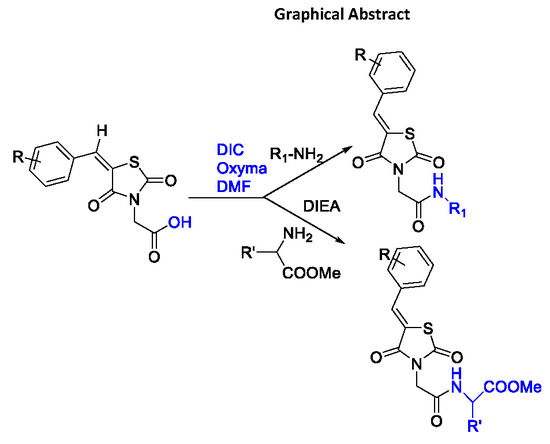
Synthesis and Antimicrobial Activity of a New Series of Thiazolidine-2,4-diones Carboxamide and Amino Acid Derivatives
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biology
3. Materials and Methods
3.1. Materials and Methods
3.2. General Procedure for the Synthesis of 2,4-Dioxothiazolidine Acid Derivatives
3.2.1. 2-(5-(4-Methylbenzylidene)-2,4-dioxothiazolidin-3-yl)acetic Acid (3b)
3.2.2. 2-(5-(4-Chlorobenzylidene)-2,4-dioxothiazolidin-3-yl)acetic Acid (3c)
3.2.3. 2-(5-(2-Chlorobenzylidene)-2,4-dioxothiazolidin-3-yl)acetic Acid (3d)
3.2.4. 2-(5-(4-Bromobenzylidene)-2,4-dioxothiazolidin-3-yl)acetic Acid (3e)
3.3. General Procedure for the Synthesis of 2,4-Dioxothiazolidine Carboxamide Derivatives 4a–s
3.3.1. 2-(5-Benzylidene-2,4-dioxothiazolidine-3-yl)-N-phenylacetamide (4a)
3.3.2. 2-(5-(3-Methoxybenzylidene)-2,4-dioxothiazolidine-3-yl)-N-phenylacetamide (4b)
3.3.3. 2-(5-(4-Bromobenzylidene)-2,4-dioxothiazolidine-3-yl)-N-phenylacetamide (4c)
3.3.4. 2-(5-(4-Methoxybenzylidene)-2,4-dioxothiazolidine-3-yl)-N-phenylacetamide (4d)
3.3.5. 2-(5-Benzylidene-2,4-dioxothiazolidine-3-yl)-N-(4-methoxyphenyl)acetamide (4e)
3.3.6. 2-(5-(3-Methoxybenzylidene)-2,4-dioxothiazolidine-3-yl)-N-(4-methoxyphenyl)acetamide (4f)
3.3.7. 2-(5-(4-Bromobenzylidene)-2,4-dioxothiazolidine-3-yl)-N-(4-methoxyphenyl)acetamide (4g)
3.3.8. 2-(5-(4-Methoxybenzylidene)-2,4-dioxothiazolidine-3-yl)-N-(4-methoxyphenyl)acetamide (4h)
3.3.9. 2-(5-Benzylidene-2,4-dioxothiazolidine-3-yl)-N-(4-bromophenyl)acetamide (4i)
3.3.10. N-(4-Bromophenyl)-2-(5-(3-methoxybenzylidene)-2,4-dioxothiazolidine-3-yl) acetamide (4j)
3.3.11. 2-(5-(4-Bromobenzylidene)-2,4-dioxothiazolidine-3-yl)-N-(4-bromophenyl)acetamide (4k)
3.3.12. N-(4-Bromophenyl)-2-(5-(4-methoxybenzylidene)-2,4-dioxothiazolidine-3-yl)acetamide (4l)
3.3.13. 2-(5-Benzylidene-2,4-dioxothiazolidine-3-yl)-N-(2-morpholinoethyl)acetamide (4m)
3.3.14. 2-(5-(2-Chlorobenzylidene)-2,4-dioxothiazolidine-3-yl)-N-(2-morpholinoethyl)acetamide (4n)
3.3.15. 2-(5-(4-Bromobenzylidene)-2,4-dioxothiazolidine-3-yl)-N-(2-morpholinoethyl)acetamide (4o)
3.3.16. 2-(5-(4-Methylbenzylidene)-2,4-dioxothiazolidine-3-yl)-N-(2-morpholinoethyl)acetamide (4p)
3.3.17. 2-(5-(4-Methoxylbenzylidene)-2,4-dioxothiazolidine-3-yl)-N-(2-morpholinoethyl)acetamide (4q)
3.3.18. 2-(5-(4-Chlorobenzylidene)-2,4-dioxothiazolidine-3-yl)-N-(2-morpholinoethyl)acetamide (4r)
3.3.19. 2-(5-(3-Methoxybenzylidene)-2,4-dioxothiazolidine-3-yl)-N-(2-morpholinoethyl)acetamide (4s)
3.4. General Procedure for the Synthesis of Amino Acid Ester Derivatives 5a–o
3.4.1. 2-(5-Benzylidene-2,4-dioxothiazolidine-3-yl) acetyl)glycinate (5a)
3.4.2. Methyl-(2-(5-(4-chlorobenzylidene)-2,4-dioxothiazolidine-3-yl)acetyl)glycinate (5b)
3.4.3. Methyl-(2-(5-(4-bromobenzylidene)-2,4-dioxothiazolidine-3-yl) acetyl)glycinate (5c)
