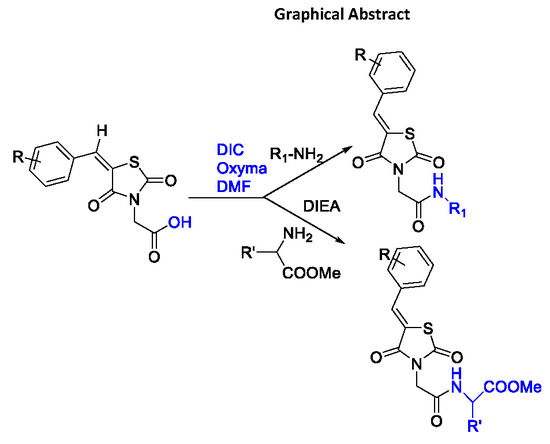
Synthesis and Antimicrobial Activity of a New Series of Thiazolidine-2,4-diones Carboxamide and Amino Acid Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biology
3. Materials and Methods
3.1. Materials and Methods
3.2. General Procedure for the Synthesis of 2,4-Dioxothiazolidine Acid Derivatives
3.2.1. 2-(5-(4-Methylbenzylidene)-2,4-dioxothiazolidin-3-yl)acetic Acid (3b)
3.2.2. 2-(5-(4-Chlorobenzylidene)-2,4-dioxothiazolidin-3-yl)acetic Acid (3c)
3.2.3. 2-(5-(2-Chlorobenzylidene)-2,4-dioxothiazolidin-3-yl)acetic Acid (3d)
3.2.4. 2-(5-(4-Bromobenzylidene)-2,4-dioxothiazolidin-3-yl)acetic Acid (3e)
3.3. General Procedure for the Synthesis of 2,4-Dioxothiazolidine Carboxamide Derivatives 4a–s
3.3.1. 2-(5-Benzylidene-2,4-dioxothiazolidine-3-yl)-N-phenylacetamide (4a)
3.3.2. 2-(5-(3-Methoxybenzylidene)-2,4-dioxothiazolidine-3-yl)-N-phenylacetamide (4b)
3.3.3. 2-(5-(4-Bromobenzylidene)-2,4-dioxothiazolidine-3-yl)-N-phenylacetamide (4c)
3.3.4. 2-(5-(4-Methoxybenzylidene)-2,4-dioxothiazolidine-3-yl)-N-phenylacetamide (4d)
3.3.5. 2-(5-Benzylidene-2,4-dioxothiazolidine-3-yl)-N-(4-methoxyphenyl)acetamide (4e)
3.3.6. 2-(5-(3-Methoxybenzylidene)-2,4-dioxothiazolidine-3-yl)-N-(4-methoxyphenyl)acetamide (4f)
3.3.7. 2-(5-(4-Bromobenzylidene)-2,4-dioxothiazolidine-3-yl)-N-(4-methoxyphenyl)acetamide (4g)
3.3.8. 2-(5-(4-Methoxybenzylidene)-2,4-dioxothiazolidine-3-yl)-N-(4-methoxyphenyl)acetamide (4h)
3.3.9. 2-(5-Benzylidene-2,4-dioxothiazolidine-3-yl)-N-(4-bromophenyl)acetamide (4i)
3.3.10. N-(4-Bromophenyl)-2-(5-(3-methoxybenzylidene)-2,4-dioxothiazolidine-3-yl) acetamide (4j)
3.3.11. 2-(5-(4-Bromobenzylidene)-2,4-dioxothiazolidine-3-yl)-N-(4-bromophenyl)acetamide (4k)
3.3.12. N-(4-Bromophenyl)-2-(5-(4-methoxybenzylidene)-2,4-dioxothiazolidine-3-yl)acetamide (4l)
3.3.13. 2-(5-Benzylidene-2,4-dioxothiazolidine-3-yl)-N-(2-morpholinoethyl)acetamide (4m)
3.3.14. 2-(5-(2-Chlorobenzylidene)-2,4-dioxothiazolidine-3-yl)-N-(2-morpholinoethyl)acetamide (4n)
3.3.15. 2-(5-(4-Bromobenzylidene)-2,4-dioxothiazolidine-3-yl)-N-(2-morpholinoethyl)acetamide (4o)
3.3.16. 2-(5-(4-Methylbenzylidene)-2,4-dioxothiazolidine-3-yl)-N-(2-morpholinoethyl)acetamide (4p)
3.3.17. 2-(5-(4-Methoxylbenzylidene)-2,4-dioxothiazolidine-3-yl)-N-(2-morpholinoethyl)acetamide (4q)
3.3.18. 2-(5-(4-Chlorobenzylidene)-2,4-dioxothiazolidine-3-yl)-N-(2-morpholinoethyl)acetamide (4r)
3.3.19. 2-(5-(3-Methoxybenzylidene)-2,4-dioxothiazolidine-3-yl)-N-(2-morpholinoethyl)acetamide (4s)
3.4. General Procedure for the Synthesis of Amino Acid Ester Derivatives 5a–o
3.4.1. 2-(5-Benzylidene-2,4-dioxothiazolidine-3-yl) acetyl)glycinate (5a)
3.4.2. Methyl-(2-(5-(4-chlorobenzylidene)-2,4-dioxothiazolidine-3-yl)acetyl)glycinate (5b)
3.4.3. Methyl-(2-(5-(4-bromobenzylidene)-2,4-dioxothiazolidine-3-yl) acetyl)glycinate (5c)
