton from mulinum crassifolium. Tetrahedron 1990, 46, 5413–5420. [Google Scholar] [CrossRef]
- Loyola, L.A.; Morales, G.; Rodriguez, B.; Jimenez-Barbero, J.; Pedreros, S.; de la Torre, M.C.; Perales, A. Mulinenic acid, a rearranged diterpenoid from Mulinum crassifolium. J. Nat. Prod. 1991, 54, 1404–1408. [Google Scholar] [CrossRef]
- Loyola, L.A.; Borquez, J.; Morales, G.; San Martin, A. Mulinolic acid, a diterpenoid from Mulinum crassifolium. Phytochemistry 1996, 43, 165–168. [Google Scholar] [CrossRef]
- Loyola, L.A.; Borquez, J.; Morales, G.; San Martin, A. A new diterpenoid from Mulinum crassifolium. Bol. Soc. Chil. Quim. 1997, 42, 311–315. [Google Scholar]
- Chiaramello, A.I.; Ardanaz, C.E.; Garcia, E.; Rossomando, P. Mulinane-type diterpenoids from Mulinum spinosum. Phytochemistry 2003, 63, 883–886. [Google Scholar] [CrossRef]
- Borquez, J.; Ardiles, A.; Loyola, L.A.; Peña-Rodriguez, L.M.; Molina-Salinas, G.M.; Vallejos, J.; Collado, I.G.; Simirgiotis, M.J. Further mulinane and azorellane diterpenoids isolated from mulinum crassifolium and Azorella compacta. Molecules 2014, 19, 3898–3908. [Google Scholar] [CrossRef]
- Martins, C.P.B.; Bromirski, M.; Conaway, M.C.P.; Makarov, A.A. Orbitrap mass spectrometry: Evolution and Applicability. In Comprensive Analitycal Chemistry; Perez, S., Eichhorn, P., Barcelo, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 71. [Google Scholar]
- Areche, C.; Sepulveda, B.; Martin, A.S.; Garcia-Beltran, O.; Simirgiotis, M.; Cañete, A. An unusual mulinane diterpenoid from the Chilean plant Azorella trifurcata (Gaertn) Pers. Org. Biomol. Chem. 2014, 12, 6406–6413. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.; Ramirez, J.E.; Areche, C.; Sepúlveda, B.; Simirgiotis, M.J. HPLC-UV-MS profiles of phenolic compounds and antioxidant activity of fruits from three citrus species consumed in Northern Chile. Molecules 2014, 19, 17400–17421. [Google Scholar] [CrossRef]
- Berovic, M.; Legisa, M. Citric acid production. Biotechnol. Annu. Rev. 2007, 13, 303–343. [Google Scholar] [PubMed]
- Simirgiotis, M.J.; Quispe, C.; Bórquez, J.; Areche, C.; Sepúlveda, B. Fast detection of phenolic compounds in extracts of easter pears (pyrus communis) from the atacama desert by ultrahigh-performance liquid chromatography and mass spectrometry (UHPLC-Q/Orbitrap/MS/MS). Molecules 2016, 21, 92. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J. Antioxidant capacity and HPLC-DAD-MS profiling of Chilean Peumo (Cryptocarya alba) fruits and comparison with German Peumo (Crataegus monogyna) from Southern Chile. Molecules 2013, 18, 2061–2080. [Google Scholar] [CrossRef] [PubMed]
- Willems, J.L.; Khamis, M.M.; Mohammed Saeid, W.; Purves, R.W.; Katselis, G.; Low, N.H.; EI-Aneed, A. Analysis of a series of chlorogenic acid isomers using differential ion mobility and tandem mass spectrometry. Anal. Chim. Acta 2016, 933, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef]
- Khallouki, F.; Ricarte, I.; Breuer, A.; Owen, R.W. Characterization of phenolic compounds in mature Moroccan Medjool date palm fruits (Phoenix dactylifera) by HPLC-DAD-ESI-MS. J. Food Compos. Anal. 2018, 70, 63–71. [Google Scholar] [CrossRef]
