Chelerythrine Chloride Downregulates β-Catenin and Inhibits Stem Cell Properties of Non-Small Cell Lung Carcinoma
Abstract
:1. Introduction
2. Results
2.1. Cheleythrine Chloride Inhibited the Growth of NCI-H1703, SK-LU-1, and Human Lung Cancer Stem Cells (HLCSC)
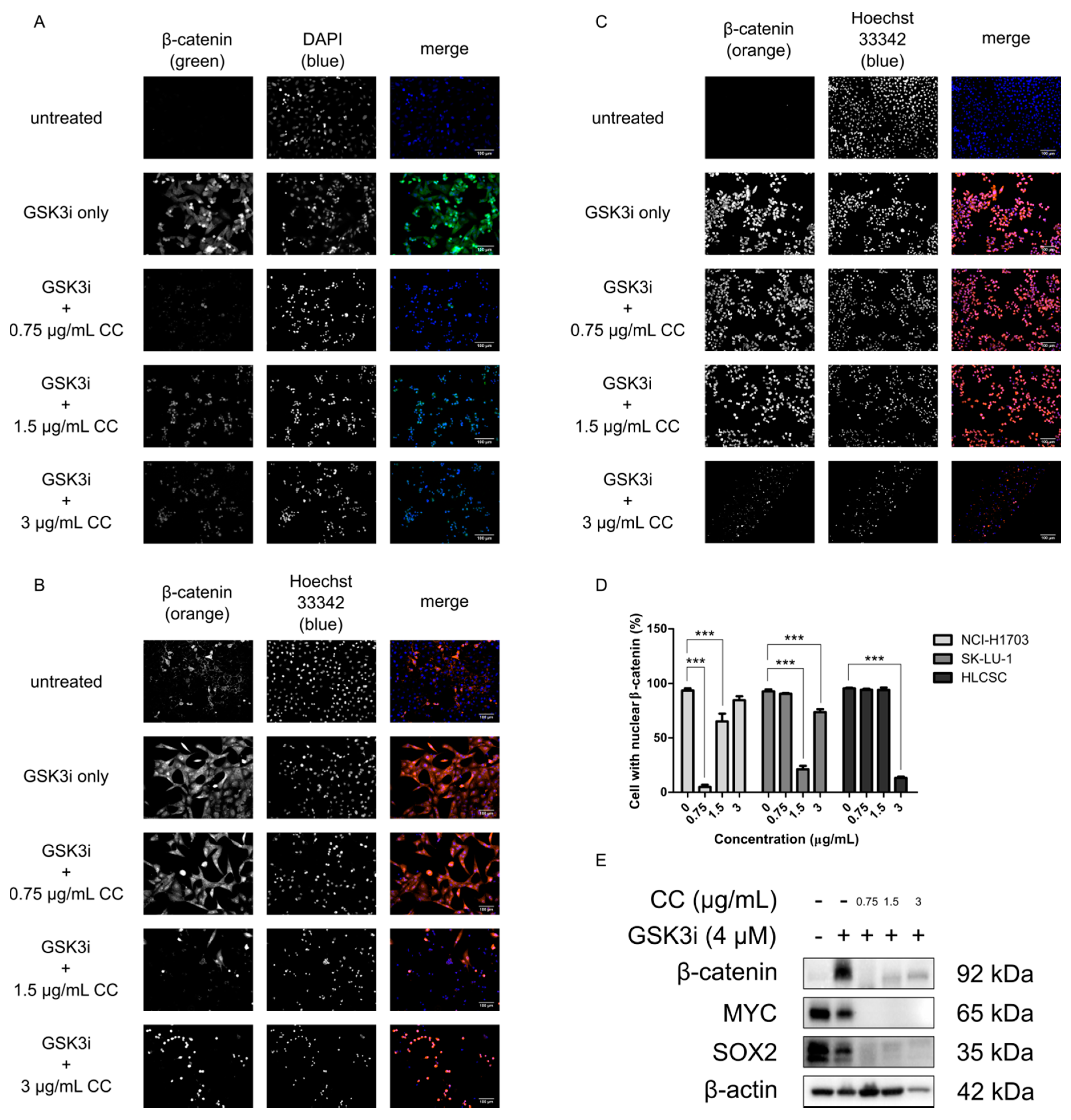
2.2. Chelerythrine Chloride Reduces the Global Expression and the Proportion of Cells with Positive Nuclear β-Catenin
2.3. Chelerythrine Chloride Partly Induces Apoptosis and Inhibits the Functions of CSC
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Chemicals
4.3. Cell Viability, Cytotoxicity and Anti-Proliferative Assay
4.4. Immunocytochemistry
4.5. SDS-PAGE and Western Blotting
4.6. Soft Agar Colony Formation Assay
4.7. Spheroid Generation
4.8. Migration and Invasion Assay
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Son, N.T. Genus Miliusa: A review of phytochemistry and pharmacology. Evid.-Based Complement. Altern. Med. 2019, 2019, 8314693. [Google Scholar]
- Guo, Z. The modification of natural products for medical use. Acta Pharm Sin. B 2017, 7, 119–136. [Google Scholar] [CrossRef] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Ceja, K.A.; Chirino, Y.I. Current FDA-approved treatments for non-small cell lung cancer and potential biomarkers for its detection. Biomed. Pharm. 2017, 90, 24–37. [Google Scholar] [CrossRef]
- Kim, E.S. Chemotherapy resistance in lung cancer. Adv. Exp. Med. Biol. 2016, 893, 189–209. [Google Scholar]
- Sosa Iglesias, V.; Giuranno, L.; Dubois, L.J.; Theys, J.; Vooijs, M. Drug resistance in non-small cell lung cancer: A potential for NOTCH targeting? Front. Oncol. 2018. [Google Scholar] [CrossRef] [Green Version]
- Remesh, A. Toxicities of anticancer drugs and its management. Int. J. Basic Clin. Pharm. 2017, 1, 2–12. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.B.; Sullivan, R.J.; Menzies, A.M. Immune checkpoint inhibitors in challenging populations. Cancer 2017, 123, 1904–1911. [Google Scholar] [CrossRef] [Green Version]
- Seung, S.J.; Hurry, M.; Hassan, S.; Walton, R.N.; Evans, W.K. Cost-of-illness study for non-small-cell lung cancer using real-world data. Curr. Oncol. 2019, 26, 102–107. [Google Scholar] [CrossRef] [Green Version]
- Heng, W.S.; Gosens, R.; Kruyt, F.A.E. Lung cancer stem cells: Origin, features, maintenance mechanisms and therapeutic targeting. Biochem. Pharm. 2019, 160, 121–133. [Google Scholar] [CrossRef]
