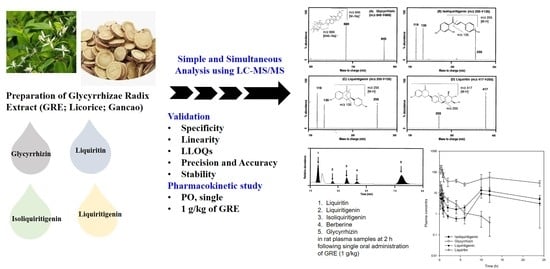
Simultaneous Determination and Pharmacokinetic Characterization of Glycyrrhizin, Isoliquiritigenin, Liquiritigenin, and Liquiritin in Rat Plasma Following Oral Administration of Glycyrrhizae Radix Extract
Abstract
1. Introduction
2. Results
2.1. LC–MS/MS Analysis
2.1.1. MS/MS Analysis
2.1.2. Specificity
2.1.3. Linearity and LLOQs
2.1.4. Accuracy and Precision
2.1.5. Matrix Effect and Recovery
2.1.6. Stability
2.2. Contents of Glycyrrhizin, Liquiritin, Isoliquiritigenin, and Liquiritigenin in GRE
2.3. Plasma Concentration of Glycyrrhizin, Liquiritin, Isoliquiritigenin, and Liquiritigenin
2.4. Biotransformation in the Rat Intestine
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Preparation of Calibration Curve and Quality Control Samples
4.4. LC–MS/MS Analysis of Glycyrrhizin, Liquiritin, Isoliquiritigenin, and Liquiritigenin
4.4.1. LC–MS/MS Condition
4.4.2. Specificity
4.4.3. Linearity
4.4.4. Accuracy and Precision
4.4.5. Extraction Recovery and Matrix Effect
4.4.6. Stability
4.5. Determination of Glycyrrhizin, Isoliquiritigenin, Liquiritigenin, and Liquiritin in GRE
4.6. Pharmacokinetic Study
4.7. Biotransformation of Isoliquiritigenin and Liquiritigenin from Liquiritin
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, T.; Ding, L.; Jin, H.; Shi, R.; Li, Y.; Wu, J.; Li, Y.; Zhu, L.; Ma, Y. Simultaneous quantification of catechin, epicatechin, liquiritin, isoliquiritin, liquiritigenin, isoliquiritigenin, piperine and glycyrrhetinic acid in rat plasma by hplc-ms/ms: Application to a pharmacokinetic study of longhu rendan pills. Biomed. Chromatogr. 2016, 30, 1166–1174. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, Y.; Wang, W.; Hou, J. Identification and simultaneous determination of glycyrrhizin, formononetin, glycyrrhetinic acid, liquiritin, isoliquiritigenin, and licochalcone a in licorice by lc-ms/ms. Acta Chromatogr. 2014, 26, 507–516. [Google Scholar] [CrossRef]
- Qiao, X.; Ji, S.; Yu, S.W.; Lin, X.H.; Jin, H.W.; Duan, Y.K.; Zhang, L.R.; Guo, D.A.; Ye, M. Identification of key licorice constituents which interact with cytochrome p450: Evaluation by lc/ms/ms cocktail assay and metabolic profiling. AAPS J. 2014, 16, 101–113. [Google Scholar] [CrossRef]
- Kobayashi, S.; Miyamoto, T.; Kimura, I.; Kimura, M. Inhibitory effect of isoliquiritin, a compound in licorice root, on angiogenesis in vivo and tube formation in vitro. Biol. Pharm. Bull. 1995, 18, 1382–1386. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yao, Y.; An, R.; You, L.; Wang, X. Simultaneous determination of puerarin, daidzein, baicalin, wogonoside and liquiritin of gegenqinlian decoction in rat plasma by ultra-performance liquid chromatography-mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 1820–1826. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.J.; Qiu, S.-X.; Wufuer, A.; Shum, L. Simultaneous determination of glycyrrhizin, a marker component in radix glycyrrhizae, and its major metabolite glycyrrhetic acid in human plasma by lc–ms/ms. J. Chromatogr. B 2005, 814, 201–207. [Google Scholar] [CrossRef]