- Zhang, S.; Qi, Y.; Xu, Y.; Han, X.; Peng, J.; Liu, K.; Sun, C.K. Protective effect of flavonoid-rich extract from Rosa laevigata Michx on cerebral ischemia-reperfusion injury through suppression of apoptosis and inflammation. Neurochem. Int. 2013, 63, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Tálos-Nebehaj, E.; Albert, L.; Németh, L. Antioxidant efficiency of Beech (Fagus sylvatica L.) bark polyphenols assessed by chemometric methods. Ind. Crops Prod. 2017, 108, 26–35. [Google Scholar] [CrossRef]
- Chen, C.Y.; Kao, C.L.; Liu, C.M. The Cancer Prevention, Anti-Inflammatory and Anti-Oxidation of Bioactive Phytochemicals Targeting the TLR4 Signaling Pathway. Int. J. Mol. Sci. 2018, 19, 2729. [Google Scholar] [CrossRef]
- Clifford, M.N.; Jaganath, I.B.; Ludwig, I.A.; Crozier, A. Chlorogenic acids and the acyl-quinic acids: Discovery, biosynthesis, bioavailability and bioactivity. Nat. Prod. Rep. 2017, 34, 1391–1421. [Google Scholar] [CrossRef] [PubMed]
- Hadrich, F.; Sayadi, S. Apigetrin inhibits adipogenesis in 3T3-L1 cells by downregulating PPARγ and CEBP-α. Lipids Health Dis. 2018, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.; Areche, C.; Sepúlveda, B.; Kennelly, E.J.; Simirgiotis, M.J. Anthocyanin characterization, total phenolic quantification and antioxidant features of some chilean edible berry extracts. Molecules 2014, 19, 10936–10955. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, J.; Samojedny, A.; Paul, M.; Pietsz, G.; Wilczok, T. Effect of apigenin, kaempferol and resveratrol on the expression of interleukin-1beta and tumor necrosis factor-alpha genes in J774.2 macrophages. Pharmacol. Rep. 2005, 57, 390–394. [Google Scholar] [PubMed]
- Cai, J.; Zhao, X.L.; Liu, A.W.; Nian, H.; Zhang, S.H. Apigenin inhibits hepatoma cell growth through alteration of gene expression patterns. Phytomedicine 2011, 18, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Lee, G.S. Isorhamnetin and hyperoside derived from water dropwort inhibits inflammasome activation. Phytomedicine 2017, 24, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Yao, R.; Liu, Y.; Wang, Z.; Huang, Z.; Du, B.; Zhang, D.; Wu, L.; Xiao, L.; Zhang, Y. Isorhamnetin protects against cardiac hypertrophy through blocking PI3K-AKT pathway. Mol. Cell. Biochem. 2017, 429, 167–177. [Google Scholar] [CrossRef]
- Mota, K.S.; Dias, G.E.; Pinto, M.E.; Luis-Ferreira, A.; Souza-Brito, A.R.; Hiruma-Lima, C.A.; Barbosa-Filho, J.M.; Batista, L.M. Flavonoids with gastroprotective activity. Molecules 2009, 14, 979–1012. [Google Scholar] [CrossRef]
- Loyola, L.A.; Bórquez, J.; Morales, J.; San-Martín, A.; Darias, J.; Flores, N.; Giménez, A. Mulinane-type diterpenoids from Azorella compacta display antiplasmodial activity. Phytochemistry 2004, 65, 1931–1935. [Google Scholar] [CrossRef]
- Lewis, D.A.; Hanson, D. Anti-ulcer drugs of plant origin. In Progress in Medicinal Chemistry, 3rd ed.; Ellis, G.P., West, G.B., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; pp. 201–231. [Google Scholar]