- Han, N.; Yang, Z.; Liu, Z.; Liu, H.; Yin, J. Research progress on natural benzophenanthridine alkaloids and their pharmacological functions: A review. Nat. Prod. Commun. 2016, 11, 1934578X1601100838. [Google Scholar] [CrossRef] [Green Version]
- Ni, H.; Martínez, Y.; Guan, G.; Rodríguez, R.; Más, D.; Peng, H.; Valdivié Navarro, M.; Liu, G. Analysis of the impact of isoquinoline alkaloids, derived from Macleaya cordata extract, on the development and innate immune response in swine and poultry. BioMed Res. Int. 2016, 2016, 1352146. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.; Zhao, M.; Li, X. Extraction of chelerythrine and its effects on pathogenic fungus spore germination. Pharm. Mag. 2017, 13, 600–606. [Google Scholar]
- Herbert, J.M.; Augereau, J.M.; Gleye, J.; Maffrand, J.P. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem. Biophys. Res. Commun. 1990, 172, 993–999. [Google Scholar] [CrossRef]
- Chmura, S.J.; Dolan, M.E.; Cha, A.; Mauceri, H.J.; Kufe, D.W.; Weichselbaum, R.R. In vitro and in vivo activity of protein kinase c inhibitor chelerythrine chloride induces tumor cell toxicity and growth delay in vivo. Clin. Cancer Res. 2000, 6, 737–742. [Google Scholar]
- Kemény-Beke, Á.; Aradi, J.; Damjanovich, J.; Beck, Z.; Facskó, A.; Berta, A.; Bodnár, A. Apoptotic response of uveal melanoma cells upon treatment with chelidonine, sanguinarine and chelerythrine. Cancer Lett. 2006, 237, 67–75. [Google Scholar] [CrossRef]
- Vrba, J.; Doležel, P.; Vičar, J.; Modrianský, M.; Ulrichová, J. Chelerythrine and dihydrochelerythrine induce G1 phase arrest and bimodal cell death in human leukemia HL-60 cells. Toxicol. Vitr. 2008, 22, 1008–1017. [Google Scholar] [CrossRef]
- Malíková, J.; Zdařilová, A.; Hlobilková, A.; Ulrichová, J. The effect of chelerythrine on cell growth, apoptosis, and cell cycle in human normal and cancer cells in comparison with sanguinarine. Cell Biol. Toxicol. 2006, 22, 439–453. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, Y.; Zhang, J.; Wei, X. Induction of apoptosis by chelerythrine chloride through mitochondrial pathway and Bcl-2 family proteins in human hepatoma SMMC-7721 Cell. Arch. Pharm. Res. 2011, 34, 791–800. [Google Scholar] [CrossRef]