3.4.4. Methyl-4-(2-(5-benzylidene-2,4-dioxothiazolidine-3-yl)acetamido)butanoate (5d)
3.4.5. Methyl-4-(2-(5-(4-chlorobenzylidene)-2,4-dioxothiazolidine-3-yl)acetamide)butanoate (5e)
3.4.6. Methyl-4-(2-(5-(4-bromobenzylidene)-2,4-dioxothiazolidine-3-yl)acetamido)butanoate (5f)
3.4.7. Methyl-2-(5-benzylidene-2,4-dioxothiazolidine-3-yl)acetyl)valinate (5g)
3.4.8. Methyl-(2-(5-(4-chlorobenzylidene)-2,4-dioxothiazolidine-3-yl)acetyl)valinate (5h)
3.4.9. Methyl-2-(5-(4-bromobenzylidene)-2,4-dioxothiazolidine-3-yl)acetyl)valinate (5i)
3.4.10. Methyl-2-(5-benzylidene-2,4-dioxothiazolidine-3-yl)acetyl)alaninate (5j)
3.4.11. Methyl-2-(5-(4-chlorobenzylidene)-2,4-dioxothiazolidine-3-yl)acetyl)alaninate (5k)
3.4.12. Methyl-2-(5-(4-bromobenzylidene)-2,4-dioxothiazolidine-3-yl) acetyl)alaninate (5l)
3.4.13. Methyl-(2-(5-benzylidene-2,4-dioxothiazolidine-3-yl) acetyl)phenylalaninate (5m)
3.4.14. Methyl-2-(5-(4-chlorobenzylidene)-2,4-dioxothiazolidine-3yl)acetyl)phenylalaninate (5n)
3.4.15. Methyl-(2-(5-(4-bromobenzylidene)-2,4-dioxothiazolidine-3-yl)acetyl)phenylalaninate (5o)
3.5. Antimicrobial Activity
3.5.1. Microbial Preparation
3.5.2. Well Diffusion Technique
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Asati, V.; Mahapatra, D.K.; Bharti, S.K. Thiazolidine-2,4-diones as multi-targeted scaffold in medicinal chemistry: Potential anticancer agents. Eur. J. Med. Chem. 2014, 87, 814–833. [Google Scholar] [CrossRef] [PubMed]
- Chadha, N.; Bahia, M.S.; Kaur, M.; Silakari, O. Thiazolidine-2,4-dione derivatives: Programmed chemical weapons for key protein targets of various pathological conditions. Bioorg. Med. Chem. 2015, 23, 2953–2974. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.S.; Vora, D.K.; Ramaa, C.S. Thiazolidine-2,4-diones: Progress towards multifarious applications. Bioorg. Med. Chem. 2013, 21, 1599–1620. [Google Scholar] [CrossRef] [PubMed]
- Ayhan-Kilcigil, G.; Altanlar, N. Synthesis of 3-substituted phenacyl-5-[2-phenyl-4H-4-oxo-1-benzopyran-6-yl)methylenyl]-thiazolidine-2,4-diones and evaluation of their antimicrobial activity. Arzneim. Forsch. Drug Res. 2000, 50, 154. [Google Scholar] [CrossRef]
- Tuncbilek, M.; Altanlar, N. Synthesis of new 3-(pubstituted phenacyl)-5-[3′-(4H-4-oxo-1-benzopyran-2-yl)-benzylidene]-2,4-thiazolidinediones and their antimicrobial activity. Arch. Pharm. Chem. Life Sci. 2006, 339, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Bozdağ-Dundar, O.; Ozen, O.; Arzu, M.; Nurten, A.; Onur, A.; Engin, K.; Rahmiye, E. Synthesis and antimicrobial activity of some new thiazolyl thiazolidine-2,4-dione derivatives. Bioorg. Med. Chem. 2007, 15, 6012–6017. [Google Scholar] [CrossRef] [PubMed]
- Alegaon, S.G.; Alagawadi, K.R.; Pawar, S.M.; Vinod, D.; Rajput, U. Synthesis, characterization, and biological evaluation of thiazolidine-2,4-dione derivatives. Med. Chem. Res. 2014, 23, 987–994. [Google Scholar] [CrossRef]