- Areche, C.; Rojas-Alvarez, F.; Campos-Briones, C.; Lima, C.; Perez, E.G.; Sepulveda, B. Further mulinane diterpenoids from Azorella compacta. J. Pharm. Pharmacol. 2013, 65, 1231–1238. [Google Scholar] [CrossRef]
- Salgado, F.; Areche, C.; Sepulveda, B.; Caceres, F.; Quispe, C.; Quispe, L.; Cano, T.; Simirgiotis, M. A new mulinane diterpenoid from the cushion shrub Azorella compacta growing in Perú. Pharmacogn. Mag. 2014, 10, S543–S548. [Google Scholar] [PubMed]
- Tundis, R.; Loizzo, M.R.; Bonesi, M.; Menichini, F.; Conforti, F.; Statti, G.; Menichini, F. Natural products as gastroprotective and antiulcer agents: Recent development. Nat. Prod. Commun. 2008, 3, 2129. [Google Scholar] [CrossRef]
- Sumbul, S.; Ahmad, M.A.; Asif, M.; Akhtar, M. Role of phenolic compounds in peptic ulcer: An overview. J. Pharm. Bioallied Sci. 2011, 3, 361–367. [Google Scholar] [PubMed]
- Khan, M.S.A.; Khundmiri, S.U.K.; Khundmiri, S.R.; Al-Sanea, M.M.; Mok, P.L. Fruit-derived polysaccharides and terpenoids: Recent update on the gastroprotective effects and mechanisms. Front. Pharmacol. 2018, 9, 569. [Google Scholar] [CrossRef] [PubMed]
- Alarcon de la Lastra, C.; Lopez, A.; Motilva, V. Gastroprotective and prostaglandin E2 generation in rats by flavonoids of Dittrichia viscosa. Planta Med. 1993, 59, 497–501. [Google Scholar] [CrossRef]
- Alanko, J.; Riutta, A.; Holm, P.; Mucha, I.; Vapata, H.; Metsa-Ketela, T. Modulation of arachidonic acid metabolism by phenols: Relation to their structure and antioxidant/pro-oxidant properties. Free Radic. Biol. Med. 1999, 26, 193–201. [Google Scholar] [CrossRef]
- Borrelli, F.; Izzo, A.A. The plant kingdom as a source of anti-ulcer remedies. Phytother. Res. 2000, 14, 581–591. [Google Scholar] [CrossRef]
- Barros, M.P.; Lemos, M.; Maistro, E.L.; Leite, M.F.; Souza, J.P.B.; Bastos, J.K.; Andrade, S.F. Evaluation of antiulcer activity of the main phenolic acids found in Brazilian Green Propolis. J. Ethnopharmacol. 2008, 120, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.I.; Nugroho, A.; Bachri, M.S.; Choi, J.; Lee, K.R.; Choi, J.S.; Kim, W.B.; Lee, K.T.; Lee, J.D.; Park, H.J. Antiulcerogenic effect and HPLC analysis of the caffeoylquinic acid-rich extract from Ligularia stenocephala. Biol. Pharm. Bull. 2010, 33, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Shimoyana, A.T.; Santin, J.R.; Machado, I.D.; Silva, A.M.O.; Melo, I.L.P.; Mancini-Filho, J.; Farsky, S.H.P. Antiulcerogenic activity of chlorogenic acid in different models of gastric ulcer. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2013, 386, 5–14. [Google Scholar] [CrossRef]
- Parra, T.; Benites, J.; Ruiz, L.M.; Sepulveda, B.; Simirgiotis, M.; Areche, C. Gastroprotective activity of ent-beyerene derivatives in mice: Effects on gastric secretion, endogenous prostaglandins and non-protein sulfhydryls. Bioorg. Med. Chem. Lett. 2015, 25, 2813–2817. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of Mulinum crassifolium are available from the authors. |
| Compound | n | Lesion Index (mm) | % Lesion Reduction | Dose (mg/Kg) |