- Chen, X.-M.; Zhang, M.; Fan, P.-L.; Qin, Y.-H.; Zhao, H.-W. Chelerythrine chloride induces apoptosis in renal cancer HEK-293 and SW-839 cell lines. Oncol. Lett. 2016, 11, 3917–3924. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Yang, Y.; Li, F.; Huang, L.; Han, Z.; Wang, G.; Yu, H.; Li, H. Chelerythrine induced cell death through ROS-dependent ER stress in human prostate cancer cells. Onco Targets 2018, 11, 2593–2601. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Zhuo, R.; Dai, J.; Wang, X.; Huang, X.; Wang, H.; Xu, D. Chelerythrine induces apoptosis via ROS-mediated endoplasmic reticulum stress and STAT3 pathways in human renal cell carcinoma. J. Cell. Mol. Med. 2019, 24, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Han, B.; Sha, H.; Shi, Z.; Yang, X.; Feng, J. Effects of a protein kinase C inhibitor combined with cisplatin on non-small cell lung cancer. Zhonghua Jie He He Hu Xi Za Zhi 2010, 33, 284–288. [Google Scholar] [PubMed]
- Lin, W.; Huang, J.; Yuan, Z.; Feng, S.; Xie, Y.; Ma, W. Protein kinase C inhibitor chelerythrine selectively inhibits proliferation of triple-negative breast cancer cells. Sci. Rep. 2017, 7, 2022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willert, K.; Jones, K.A. Wnt signaling: Is the party in the nucleus? Genes Dev. 2006, 20, 1394–1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, W.; Kim, M.; Jho, E. Wnt/β-catenin signalling: From plasma membrane to nucleus. Biochem. J. 2013, 450, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Mucenski, M.L.; Wert, S.E.; Nation, J.M.; Loudy, D.E.; Huelsken, J.; Birchmeier, W.; Morrisey, E.E.; Whitsett, J.A. β-Catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J. Biol. Chem. 2003, 278, 40231–40238. [Google Scholar] [CrossRef] [Green Version]
- Frank, D.B.; Peng, T.; Zepp, J.; Snitow, M.; Vincent, T.; Penkala, I.J.; Cui, Z.; Herriges, M.J.; Morley, M.P.; Zhou, S.; et al. Emergence of a wave of Wnt signaling that regulates lung alveologenesis through controlling epithelial self-renewal and differentiation. Cell Rep. 2016, 17, 2312–2325. [Google Scholar] [CrossRef] [Green Version]
- Zacharias, W.J.; Frank, D.B.; Zepp, J.A.; Morley, M.P.; Alkhaleel, F.A.; Kong, J.; Zhou, S.; Cantu, E.; Morrisey, E.E. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature 2018, 555, 251–255. [Google Scholar] [CrossRef]
- Stewart, D.J. Wnt signaling pathway in non–small cell lung cancer. JNCI J. Natl. Cancer Inst. 2014, 106, djt356. [Google Scholar] [CrossRef]
- Rapp, J.; Jaromi, L.; Kvell, K.; Miskei, G.; Pongracz, J.E. WNT signaling—Lung cancer is no exception. Respir. Res. 2017, 18, 167. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.; Wang, H.; Zhang, S. Negative regulators of Wnt signaling in non-small cell lung cancer: Theoretical basis and therapeutic potency. Biomed. Pharm. 2019, 118, 109336. [Google Scholar] [CrossRef]