- Khan, F.A.K.; Patil, R.H.; Shinde, D.B.; Sangshett, J.N. Design and synthesis of 4′-((5-benzylidene-2,4-dioxothiazolidin-3-yl)methyl)biphenyl-2-carbonitrile analogs as bacterial Peptide deformylase inhibitors. Chem. Biol. Drug. Des. 2016, 88, 938–944. [Google Scholar] [CrossRef]
- Trotsko, N.; Kosikowska, U.; Paneth, A.; Plech, T.; Malm, A.; Wujec, M. Synthesis and antibacterial activity of New Thiazolidine-2,4-dione-based chlorophenylthiosemi carbazone hybrids. Molecules 2018, 23, 1023. [Google Scholar] [CrossRef] [Green Version]
- Moorthy, P.; Ekambaram, S.P.; Perumal, S.S. Synthesis, characterization and antimicrobial evaluation of imidazolylthiazolidinedione derivatives. Arabian J. Chem. 2014, 8, 1–7. [Google Scholar]
- Alagawadi, K.R.; Alegaon, S.G. Synthesis, characterization and antimicrobial activity evaluation of new 2,4-Thiazolidinediones bearing Imidazo [2, 1-b] [1, 3, 4] thiadiazole moiety. Arabian J. Chem. 2011, 4, 465–472. [Google Scholar] [CrossRef] [Green Version]
- Aneja, D.K.; Lohan, P.; Arora, S.; Sharma, C.; Aneja, K.R.; Prakash, O. Synthesis of new pyrazolyl-2,4-thiazolidinediones as antibacterial and antifungal agents. Org. Med. Chem. Lett. 2011, 1, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, M.; Takagi, M.; Noritake, C.; Kagabu, S. 2,4-Dioxo-1,3-thiazolidine derivatives as a lead for new fungicides. J. Pestic. Sci. 2008, 33, 357–363. [Google Scholar] [CrossRef] [Green Version]
- Barros, C.D.; Amato, A.A.; de Oliveira, T.B.; Iannini, K.B.R.; da Silva, A.L.; da Silva, T.G.; Leite, E.S.; Hernandes, M.Z.; de Lima, M.D.A.; Galdino, S.L.; et al. Synthesis and anti-inflammatory activity of new arylidene-thiazolidine-2,4-diones as PPARγ ligands. Bioorg. Med. Chem. 2010, 18, 3805–3811. [Google Scholar] [CrossRef]
- Maccari, R.; Vitale, R.M.; Ottanà, R.; Rocchiccioli, M.; Marrazzo, A.; Cardile, V.; Graziano, A.C.E.; Amodeo, P.; Mura, U.; Corso, A.D. Structure-activity relationships and molecular modelling of new 5-arylidene-4-thiazolidinone derivatives as aldose reductase inhibitors and potential anti-inflammatory agents. Eur. J. Med. Chem. 2014, 81, 1–14. [Google Scholar] [CrossRef]
- Youssef, M.; White, M.S.; Villanueva, E.B.; El-Ashmawy, I.M.; Klegeris, A. Synthesis and biological evaluation of novel pyrazolyl-2,4-thiazolidinediones as anti-inflammatory and neuroprotective agents. Bioorg. Med. Chem. 2010, 18, 2019–2028. [Google Scholar] [CrossRef]
- Ottanà, R.; Maccari, R.; Giglio, M.; Del Corso, A.; Cappiello, M.; Mura, U.; Cosconati, S.; Marinelli, L.; Novellino, E.; Sartini, S.; et al. Identification of 5-arylidene-4-thiazolidinone derivatives endowed with dual activity as aldose reductase inhibitors and antioxidant agents for the treatment of diabetic complications. Eur. J. Med. Chem. 2011, 46, 2797–2806. [Google Scholar] [CrossRef]