|---|---|---|---|---|
| EtOAc-E | 7 | 40.6 ± 1.5 ** | 33 * | 100 |
| INF-E | 7 | 15.5 ± 1.1 | 74 * | 100 |
| Lansoprazole | 7 | 12.9 ± 2.9 | 78 * | 30 |
| Control | 7 | 60.6 ± 1.9 | - | - |
| Peak # | RT (min) | UV Max | Tentative Identification | [M − H]− | Theoretical Mass (m/z) | Measured Mass (m/z) | Accuracy (δppm) | MS Ions |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.35 | - | Quinic acid | C7H11O6− | 191.05611 | 191.05574 | −1.93 | 109.02866 (C6H5O2−); 173.04552 (C7H9O5−) |
| 2 | 1.45 | - | Malic acid | C4H5O5− | 133.01425 | 133.01364 | −4–59 | 115.00294 (C4H3O4−, M−-H2O) |
| 3 | 1.87 | 210 | Citric acid | C6H7O7− | 191.01973 | 191.01938 | −1.83 | 111.00797 (C5H3O3−) |
| 4 | 2.42 | 245 | 3-O-glucoside-4-methoxybenzoic acid | C14H17O9− | 329.08781 | 329.08801 | 0.60 | 167.0345 (C8H7O4−) |
| 5 | 3.47 | 245 | 3,4-dihydroxybenzoic acid | C7H5O4− | 153.01933 | 153.01888 | −2.94 | 109.02893 (C6H5O2-) |
| 6 | 4.35 | 245 | 3-O-glucosyl-4-hydroxybenzoic acid | C14H17O9− | 315.07216 | 315.07251 | 1.11 | 153.01856 (C7H5O4−) |
| 7 | 5.75 | 245 | 3 or 4-O-glycosylbenzoic acid | C13H15O8− | 299.07724 | 299.07748 | 0.80 | 137.0236 (C7H5O3−) |
| 8 | 7.85 | 236–329 | Chlorogenic acid, (1- Caffeoylquinic acid) | C16H17O9− | 353.08781 | 353.08813 | 0.90 | 191.05559 (C7H11O6−) Quinic acid |
| 9 | 8.23 | 236–329 | 3-O-diglucosyl-4-methoxy-3-hydroxybenzoic acid | C20H27O14− | 491.14063 | 491.14096 | 0.67 | 167.0341(C8H7O4−) |
| 10 | 8.62 | 236–325 | Caffeoyl glucoside | C15H17O9− | 341.08781 | 341.08813 | 0.93 | 179.0564 (C6H11O6−); 135.0445 (C8H7O2−) |
| 11 | 8.97 | 236–329 | 4,5-dicaffeoylquinic acid | C22H27O14− | 515.14063 | 515.14087 | 0.46 | 191.05559 (C7H11O6−) Quinic acid |
| 12 | 9.09 | 245 | 4-O-(3-O-glucosyl-4-hydroxybenzoyl)-quinic acid | C20H25O14− | 489.12498 | 489.12521 | 0.47 | 191.05586 (C7H11O6−) Quinic acid |
| 13 | 9.36 | 236–329 | 3-Caffeoylquinic acid | C16H17O9− | 353.08781 | 353.08820 | 1.10 | 191.05579 (C7H11O6−) Quinic acid |
| 14 | 9.44 | 245 | 4-Methoxybenzoic acid 3-O-rutinoside | C20H27O13− | 475.14571 | 475.14571 | 0.0 | 151.03946 (C8H7O3−) |
| 15 | 10.62 | 236–329 | 5-caffeoylquinic acid× | C16H17O9− | 353.08781 | 353.08820 | 1.10 | 191.05579 (C7H11O6−) Quinic acid |
| 16 | 10.86 | 279 | Syringic acid hexoside | C15H19O10− | 359.09837 | 359.09872 | 0.97 | 197.0445 (C9H9O5−) |
| 17 | 10.95 | 236–325 | Caffeic acid× | C9H7O4− | 179.03498 | 179.03470 | −1.56 | 135.04457 (C8H7O2−) |
| 18 | 11.18 | 236–329 | p-Coumaroylquinic acid | C16H17O8− | 337.09289 | 337.09320 | 0.91 | 191.05573 (C7H11O6−) Quinic acid |
| 19 | 11.28 | 236–329 | Feruloyl-quinic acid | C17H19O9− | 367.10346 | 367.10391 | 1.22 | 191.05577 (C7H11O6−) Quinic acid |
| 20 | 11.62 | 267–335 | Apigenin 7-O-glucoside | C21H19O10− | 431.09837 | 431.09885 | 1.11 | 269.0459 (C15H9O5−) apigenin |
| 21 | 11.76 | 233–325 | p-Coumaric acid× | C9H7O3− | 163.04007 | 163.03973 | −2.08 | 119.04955 (C8H7O3−, M−-CO2) |
| 22 | 12.56 | 254–354 | Isorhamnetin-3-O-rutinoside | C16H11O7− | 623.16162 | 623.16162 | 0.00 | 315.05118 |