- Tammela, T.; Sanchez-Rivera, F.J.; Cetinbas, N.M.; Wu, K.; Joshi, N.S.; Helenius, K.; Park, Y.; Azimi, R.; Kerper, N.R.; Wesselhoeft, R.A.; et al. A Wnt-producing niche drives proliferative potential and progression in lung adenocarcinoma. Nature 2017, 545, 355–359. [Google Scholar] [CrossRef] [Green Version]
- Krieghoff, E.; Behrens, J.; Mayr, B. Nucleo-cytoplasmic distribution of β-catenin is regulated by retention. J. Cell Sci. 2006, 119, 1453–1463. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Xie, S.; Zhang, W.; Zhang, C.; Gao, C.; Sun, Q.; Cai, Y.; Xu, Z.; Xiao, M.; Xu, Y.; et al. Twa1/Gid8 is a β-catenin nuclear retention factor in Wnt signaling and colorectal tumorigenesis. Cell Res. 2017, 27, 1422–1440. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495. [Google Scholar] [CrossRef]
- Veeresham, C. Natural products derived from plants as a source of drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200–201. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Mahmood, T.; Kanwal, S.; Ali, B.; Shah, S.A.; Khalil, A.T. Plant-derived anticancer agents: A green anticancer approach. Asian Pac. J. Trop. Biomed. 2017, 7, 1129–1150. [Google Scholar] [CrossRef]
- Mathur, S.; Hoskins, C. Drug development: Lessons from nature. Biomed. Rep. 2017, 6, 612–614. [Google Scholar] [CrossRef] [Green Version]
- He, B.; You, L.; Uematsu, K.; Xu, Z.; Lee, A.Y.; Matsangou, M.; McCormick, F.; Jablons, D.M. A monoclonal antibody against WNT-1 induces apoptosis in human cancer cells. Neoplasia 2004, 6, 7–14. [Google Scholar] [CrossRef] [Green Version]
- You, L.; He, B.; Xu, Z.; Uematsu, K.; Mazieres, J.; Mikami, I.; Reguart, N.; Moody, T.W.; Kitajewski, J.; McCormick, F.; et al. Inhibition of Wnt-2-mediated signaling induces programmed cell death in non-small-cell lung cancer cells. Oncogene 2004, 23, 6170–6174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ochieng, J.K.; Schilders, K.; Kool, H.; Boerema-De Munck, A.; Buscop-Van Kempen, M.; Gontan, C.; Smits, R.; Grosveld, F.G.; Wijnen, R.M.H.; Tibboel, D.; et al. Sox2 regulates the emergence of lung basal cells by directly activating the transcription of Trp63. Am. J. Respir. Cell Mol. Biol. 2014, 51, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.R.; de Laar, E.V.; Cabanero, M.; Tarumi, S.; Hasenoeder, S.; Wang, D.; Virtanen, C.; Suzuki, T.; Bandarchi, B.; Sakashita, S.; et al. SOX2 and PI3K cooperate to induce and stabilize a squamous-committed stem cell injury state during lung squamous cell carcinoma pathogenesis. PLoS Biol. 2016, 14, e1002581. [Google Scholar] [CrossRef]