- Pandey, J.; Ali, A.; Gupta, A.K. Synthesis and evaluation of some new 2-(5-(4-benzamidobenzylidene)-2,4-dioxothiazolidin-3-yl) acetic acid analogs as aldose reductase inhibitors. Asian J. Pharm. Clin. Res. 2017, 10, 62–66. [Google Scholar]
- Kumar, K.S.; Reddy, B.M.; Babu, V.H. Synthesis of some novel 2,4-thiazolidinedione incorporated pyrazole derivatives as anti-cancer agent. Int. J. Pharm. Pharm. Sci. 2014, 6, 831–834. [Google Scholar]
- Trotsko, N.; Przekora, A.; Zalewska, J.; Ginalska, G.; Paneth, A.; Wujec, M. Synthesis and in vitro antiproliferative and antibacterial activity of new thiazolidine-2,4-dione derivatives. J. Enzyme. Inhib. Med. Chem. 2017, 33, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Ozen, C.; C-Unlusoy, M.; Aliary, N.; Ozturk, M.; B-Dundar, O. Thiazolidinedione or Rhodanine: A study on synthesis and anti-cancer activity comparison of novel thiazole derivatives. J. Pharm. Sci. 2017, 20, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Asati, V.; Bharti, S.K. Design, synthesis and molecular modeling studies of novel thiazolidine-2,4-dione derivatives as potential anti-cancer agents. J. Mol. Structure. 2018, 1154, 406–417. [Google Scholar] [CrossRef]
- Pavase1, L.S.; Mane, D.V. Synthesis and anticancer studies of 1-4-(2,4-Dioxo-thiazolidin-5-ylidenemethyl)-benzenesulfonyl]-pyrrolidine-2-carboxylic acid derivatives. Chem. Biol. Inter. 2017, 7, 183–194. [Google Scholar]
- Liu, K.; Rao, W.; Parikh, H.; Li, Q.; Guo, T.; Grant, S.; Kellogg, G.; Zhang, S. 3,5-Disubstituted-thiazolidine-2,4-dione analogs as anticancer agents: Design, synthesis and biological characterization. Eur. J. Med. Chem. 2012, 47, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Reddy, T.S.; Kumar, N.P.; Senwar, K.R.; Bhargava, S.K.; Shankaraiah, N. Conventional and microwave-assisted synthesis of new 1H-benzimidazole-thiazolidinedione derivatives: A potential anticancer scaffold. Eur. J. Med. Chem. 2017, 138, 234–245. [Google Scholar] [CrossRef]
- Sharma, R.K.; Younis, Y.; Mugumbate, G.; Njoroge, M.; Gut, J.; Rosenthal, P.J.; Chibale, K. Synthesis and structure reactivity-relationship studies of thiazolidinediones as antiplasmodial inhibitors of the Plasmodium falciparum cysteine protease falcipain-2. Eur. J. Med. Chem. 2015, 90, 507–518. [Google Scholar] [CrossRef]
- Datar, P.A.; Aher, S.B. Design and synthesis of novel thiazolidine-2,4-diones as hypoglycemic agents. J. Saudi Chem. Soc. 2016, 20, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Shrivastava, S.K.; Batham, A.; Sinha, S.K.; Parida, T.K.; Garabadu, D.; Choubey, P.K. Design, synthesis and evaluation of novel thiazolidinedione derivatives as anti-hyperglycemic and anti-hyperlipidemic agents. Med. Chem. Res. 2016, 25, 2258–2266. [Google Scholar] [CrossRef]
- Naim, M.J.; Alam, M.J.; Nawaz, F.; Naidu, V.G.M.; Aaghaz, S.; Sahu, M.; Siddiqui, N.; Alam, O. Synthesis, molecular docking and anti-diabetic evaluation of 2,4-thiazolidinedione based amide derivatives. Bioorg. Chem. 2017, 73, 24–36. [Google Scholar] [CrossRef]