3.4.4. Methyl-4-(2-(5-benzylidene-2,4-dioxothiazolidine-3-yl)acetamido)butanoate (5d)
3.4.5. Methyl-4-(2-(5-(4-chlorobenzylidene)-2,4-dioxothiazolidine-3-yl)acetamide)butanoate (5e)
3.4.6. Methyl-4-(2-(5-(4-bromobenzylidene)-2,4-dioxothiazolidine-3-yl)acetamido)butanoate (5f)
3.4.7. Methyl-2-(5-benzylidene-2,4-dioxothiazolidine-3-yl)acetyl)valinate (5g)
3.4.8. Methyl-(2-(5-(4-chlorobenzylidene)-2,4-dioxothiazolidine-3-yl)acetyl)valinate (5h)
3.4.9. Methyl-2-(5-(4-bromobenzylidene)-2,4-dioxothiazolidine-3-yl)acetyl)valinate (5i)
3.4.10. Methyl-2-(5-benzylidene-2,4-dioxothiazolidine-3-yl)acetyl)alaninate (5j)
3.4.11. Methyl-2-(5-(4-chlorobenzylidene)-2,4-dioxothiazolidine-3-yl)acetyl)alaninate (5k)
3.4.12. Methyl-2-(5-(4-bromobenzylidene)-2,4-dioxothiazolidine-3-yl) acetyl)alaninate (5l)
3.4.13. Methyl-(2-(5-benzylidene-2,4-dioxothiazolidine-3-yl) acetyl)phenylalaninate (5m)
3.4.14. Methyl-2-(5-(4-chlorobenzylidene)-2,4-dioxothiazolidine-3yl)acetyl)phenylalaninate (5n)
3.4.15. Methyl-(2-(5-(4-bromobenzylidene)-2,4-dioxothiazolidine-3-yl)acetyl)phenylalaninate (5o)
3.5. Antimicrobial Activity
3.5.1. Microbial Preparation
3.5.2. Well Diffusion Technique
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Asati, V.; Mahapatra, D.K.; Bharti, S.K. Thiazolidine-2,4-diones as multi-targeted scaffold in medicinal chemistry: Potential anticancer agents. Eur. J. Med. Chem. 2014, 87, 814–833. [Google Scholar] [CrossRef] [PubMed]
- Chadha, N.; Bahia, M.S.; Kaur, M.; Silakari, O. Thiazolidine-2,4-dione derivatives: Programmed chemical weapons for key protein targets of various pathological conditions. Bioorg. Med. Chem. 2015, 23, 2953–2974. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.S.; Vora, D.K.; Ramaa, C.S. Thiazolidine-2,4-diones: Progress towards multifarious applications. Bioorg. Med. Chem. 2013, 21, 1599–1620. [Google Scholar] [CrossRef] [PubMed]
- Ayhan-Kilcigil, G.; Altanlar, N. Synthesis of 3-substituted phenacyl-5-[2-phenyl-4H-4-oxo-1-benzopyran-6-yl)methylenyl]-thiazolidine-2,4-diones and evaluation of their antimicrobial activity. Arzneim. Forsch. Drug Res. 2000, 50, 154. [Google Scholar] [CrossRef]
- Tuncbilek, M.; Altanlar, N. Synthesis of new 3-(pubstituted phenacyl)-5-[3′-(4H-4-oxo-1-benzopyran-2-yl)-benzylidene]-2,4-thiazolidinediones and their antimicrobial activity. Arch. Pharm. Chem. Life Sci. 2006, 339, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Bozdağ-Dundar, O.; Ozen, O.; Arzu, M.; Nurten, A.; Onur, A.; Engin, K.; Rahmiye, E. Synthesis and antimicrobial activity of some new thiazolyl thiazolidine-2,4-dione derivatives. Bioorg. Med. Chem. 2007, 15, 6012–6017. [Google Scholar] [CrossRef] [PubMed]
- Alegaon, S.G.; Alagawadi, K.R.; Pawar, S.M.; Vinod, D.; Rajput, U. Synthesis, characterization, and biological evaluation of thiazolidine-2,4-dione derivatives. Med. Chem. Res. 2014, 23, 987–994. [Google Scholar] [CrossRef]