- Hu, F.W.; Yu, C.C.; Hsieh, P.L.; Liao, Y.W.; Lu, M.Y.; Chu, P.M. Targeting oral cancer stemness and chemoresistance by isoliquiritigenin-mediated grp78 regulation. Oncotarget 2017, 8, 93912–93923. [Google Scholar] [CrossRef]
- Liao, M.; Zhao, Y.; Huang, L.; Cheng, B.; Huang, K. Isoliquiritigenin and liquiritin from glycyrrhiza uralensis inhibit α-synuclein amyloid formation, rcs advances, 2016. RCS Adv. 2016, 6, 86640–86649. [Google Scholar]
- Peng, F.; Du, Q.; Peng, C.; Wang, N.; Tang, H.; Xie, X.; Shen, J.; Chen, J. A review: The pharmacology of isoliquiritigenin. Phytother. Res. 2015, 29, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Maggiolini, M.; Statti, G.; Vivacqua, A.; Gabriele, S.; Rago, V.; Loizzo, M.; Menichini, F.; Amdò, S. Estrogenic and antiproliferative activities of isoliquiritigenin in mcf7 breast cancer cells. J. Steroid. Biochem. 2002, 82, 315–322. [Google Scholar] [CrossRef]
- Cuendet, M.; Guo, J.; Luo, Y.; Chen, S.; Oteham, C.P.; Moon, R.C.; Van Breemen, R.B.; Marler, L.E.; Pezzuto, J.M. Cancer chemopreventive activity and metabolism of isoliquiritigenin, a compound found in licorice. Cancer Prev. Res. 2010, 3, 221–232. [Google Scholar] [CrossRef]
- Cuendet, M.; Oteham, C.P.; Moon, R.C.; Pezzuto, J.M. Quinone reductase induction as a biomarker for cancer chemoprevention. J. Nat. Prod. 2006, 69, 460–463. [Google Scholar] [CrossRef]
- Jang, D.S.; Park, E.J.; Hawthorne, M.E.; Vigo, J.S.; Graham, J.G.; Cabieses, F.; Santarsiero, B.D.; Mesecar, A.D.; Fong, H.H.; Mehta, R.G. Potential cancer chemopreventive constituents of the seeds of dipteryx o dorata (tonka bean). J. Nat. Prod. 2003, 66, 583–587. [Google Scholar] [CrossRef]
- Zhang, X.; Qiao, H.; Zhang, T.; Shi, Y.; Ni, J. Enhancement of gastrointestinal absorption of isoliquiritigenin by nanostructured lipid carrier. Adv. Powder Technol. 2014, 25, 1060–1068. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.-S. Determination of liquiritigenin, liquiritin, isoliquiritigenin and isoliquiritin in extract of traditional chinese medicine sijunzi decoction by high-performance liquid chromatography. J. Chinese Pharma. Sci. 2005, 14, 227. [Google Scholar]
- Zhang, Y.; Cao, J.; Wang, Y.; Xiao, S. Simultaneous determination of glycyrrhizin and 15 flavonoids in licorice and blood by high performance liquid chromatography with ultraviolet detector. ISRN Anal. Chem. 2013, 10, 1–7. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, C.; Wang, P.; Lin, X.; Yang, Y.; Li, D.; Li, H.; Wu, X.; Liu, H. Pharmacokinetic comparisons of different combinations of shaoyao-gancao-decoction in rats: Simultaneous determination of ten active constituents by hplc–ms/ms. J. Chromatogr. B 2013, 932, 76–87. [Google Scholar] [CrossRef]
- Mao, Y.; Peng, L.; Kang, A.; Xie, T.; Xu, J.; Shen, C.; Ji, J.; Di, L.; Wu, H.; Shan, J. Influence of jiegeng on pharmacokinetic properties of flavonoids and saponins in gancao. Molecules 2017, 22, 1587. [Google Scholar] [CrossRef]