- Ferone, G.; Song, J.-Y.; Sutherland, K.D.; Bhaskaran, R.; Monkhorst, K.; Lambooij, J.-P.; Proost, N.; Gargiulo, G.; Berns, A. SOX2 is the determining oncogenic switch in promoting lung squamous cell carcinoma from different cells of origin. Cancer Cell 2016, 30, 519–532. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, S.; Chen, H.; Que, J.; Brockway, B.L.; Drake, J.A.; Snyder, J.C.; Randell, S.H.; Stripp, B.R. β-Catenin–SOX2 signaling regulates the fate of developing airway epithelium. J. Cell Sci. 2012, 125, 932–942. [Google Scholar] [CrossRef] [Green Version]
- Golstein, P.; Kroemer, G. Cell death by necrosis: Towards a molecular definition. Trends Biochem. Sci. 2007, 32, 37–43. [Google Scholar] [CrossRef]
- Covarrubias, L.; Hernández-García, D.; Schnabel, D.; Salas-Vidal, E.; Castro-Obregón, S. Function of reactive oxygen species during animal development: Passive or active? Dev. Biol. 2008, 320, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Milkovic, L.; Cipak Gasparovic, A.; Cindric, M.; Mouthuy, P.-A.; Zarkovic, N. Short overview of ROS as cell function regulators and their implications in therapy concepts. Cells 2019, 8, 793. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.-H.; Cao, W.-X.; Wang, Z.-Y.; Lu, J.-H.; Liu, B.; Chen, X.; Lu, J.-J. Induction of reactive oxygen species-stimulated distinctive autophagy by chelerythrine in non-small cell lung cancer cells. Redox Biol. 2017, 12, 367–376. [Google Scholar] [CrossRef]
- Chen, R.-H.; Ding, W.V.; McCormick, F. Wnt Signaling to β-catenin Involves Two Interactive Components glycogen synthase kinase-3β inhibition and activation of protein kinase c. J. Biol. Chem. 2000, 275, 17894–17899. [Google Scholar] [CrossRef] [Green Version]
- Goode, N.; Hughes, K.; Woodgett, J.R.; Parker, P.J. Differential regulation of glycogen synthase kinase-3 beta by protein kinase C isotypes. J. Biol. Chem. 1992, 267, 16878–16882. [Google Scholar]
- He, T.C.; Sparks, A.B.; Rago, C.; Hermeking, H.; Zawel, L.; da Costa, L.T.; Morin, P.J.; Vogelstein, B.; Kinzler, K.W. Identification of c-MYC as a target of the APC pathway. Science 1998, 281, 1509–1512. [Google Scholar] [CrossRef]
- Weaver, B.A. How taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef]
- Sak, K. Chemotherapy and dietary phytochemical agents. Chemother. Res. Pr. 2012, 2012, 282570. [Google Scholar] [CrossRef] [Green Version]