- Khan, F.A.K.; Patil, R.H.; Shinde, D.B.; Sangshett, J.N. Design and synthesis of 4′-((5-benzylidene-2,4-dioxothiazolidin-3-yl)methyl)biphenyl-2-carbonitrile analogs as bacterial Peptide deformylase inhibitors. Chem. Biol. Drug. Des. 2016, 88, 938–944. [Google Scholar] [CrossRef]
- Trotsko, N.; Kosikowska, U.; Paneth, A.; Plech, T.; Malm, A.; Wujec, M. Synthesis and antibacterial activity of New Thiazolidine-2,4-dione-based chlorophenylthiosemi carbazone hybrids. Molecules 2018, 23, 1023. [Google Scholar] [CrossRef]
- Moorthy, P.; Ekambaram, S.P.; Perumal, S.S. Synthesis, characterization and antimicrobial evaluation of imidazolylthiazolidinedione derivatives. Arabian J. Chem. 2014, 8, 1–7. [Google Scholar]
- Alagawadi, K.R.; Alegaon, S.G. Synthesis, characterization and antimicrobial activity evaluation of new 2,4-Thiazolidinediones bearing Imidazo [2, 1-b] [1, 3, 4] thiadiazole moiety. Arabian J. Chem. 2011, 4, 465–472. [Google Scholar] [CrossRef]
- Aneja, D.K.; Lohan, P.; Arora, S.; Sharma, C.; Aneja, K.R.; Prakash, O. Synthesis of new pyrazolyl-2,4-thiazolidinediones as antibacterial and antifungal agents. Org. Med. Chem. Lett. 2011, 1, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Takagi, M.; Noritake, C.; Kagabu, S. 2,4-Dioxo-1,3-thiazolidine derivatives as a lead for new fungicides. J. Pestic. Sci. 2008, 33, 357–363. [Google Scholar] [CrossRef]
- Barros, C.D.; Amato, A.A.; de Oliveira, T.B.; Iannini, K.B.R.; da Silva, A.L.; da Silva, T.G.; Leite, E.S.; Hernandes, M.Z.; de Lima, M.D.A.; Galdino, S.L.; et al. Synthesis and anti-inflammatory activity of new arylidene-thiazolidine-2,4-diones as PPARγ ligands. Bioorg. Med. Chem. 2010, 18, 3805–3811. [Google Scholar] [CrossRef]
- Maccari, R.; Vitale, R.M.; Ottanà, R.; Rocchiccioli, M.; Marrazzo, A.; Cardile, V.; Graziano, A.C.E.; Amodeo, P.; Mura, U.; Corso, A.D. Structure-activity relationships and molecular modelling of new 5-arylidene-4-thiazolidinone derivatives as aldose reductase inhibitors and potential anti-inflammatory agents. Eur. J. Med. Chem. 2014, 81, 1–14. [Google Scholar] [CrossRef]
- Youssef, M.; White, M.S.; Villanueva, E.B.; El-Ashmawy, I.M.; Klegeris, A. Synthesis and biological evaluation of novel pyrazolyl-2,4-thiazolidinediones as anti-inflammatory and neuroprotective agents. Bioorg. Med. Chem. 2010, 18, 2019–2028. [Google Scholar] [CrossRef]
- Ottanà, R.; Maccari, R.; Giglio, M.; Del Corso, A.; Cappiello, M.; Mura, U.; Cosconati, S.; Marinelli, L.; Novellino, E.; Sartini, S.; et al. Identification of 5-arylidene-4-thiazolidinone derivatives endowed with dual activity as aldose reductase inhibitors and antioxidant agents for the treatment of diabetic complications. Eur. J. Med. Chem. 2011, 46, 2797–2806. [Google Scholar] [CrossRef]