- Shan, J.; Qian, W.; Peng, L.; Chen, L.; Kang, A.; Xie, T.; Di, L. A comparative pharmacokinetic study by uhplc-ms/ms of main active compounds after oral administration of zushima-gancao extract in normal and adjuvant-induced arthritis rats. Molecules 2018, 23, 227. [Google Scholar] [CrossRef]
- Zimmer, D. New us fda draft guidance on bioanalytical method validation versus current fda and ema guidelines: Chromatographic methods and isr. Bioanalysis 2014, 6, 13–19. [Google Scholar] [CrossRef]
- Lin, S.J.; Tseng, H.H.; Wen, K.C.; Suen, T.T. Determination of gentiopicroside, mangiferin, palmatine, berberine, baicalin, wogonin and glycyrrhizin in the traditional chinese medicinal preparation sann-joong-kuey-jian-tang by high-performance liquid chromatography. J. Chromatogr. A 1996, 730, 17–23. [Google Scholar] [CrossRef]
- Okamura, N.; Miki, H.; Ishida, S.; Ono, H.; Yano, A.; Tanaka, T.; Ono, Y.; Yagi, A. Simultaneous determination of baicalin, wogonoside, baicalein, wogonin, berberine, coptisine, palmatine, jateorrhizine and glycyrrhizin in kampo medicines by ion-pair high-performance liquid chromatography. Biol. Pharm. Bull. 1999, 22, 1015–1021. [Google Scholar] [CrossRef]
- Cantelli-Forti, G.; Maffei, F.; Hrelia, P.; Bugamelli, F.; Bernardi, M.; D’Intino, P.; Maranesi, M.; Raggi, M. Interaction of licorice on glycyrrhizin pharmacokinetics. Environ. Health Perspect. 1994, 102, 65. [Google Scholar] [CrossRef]
- ISHIDA, S.; SAKIYA, Y.; ICHIKAWA, T.; TAIRA, Z. Dose-dependent pharmacokinetics of glycyrrhizin in rats. Chem. Pharm. Bull. 1992, 40, 1917–1920. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, Y.; Ho, W.S. Combination of liquiritin, isoliquiritin and isoliquirigenin induce apoptotic cell death through upregulating p53 and p21 in the a549 non-small cell lung cancer cells. Oncol Rep. 2014, 31, 298–304. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, H.; Liu, Y.; Dong, H.; Lv, B.; Fang, M.; Zhao, H. Metabolic routes along digestive system of licorice: Multicomponent sequential metabolism method in rat. Biomed. Chromatogr. 2016, 30, 902–912. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, L.; Chai, C.; Qian, X.C.; Li, W.; Li, J.S.; Di, L.Q.; Cai, B.C. Effects of food and gender on the pharmacokinetics of ginkgolides a, b, c and bilobalide in rats after oral dosing with ginkgo terpene lactones extract. J. Pharm. Biomed. Anal. 2014, 100, 138–144. [Google Scholar] [CrossRef]
- Guo, P.; Dong, L.; Yan, W.; Wei, J.; Wang, C.; Zhang, Z. Simultaneous determination of linarin, naringenin and formononetin in rat plasma by lc-ms/ms and its application to a pharmacokinetic study after oral administration of bushen guchi pill. Biomed. Chromatogr. 2015, 29, 246–253. [Google Scholar] [CrossRef]