- Bhat, B.A.; Ponnala, S.; Sahu, D.P.; Tiwari, P.; Tripathi, B.K.; Srivastava, A.K. Synthesis and anti-hyperglycemic activity profiles of novel thiazolidinedione derivatives. Bioorg. Med. Chem. 2004, 12, 5857–5864. [Google Scholar] [CrossRef]
- Mahapatra, M.K.; Kumar, R.; Kumar, M. Exploring sulfonate esters of 5-arylidene thiazolidine-2,4-diones as PTP1B inhibitors with anti-hyperglycemic activity. Med. Chem. Res. 2018, 27, 476–487. [Google Scholar] [CrossRef]
- Yoshioka, T.; Fujita, T.; Kanai, T.; Aizava, Y.; Kurumada, T.; Hasegava, K.; Horikoshi, H. Studies on hindered phenols and analogues. 1. Hypolipidemic and hypoglycemic agents with ability to inhibit lipid peroxidation. J. Med. Chem. 1989, 32, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Rakowitz, D.; Maccari, R.; Ottaná, R.; Vigorita, M.G. In vitro aldose reductase inhibitory activity of 5-benzyl-2,4-thiazolidinediones. Bioorg. Med. Chem. 2006, 14, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Momose, Y.; Maekawa, T.; Odaka, H.; Ikeda, H.; Sohdad, T. Novel 5-Substituted-1H-tetrazole derivatives as potent glucose and lipid lowering agents. Chem. Pharm. Bull. 2002, 50, 100–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, B.R.P.; Karvekar, M.D.; Adhikary, L.; Nanjan, N.J.; Suresh, B. Microwave induced synthesis of the thiazolidine-2,4-dione motif and the efficient solvent free-solid phase parallel syntheses of 5-Benzylidene-thiazolidine-2,4-dione and 5-benzylidene-2-thioxo-thiazolidine-4-one compounds. J. Hetero. Chem. 2006, 43, 897–903. [Google Scholar] [CrossRef]
- S-Funosas, R.; Prohens, R.; Barbas, R.; El-Faham, A.; Albericio, F. Oxyma: An Efficient additive for peptide synthesis to replace the benzotriazole-based HOBt and HOAt with a lower risk of explosion. Chem. Eur. J. 2009, 15, 9394–9403. [Google Scholar] [CrossRef]
- El-Faham, A.; Almarhoon, Z.; Ab-Megeed, A.; Albericio, F. OxymaPure/DIC: An efficient reagent for the synthesis of a novel series of 4-[2-(2-Acetylaminophenyl)-2-oxo-acetylamino] benzoyl amino acid ester derivatives. Molecules 2013, 18, 14747–14759. [Google Scholar] [CrossRef] [Green Version]
- Romagnoli, R.; Baraldi, P.G.; Kimatrai Salvador, M.; Camacho, M.E.; Balzarini, J.; Bermejo, J.; Estévez, F. Anticancer activity of novel hybrid molecules containing 5-benzylidene thiazolidine-2,4-dione. Eur. J. Med. Chem. 2013, 63, 544–557. [Google Scholar] [CrossRef]
- Azevedo, M.M.; Ramalho, P.; Silva, A.P.; T.-Santos, R.; P.-Vaz, C.; Rodrigues, A.G. Polyethyleneimine (PEI) and PEI-based nanoparticles: Novel bacterial and yeast biofilm inhibitors. J. Med. Microbiol. 2014, 63, 1167–1173. [Google Scholar] [CrossRef]