- Pandey, J.; Ali, A.; Gupta, A.K. Synthesis and evaluation of some new 2-(5-(4-benzamidobenzylidene)-2,4-dioxothiazolidin-3-yl) acetic acid analogs as aldose reductase inhibitors. Asian J. Pharm. Clin. Res. 2017, 10, 62–66. [Google Scholar]
- Kumar, K.S.; Reddy, B.M.; Babu, V.H. Synthesis of some novel 2,4-thiazolidinedione incorporated pyrazole derivatives as anti-cancer agent. Int. J. Pharm. Pharm. Sci. 2014, 6, 831–834. [Google Scholar]
- Trotsko, N.; Przekora, A.; Zalewska, J.; Ginalska, G.; Paneth, A.; Wujec, M. Synthesis and in vitro antiproliferative and antibacterial activity of new thiazolidine-2,4-dione derivatives. J. Enzyme. Inhib. Med. Chem. 2017, 33, 17–24. [Google Scholar] [CrossRef]
- Ozen, C.; C-Unlusoy, M.; Aliary, N.; Ozturk, M.; B-Dundar, O. Thiazolidinedione or Rhodanine: A study on synthesis and anti-cancer activity comparison of novel thiazole derivatives. J. Pharm. Sci. 2017, 20, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Asati, V.; Bharti, S.K. Design, synthesis and molecular modeling studies of novel thiazolidine-2,4-dione derivatives as potential anti-cancer agents. J. Mol. Structure. 2018, 1154, 406–417. [Google Scholar] [CrossRef]
- Pavase1, L.S.; Mane, D.V. Synthesis and anticancer studies of 1-4-(2,4-Dioxo-thiazolidin-5-ylidenemethyl)-benzenesulfonyl]-pyrrolidine-2-carboxylic acid derivatives. Chem. Biol. Inter. 2017, 7, 183–194. [Google Scholar]
- Liu, K.; Rao, W.; Parikh, H.; Li, Q.; Guo, T.; Grant, S.; Kellogg, G.; Zhang, S. 3,5-Disubstituted-thiazolidine-2,4-dione analogs as anticancer agents: Design, synthesis and biological characterization. Eur. J. Med. Chem. 2012, 47, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Reddy, T.S.; Kumar, N.P.; Senwar, K.R.; Bhargava, S.K.; Shankaraiah, N. Conventional and microwave-assisted synthesis of new 1H-benzimidazole-thiazolidinedione derivatives: A potential anticancer scaffold. Eur. J. Med. Chem. 2017, 138, 234–245. [Google Scholar] [CrossRef]
- Sharma, R.K.; Younis, Y.; Mugumbate, G.; Njoroge, M.; Gut, J.; Rosenthal, P.J.; Chibale, K. Synthesis and structure reactivity-relationship studies of thiazolidinediones as antiplasmodial inhibitors of the Plasmodium falciparum cysteine protease falcipain-2. Eur. J. Med. Chem. 2015, 90, 507–518. [Google Scholar] [CrossRef]
- Datar, P.A.; Aher, S.B. Design and synthesis of novel thiazolidine-2,4-diones as hypoglycemic agents. J. Saudi Chem. Soc. 2016, 20, 196–201. [Google Scholar] [CrossRef]
- Shrivastava, S.K.; Batham, A.; Sinha, S.K.; Parida, T.K.; Garabadu, D.; Choubey, P.K. Design, synthesis and evaluation of novel thiazolidinedione derivatives as anti-hyperglycemic and anti-hyperlipidemic agents. Med. Chem. Res. 2016, 25, 2258–2266. [Google Scholar] [CrossRef]
- Naim, M.J.; Alam, M.J.; Nawaz, F.; Naidu, V.G.M.; Aaghaz, S.; Sahu, M.; Siddiqui, N.; Alam, O. Synthesis, molecular docking and anti-diabetic evaluation of 2,4-thiazolidinedione based amide derivatives. Bioorg. Chem. 2017, 73, 24–36. [Google Scholar] [CrossRef]