- McConnell, E.L.; Basit, A.W.; Murdan, S. Measurements of rat and mouse gastrointestinal ph, fluid and lymphoid tissue, and implications for in-vivo experiments. J. Pharm. Pharmacol. 2008, 60, 63–70. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of Glycyrrhizae Radix extract (#KNUNPM GR-2015-001) are available from the authors. |






| Compounds | MRM Transitions (m/z) | Ionization Mode | Collision Energy (eV) | |
|---|---|---|---|---|
| Precursor Ion | Product Ion | |||
| Glycyrrhizin | 845 | 669 | Positive | 35 |
| Isoliquiritigenin | 255 | 135 | Negative | 15 |
| Liquiritigenin | 255 | 135 | Negative | 15 |
| Liquiritin | 417 | 255 | Negative | 20 |
| Berberine (IS) | 336 | 320 | Positive | 30 |
| Analytes | Representative Regression Equation | r2 | Linear Range (ng/mL) | LLOQ (ng/mL) |
|---|---|---|---|---|
| Glycyrrhizin | y = 0.0035x + 0.03 | 0.996 | 2–500 | 2 |
| Isoliquiritigenin | y = 0.1215x + 0.008 | 0.998 | 0.2–50 | 0.2 |
| Liquiritigenin | y = 0.1214x + 0.01 | 0.999 | 0.2–50 | 0.2 |
| Liquiritin | y = 0.2242x + 0.06 | 0.994 | 0.2–100 | 0.2 |
| Analytes | Nominal Concentration (ng/mL) | Intra-day | Inter-day | ||||
|---|---|---|---|---|---|---|---|
| Measured Concentration (ng/mL) | Precision (%) | Accuracy (%) | Measured Concentration (ng/mL) | Precision (%) | Accuracy (%) | ||
| Glycyrrhizin | 6 | 5.8 | 13.3 | 96.1 | 5.2 | 6.3 | 87.4 |
| 75 | 70.1 | 13.6 | 93.5 | 77.5 | 6.8 | 103.3 | |
| 400 | 416.8 | 13.3 | 104.2 | 410.1 | 7.4 | 102.5 | |
| Isoliquiritigenin | 0.6 | 0.7 | 4.9 | 108.6 | 0.6 | 8.9 | 101.8 |
| 7.5 | 7.9 | 10.8 | 105.6 | 7.7 | 4.7 | 102.1 | |
| 30 | 32.8 | 7.9 | 109.2 | 33.3 | 7.0 | 111.0 | |
| Liquiritigenin | 0.6 | 0.6 | 4.0 | 98.6 | 0.6 | 7.6 | 97.7 |
| 7.5 | 7.7 | 3.7 | 103.0 | 7.5 | 5.8 | 100.5 | |
| 30 | 32.6 | 3.7 | 108.6 | 33.6 | 5.6 | 112.0 | |
| Liquiritin | 0.6 | 0.6 | 9.8 | 99.2 | 0.6 | 8.8 | 103.3 |
| 10 | 9.9 | 10.0 | 99.2 | 10.0 | 5.5 | 99.9 | |
| 75 | 74.2 | 8.9 | 98.9 | 84.1 | 3.8 | 112.2 | |
| Analyte | Concentration (ng/mL) | Extraction Recovery (%) | CV (%) | Matrix Effects (%) | CV (%) | |
|---|---|---|---|---|---|---|
| Glycyrrhizin | Low QC | 6 | 89.06 ± 7.38 | 8.29 | 98.80 ± 5.89 | 5.96 |
| Medium QC | 75 | 77.2 ± 7.7 | 9.9 | 92.1 ± 3.6 | 3.9 | |
| High QC | 400 | 75.0 ± 4.9 | 6.6 | 96.4 ± 4.6 | 4.8 | |
| Isoliquiritigenin | Low QC | 0.6 | 80.2 ± 10.8 | 13.6 | 78.9 ± 3.0 | 3.8 |
| Medium QC | 7.5 | 74.6 ± 8.1 | 10.8 | 88.3 ± 4.9 | 5.5 | |
| High QC | 30 | 70.3 ± 4.9 | 7.1 | 93.6 ± 5.3 | 5.6 | |
| Liquiritigenin | Low QC | 0.6 | 99.1 ± 7.9 | 8.0 | 76.2 ± 3.1 | 4.1 |
| Medium QC | 7.5 | 88.9 ± 9.5 | 10.7 | 96.6 ± 8.1 | 8.4 | |
| High QC | 30 | 83.5 ± 6.4 | 7.7 | 104.9 ± 8.2 | 7.8 | |
| Liquiritin | Low QC | 0.6 | 79.2 ± 11.1 | 14.0 | 114.2 ± 10.4 | 9.1 |
| Medium QC | 10 | 83.2 ± 11.3 | 13.6 | 97.2 ± 14.4 | 14.8 | |
| High QC | 75 | 90.7 ± 6.4 | 7.1 | 101.8 ± 7.7 | 7.6 | |
| IS | 0.1 | 86.2 ± 2.7 | 3.1 | 108.2 ± 1.8 | 1.7 |
| Storage Conditions | Analytes | Concentration (ng/mL) | Precision % | Accuracy % | |
|---|---|---|---|---|---|
| Spiked | Measured | ||||
| Short-term stability | Glycyrrhizin | 6 | 5.6 | 6.8 | 94.0 |