| 23 | 12.90 | 236–329 | 1,3-Dicaffeoylquinic acid | C25H23O12− | 515.11950 | 515.11981 | 0.60 | 135.04446 (C8H7O2−, caffeic acid-CO2); 173.04506 (C7H9O5−, quinic acid-H2O) |
| 24 | 14.76 | 236–329 | 1,5-Dicaffeoylquinic acid× | C25H23O12− | 515.11950 | 515.11969 | 0.36 | 135.04454 (C8H7O2−); 191.05576 (C7H11O6−) |
| 25 | 16.92 | 236–329 | 1,4-Dicaffeoylquinic acid | C25H23O12− | 515.11950 | 515.11975 | 0.48 | 135.04454 (C8H7O2−); 173.04509 (C7H9O5−) |
| 26 | 17.34 | 236–329 | caffeoylferuloylquinic acid | C26H25O12− | 529.13515 | 529.13544 | 0.54 | 135.04449 (C8H7O2−); 173.04501 (C7H9O5−) |
| 27 | 18.12 | 236–329 | Caffeoyl-feruloyl-quinic acid | C26H25O12− | 529.13515 | 529.13538 | 0.43 | 135.04449 (C8H7O2−); 173.04501 (C7H9O5−) |
| 28 | 18.44 | 254–354 | Isorhamnetin× | C16H11O7− | 315.05103 | 315.05136 | 1.04 | 300.02731 (C15H8O7−, M-CH3) |
| 29 | 19.14 | 254–354 | Quercetin× | C18H15O7− | 301.03538 | 301.03571 | 1.09 | 151.00342 (C8H3O4−) |
| 30 | 19.96 | 18.20 | 13,14-Dihydroxymulin-11-en-20-oic acid | C20H31O4− | 335.22278 | 335.22302 | 0.71 | No diagnostic ions known |
| 31 | 20.89 | 217 | Hydroxy-palmitic acid | C16H31O7− | 271.22787 | 271.22803 | 0.58 | No diagnostic ions known |
| 32 | 24.52 | - | 14α-Hydroxy-mulin-12-en-11-one-20-oic acid (compound 5) | C20H29O4− | 333.20718 | 333.20758 | 1.20 | 135.04778 |
| 33 | 26.34 | 211 | 14α-acetoxy-mulin-12-en-11-one-20-oic acid | C22H33O5− | 377.23380 | 377.23335 | −1.19 | 323.32305 |
| 34 | 27.36 | 245 | 9,13-Epoxymulin-11-en-20-oic acid (compound 3) | C20H29O4− | 317.21223 | 317.21273 | −1.7 | No diagnostic ions known |
| 35 | 27.78 | 255–355 | 7,3′,4′-Trimethoxy-quercetin | C18H15O7− | 343.08233 | 343.082276 | −0.15 | 179.0432 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Areche, C.; Fernandez-Burgos, R.; de Terrones, T.C.; Simirgiotis, M.; García-Beltrán, O.; Borquez, J.; Sepulveda, B. Mulinum crassifolium Phil; Two New Mulinanes, Gastroprotective Activity and Metabolomic Analysis by UHPLC-Orbitrap Mass Spectrometry. Molecules 2019, 24, 1673. https://doi.org/10.3390/molecules24091673
Areche C, Fernandez-Burgos R, de Terrones TC, Simirgiotis M, García-Beltrán O, Borquez J, Sepulveda B. Mulinum crassifolium Phil; Two New Mulinanes, Gastroprotective Activity and Metabolomic Analysis by UHPLC-Orbitrap Mass Spectrometry. Molecules. 2019; 24(9):1673. https://doi.org/10.3390/molecules24091673
Chicago/Turabian StyleAreche, Carlos, Ronald Fernandez-Burgos, Teresa Cano de Terrones, Mario Simirgiotis, Olimpo García-Beltrán, Jorge Borquez, and Beatriz Sepulveda. 2019. "Mulinum crassifolium Phil; Two New Mulinanes, Gastroprotective Activity and Metabolomic Analysis by UHPLC-Orbitrap Mass Spectrometry" Molecules 24, no. 9: 1673. https://doi.org/10.3390/molecules24091673
APA StyleAreche, C., Fernandez-Burgos, R., de Terrones, T. C., Simirgiotis, M., García-Beltrán, O., Borquez, J., & Sepulveda, B. (2019). Mulinum crassifolium Phil; Two New Mulinanes, Gastroprotective Activity and Metabolomic Analysis by UHPLC-Orbitrap Mass Spectrometry. Molecules, 24(9), 1673. https://doi.org/10.3390/molecules24091673