- Maccari, R.; Ottana, R.; Curinga, C.; Vigorita, M.G.; Rakowitz, D.; Steindl, T.; Langer, T. Structure–activity relationships and molecular modelling of 5-arylidene-2,4-thiazolidinediones active as aldose reductase inhibitors. Bioorg. Med. Chem. 2005, 13, 2809–2823. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 4a–s and 5a–o are available from the authors. |



| Average Inhibition Zone in mm | |||||
|---|---|---|---|---|---|
| Chemical Compounds | S. aureus | Bacillus subtilis | E. coli | Ps. aeruginosa | C. albicans |
| 3a | 12 | - | 11 | 16 | 15 |
| 3b | 12 | - | 12 | 11 | 11 |
| 3c | - | - | - | 10 | - |
| 3d | - | - | 10 | 15 | 15 |
| 3e | - | - | 12 | 13 | - |
| 3f | - | - | - | 13 | - |
| 3g | 20 | - | 7 | 14 | 7 |
| 4a | - | - | - | 12 | 14 |
| 4b | - | - | - | - | 12 |
| 4c | - | - | - | - | 12 |
| 4d | - | - | - | 14 | - |
| 4e | - | - | - | - | 13 |
| 4f | - | - | - | 11 | - |
| 4g | - | - | - | 12 | 14 |
| 4h | - | - | - | 13 | - |
| 4i | - | - | - | 12 | - |
| 4k | - | - | - | - | 15 |
| 4l | - | - | - | - | 13 |
| 4m | - | - | - | 10 | - |
| 4n | - | - | - | 11 | 13 |
| 4o | - | - | - | 10 | - |
| 4p | - | - | - | 12 | 14 |
| 4q | - | - | - | 12 | 12 |
| 4r | - | - | - | 14 | - |
| 4s | - | - | 11 | 12 | 12 |
| 5a | - | - | - | - | - |
| 5b | - | - | - | - | 15 |
| 5c | - | - | - | - | 13 |
| 5d | - | - | - | - | - |
| 5e | - | - | - | 12 | - |
| 5f | - | - | - | - | - |
| 5g | - | - | 10 | 12 | 15 |
| 5h | - | - | 11 | 11 | 13 |
| 5i | - | - | 12 | 12 | - |
| 5j | - | - | - | - | - |
| 5k | - | - | 7 | 13 | 18 |
| 5l | - | - | - | - | 12 |
| 5m | - | - | - | - | 12 |
| 5n | - | - | 10 | - | - |
| 5o | - | - | 11 | 12 | 16 |
| Impenem * | 30 | 34 | 20 | 35 | |
| SXT ** | 22 | 20 | - | 30 | |
| Fluconazole | - | - | - | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd Alhameed, R.; Almarhoon, Z.; Bukhari, S.I.; El-Faham, A.; de la Torre, B.G.; Albericio, F. Synthesis and Antimicrobial Activity of a New Series of Thiazolidine-2,4-diones Carboxamide and Amino Acid Derivatives. Molecules 2020, 25, 105. https://doi.org/10.3390/molecules25010105
Abd Alhameed R, Almarhoon Z, Bukhari SI, El-Faham A, de la Torre BG, Albericio F. Synthesis and Antimicrobial Activity of a New Series of Thiazolidine-2,4-diones Carboxamide and Amino Acid Derivatives. Molecules. 2020; 25(1):105. https://doi.org/10.3390/molecules25010105
Chicago/Turabian StyleAbd Alhameed, Rakia, Zainab Almarhoon, Sarah I. Bukhari, Ayman El-Faham, Beatriz G. de la Torre, and Fernando Albericio. 2020. "Synthesis and Antimicrobial Activity of a New Series of Thiazolidine-2,4-diones Carboxamide and Amino Acid Derivatives" Molecules 25, no. 1: 105. https://doi.org/10.3390/molecules25010105
APA StyleAbd Alhameed, R., Almarhoon, Z., Bukhari, S. I., El-Faham, A., de la Torre, B. G., & Albericio, F. (2020). Synthesis and Antimicrobial Activity of a New Series of Thiazolidine-2,4-diones Carboxamide and Amino Acid Derivatives. Molecules, 25(1), 105. https://doi.org/10.3390/molecules25010105