- Bhat, B.A.; Ponnala, S.; Sahu, D.P.; Tiwari, P.; Tripathi, B.K.; Srivastava, A.K. Synthesis and anti-hyperglycemic activity profiles of novel thiazolidinedione derivatives. Bioorg. Med. Chem. 2004, 12, 5857–5864. [Google Scholar] [CrossRef]
- Mahapatra, M.K.; Kumar, R.; Kumar, M. Exploring sulfonate esters of 5-arylidene thiazolidine-2,4-diones as PTP1B inhibitors with anti-hyperglycemic activity. Med. Chem. Res. 2018, 27, 476–487. [Google Scholar] [CrossRef]
- Yoshioka, T.; Fujita, T.; Kanai, T.; Aizava, Y.; Kurumada, T.; Hasegava, K.; Horikoshi, H. Studies on hindered phenols and analogues. 1. Hypolipidemic and hypoglycemic agents with ability to inhibit lipid peroxidation. J. Med. Chem. 1989, 32, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Rakowitz, D.; Maccari, R.; Ottaná, R.; Vigorita, M.G. In vitro aldose reductase inhibitory activity of 5-benzyl-2,4-thiazolidinediones. Bioorg. Med. Chem. 2006, 14, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Momose, Y.; Maekawa, T.; Odaka, H.; Ikeda, H.; Sohdad, T. Novel 5-Substituted-1H-tetrazole derivatives as potent glucose and lipid lowering agents. Chem. Pharm. Bull. 2002, 50, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.R.P.; Karvekar, M.D.; Adhikary, L.; Nanjan, N.J.; Suresh, B. Microwave induced synthesis of the thiazolidine-2,4-dione motif and the efficient solvent free-solid phase parallel syntheses of 5-Benzylidene-thiazolidine-2,4-dione and 5-benzylidene-2-thioxo-thiazolidine-4-one compounds. J. Hetero. Chem. 2006, 43, 897–903. [Google Scholar] [CrossRef]
- S-Funosas, R.; Prohens, R.; Barbas, R.; El-Faham, A.; Albericio, F. Oxyma: An Efficient additive for peptide synthesis to replace the benzotriazole-based HOBt and HOAt with a lower risk of explosion. Chem. Eur. J. 2009, 15, 9394–9403. [Google Scholar] [CrossRef]
- El-Faham, A.; Almarhoon, Z.; Ab-Megeed, A.; Albericio, F. OxymaPure/DIC: An efficient reagent for the synthesis of a novel series of 4-[2-(2-Acetylaminophenyl)-2-oxo-acetylamino] benzoyl amino acid ester derivatives. Molecules 2013, 18, 14747–14759. [Google Scholar] [CrossRef]
- Romagnoli, R.; Baraldi, P.G.; Kimatrai Salvador, M.; Camacho, M.E.; Balzarini, J.; Bermejo, J.; Estévez, F. Anticancer activity of novel hybrid molecules containing 5-benzylidene thiazolidine-2,4-dione. Eur. J. Med. Chem. 2013, 63, 544–557. [Google Scholar] [CrossRef]
- Azevedo, M.M.; Ramalho, P.; Silva, A.P.; T.-Santos, R.; P.-Vaz, C.; Rodrigues, A.G. Polyethyleneimine (PEI) and PEI-based nanoparticles: Novel bacterial and yeast biofilm inhibitors. J. Med. Microbiol. 2014, 63, 1167–1173. [Google Scholar] [CrossRef]