- Chamberlin, S.R.; Blucher, A.; Wu, G.; Shinto, L.; Choonoo, G.; Kulesz-Martin, M.; McWeeney, S. Natural product target network reveals potential for cancer combination therapies. Front. Pharm. 2019, 10, 557. [Google Scholar] [CrossRef]
- He, M.; Yang, Z.; Zhang, L.; Song, C.; Li, Y.; Zhang, X. Additive effects of cherlerythrine chloride combination with erlotinib in human non-small cell lung cancer cells. PLoS ONE 2017, 12, e0175466. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Meth. 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Baranwal, S.; Wang, Y.; Rathinam, R.; Lee, J.; Jin, L.; McGoey, R.; Pylayeva, Y.; Giancotti, F.; Blobe, G.C.; Alahari, S.K. Molecular Characterization of the Tumor-Suppressive Function of Nischarin in Breast Cancer. JNCI J. Natl. Cancer Inst. 2011, 103, 1513–1528. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |
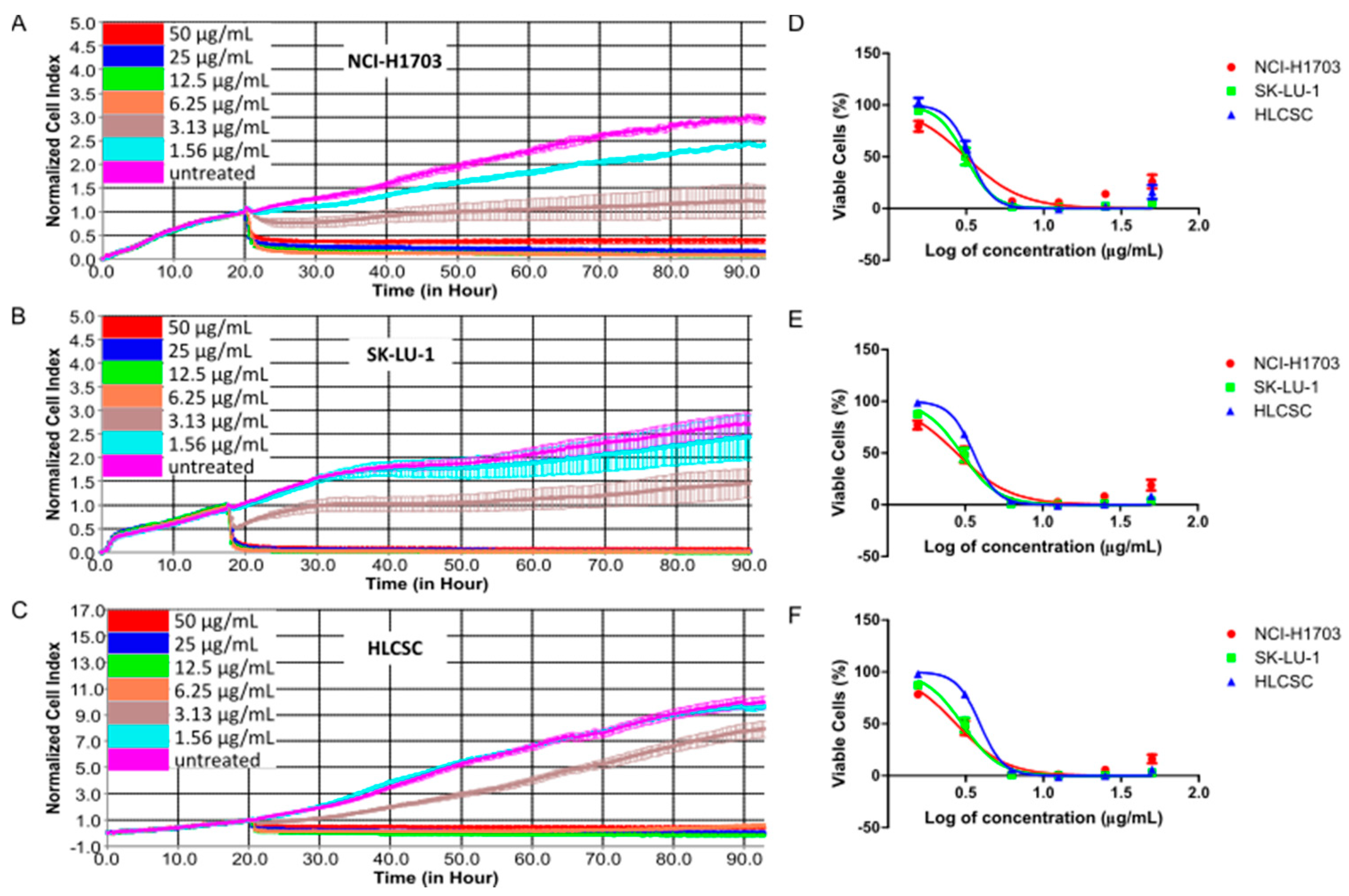

| Cell Lines | IC20 (μg/mL) 1 | ||
| 24 h | 48 h | 72 h | |
| NCI-H1703 | 1.85 ± 0.04 | 1.81 ± 0.03 | 1.89 ± 0.05 |
| SK-LU-1 | 2.35 ± 0.02 | 2.17 ± 0.04 | 2.18 ± 0.04 |
| HLCSC | 2.54 ± 0.08 | 2.73 ± 0.05 | 3.04 ± 0.08 |
| Cell Lines | IC50 (μg/mL) 2 | ||
| 24 h | 48 h | 72 h | |
| NCI-H1703 | 3.30 ± 0.08 | 3.16 ± 0.05 | 3.15 ± 0.08 |
| SK-LU-1 | 3.14 ± 0.03 | 3.17 ± 0.06 | 3.19 ± 0.06 |
| HLCSC | 3.20 ± 0.10 | 3.48 ± 0.06 | 3.88 ± 0.10 |
| Cell Lines | IC80 (μg/mL) 1 | ||
| 24 h | 48 h | 72 h | |
| NCI-H1703 | 5.87 ± 0.14 | 5.48 ± 0.09 | 5.23 ± 0.13 |
| SK-LU-1 | 4.19 ± 0.04 | 4.62 ± 0.08 | 4.65 ± 0.09 |
| HLCSC | 4.04 ± 0.12 | 4.43 ± 0.08 | 4.94 ± 0.13 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heng, W.S.; Cheah, S.-C. Chelerythrine Chloride Downregulates β-Catenin and Inhibits Stem Cell Properties of Non-Small Cell Lung Carcinoma. Molecules 2020, 25, 224. https://doi.org/10.3390/molecules25010224
Heng WS, Cheah S-C. Chelerythrine Chloride Downregulates β-Catenin and Inhibits Stem Cell Properties of Non-Small Cell Lung Carcinoma. Molecules. 2020; 25(1):224. https://doi.org/10.3390/molecules25010224
Chicago/Turabian StyleHeng, Win Sen, and Shiau-Chuen Cheah. 2020. "Chelerythrine Chloride Downregulates β-Catenin and Inhibits Stem Cell Properties of Non-Small Cell Lung Carcinoma" Molecules 25, no. 1: 224. https://doi.org/10.3390/molecules25010224
APA StyleHeng, W. S., & Cheah, S.-C. (2020). Chelerythrine Chloride Downregulates β-Catenin and Inhibits Stem Cell Properties of Non-Small Cell Lung Carcinoma. Molecules, 25(1), 224. https://doi.org/10.3390/molecules25010224