| 400 | 390.8 | 9.5 | 97.7 | ||
| Isoliquiritigenin | 0.6 | 0.6 | 12.9 | 101.8 | |
| 30 | 30.1 | 7.5 | 100.3 | ||
| Liquiritigenin | 0.6 | 0.6 | 3.3 | 93.6 | |
| 30 | 28.8 | 8.9 | 102.7 | ||
| Liquiritin | 0.6 | 0.6 | 9.1 | 92.8 | |
| 75 | 71.3 | 10.1 | 99.5 | ||
| Post-preparative stability | Glycyrrhizin | 6 | 5.8 | 4.9 | 96.2 |
| 400 | 390.8 | 3.7 | 97.7 | ||
| Isoliquiritigenin | 0.6 | 0.6 | 4.1 | 92.4 | |
| 30 | 32.2 | 3.6 | 107.3 | ||
| Liquiritigenin | 0.6 | 0.6 | 6.4 | 93.0 | |
| 30 | 32.8 | 4.8 | 109.5 | ||
| Liquiritin | 0.6 | 0.6 | 3.7 | 97.3 | |
| 75 | 76.4 | 2.5 | 101.9 | ||
| Three freeze-thaw cycle stability | Glycyrrhizin | 6 | 6.8 | 0.8 | 113.9 |
| 400 | 394.1 | 10.3 | 98.5 | ||
| Isoliquiritigenin | 0.6 | 0.5 | 5.8 | 90.9 | |
| 30 | 30.0 | 7.1 | 99.9 | ||
| Liquiritigenin | 0.6 | 0.5 | 5.1 | 90.0 | |
| 30 | 29.2 | 8.2 | 97.2 | ||
| Liquiritin | 0.6 | 0.5 | 4.3 | 87.3 | |
| 75 | 72.6 | 11.1 | 96.7 | ||
| Compounds | Content (%) |
|---|---|
| Glycyrrhizin | 1.3 ± 0.2 |
| Isoliquiritigenin | 0.014 ± 0.004 |
| Liquiritigenin | 0.027 ± 0.010 |
| Liquiritin | 0.38 ± 0.07 |
| Parameters | Glycyrrhizin | Isoliquiritigenin | Liquiritigenin | Liquiritin |
|---|---|---|---|---|
| Cmax (ng/mL) | 164.4 ± 62.0 | 16.6 ± 2.7 | 10.8 ± 3.6 | 26.8 ± 8.5 |
| AUClast (ng∙h/mL) | 1051.0 ± 487.5 | 179.2 ± 46.3 | 112.5 ± 36.4 | 39.5 ± 7.8 |
| Tmax (h) | 0.4 ± 0.1 | 8.1 ± 5.2 | 8.1 ± 5.3 | 0.4 ± 0.1 |
| T1/2 (h) | 23.1 ± 15.5 | - | - | 3.7 ± 2.2 |
| MRTlast (h) | 10.7 ± 0.7 | 12.5 ± 1.3 | 12.8 ± 1.8 | 3.3 ± 1.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.J.; Kang, B.; Yang, E.-J.; Choi, M.-K.; Song, I.-S. Simultaneous Determination and Pharmacokinetic Characterization of Glycyrrhizin, Isoliquiritigenin, Liquiritigenin, and Liquiritin in Rat Plasma Following Oral Administration of Glycyrrhizae Radix Extract. Molecules 2019, 24, 1816. https://doi.org/10.3390/molecules24091816
Han YJ, Kang B, Yang E-J, Choi M-K, Song I-S. Simultaneous Determination and Pharmacokinetic Characterization of Glycyrrhizin, Isoliquiritigenin, Liquiritigenin, and Liquiritin in Rat Plasma Following Oral Administration of Glycyrrhizae Radix Extract. Molecules. 2019; 24(9):1816. https://doi.org/10.3390/molecules24091816
Chicago/Turabian StyleHan, You Jin, Bitna Kang, Eun-Ju Yang, Min-Koo Choi, and Im-Sook Song. 2019. "Simultaneous Determination and Pharmacokinetic Characterization of Glycyrrhizin, Isoliquiritigenin, Liquiritigenin, and Liquiritin in Rat Plasma Following Oral Administration of Glycyrrhizae Radix Extract" Molecules 24, no. 9: 1816. https://doi.org/10.3390/molecules24091816
APA StyleHan, Y. J., Kang, B., Yang, E.-J., Choi, M.-K., & Song, I.-S. (2019). Simultaneous Determination and Pharmacokinetic Characterization of Glycyrrhizin, Isoliquiritigenin, Liquiritigenin, and Liquiritin in Rat Plasma Following Oral Administration of Glycyrrhizae Radix Extract. Molecules, 24(9), 1816. https://doi.org/10.3390/molecules24091816