- Maccari, R.; Ottana, R.; Curinga, C.; Vigorita, M.G.; Rakowitz, D.; Steindl, T.; Langer, T. Structure–activity relationships and molecular modelling of 5-arylidene-2,4-thiazolidinediones active as aldose reductase inhibitors. Bioorg. Med. Chem. 2005, 13, 2809–2823. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 4a–s and 5a–o are available from the authors. |



| Average Inhibition Zone in mm | |||||
|---|---|---|---|---|---|
| Chemical Compounds | S. aureus | Bacillus subtilis | E. coli | Ps. aeruginosa | C. albicans |
| 3a | 12 | - | 11 | 16 | 15 |
| 3b | 12 | - | 12 | 11 | 11 |
| 3c | - | - | - | 10 | - |
| 3d | - | - | 10 | 15 | 15 |
| 3e | - | - | 12 | 13 | - |
| 3f | - | - | - | 13 | - |
| 3g | 20 | - | 7 | 14 | 7 |
| 4a | - | - | - | 12 | 14 |
| 4b | - | - | - | - | 12 |
| 4c | - | - | - | - | 12 |
| 4d | - | - | - | 14 | - |
| 4e | - | - | - | - | 13 |
| 4f | - | - | - | 11 | - |
| 4g | - | - | - | 12 | 14 |
| 4h | - | - | - | 13 | - |
| 4i | - | - | - | 12 | - |
| 4k | - | - | - | - | 15 |
| 4l | - | - | - | - | 13 |
| 4m | - | - | - | 10 | - |
| 4n | - | - | - | 11 | 13 |
| 4o | - | - | - | 10 | - |
| 4p | - | - | - | 12 | 14 |
| 4q | - | - | - | 12 | 12 |
| 4r | - | - | - | 14 | - |
| 4s | - | - | 11 | 12 | 12 |
| 5a | - | - | - | - | - |
| 5b | - | - | - | - | 15 |
| 5c | - | - | - | - | 13 |
| 5d | - | - | - | - | - |
| 5e | - | - | - | 12 | - |
| 5f | - | - | - | - | - |
| 5g | - | - | 10 | 12 | 15 |
| 5h | - | - | 11 | 11 | 13 |
| 5i | - | - | 12 | 12 | - |
| 5j | - | - | - | - | - |
| 5k | - | - | 7 | 13 | 18 |
| 5l | - | - | - | - | 12 |
| 5m | - | - | - | - | 12 |
| 5n | - | - | 10 | - | - |
| 5o | - | - | 11 | 12 | 16 |
| Impenem * | 30 | 34 | 20 | 35 | |
| SXT ** | 22 | 20 | - | 30 | |
| Fluconazole | - | - | - | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd Alhameed, R.; Almarhoon, Z.; Bukhari, S.I.; El-Faham, A.; de la Torre, B.G.; Albericio, F. Synthesis and Antimicrobial Activity of a New Series of Thiazolidine-2,4-diones Carboxamide and Amino Acid Derivatives. Molecules 2020, 25, 105. https://doi.org/10.3390/molecules25010105
Abd Alhameed R, Almarhoon Z, Bukhari SI, El-Faham A, de la Torre BG, Albericio F. Synthesis and Antimicrobial Activity of a New Series of Thiazolidine-2,4-diones Carboxamide and Amino Acid Derivatives. Molecules. 2020; 25(1):105. https://doi.org/10.3390/molecules25010105
Chicago/Turabian StyleAbd Alhameed, Rakia, Zainab Almarhoon, Sarah I. Bukhari, Ayman El-Faham, Beatriz G. de la Torre, and Fernando Albericio. 2020. "Synthesis and Antimicrobial Activity of a New Series of Thiazolidine-2,4-diones Carboxamide and Amino Acid Derivatives" Molecules 25, no. 1: 105. https://doi.org/10.3390/molecules25010105
APA StyleAbd Alhameed, R., Almarhoon, Z., Bukhari, S. I., El-Faham, A., de la Torre, B. G., & Albericio, F. (2020). Synthesis and Antimicrobial Activity of a New Series of Thiazolidine-2,4-diones Carboxamide and Amino Acid Derivatives. Molecules, 25(1), 105. https://doi.org/10.3390/molecules25010105