Glycomic Signatures of Plasma IgG Improve Preoperative Prediction of the Invasiveness of Small Lung Nodules
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Purification of IgG from Plasma
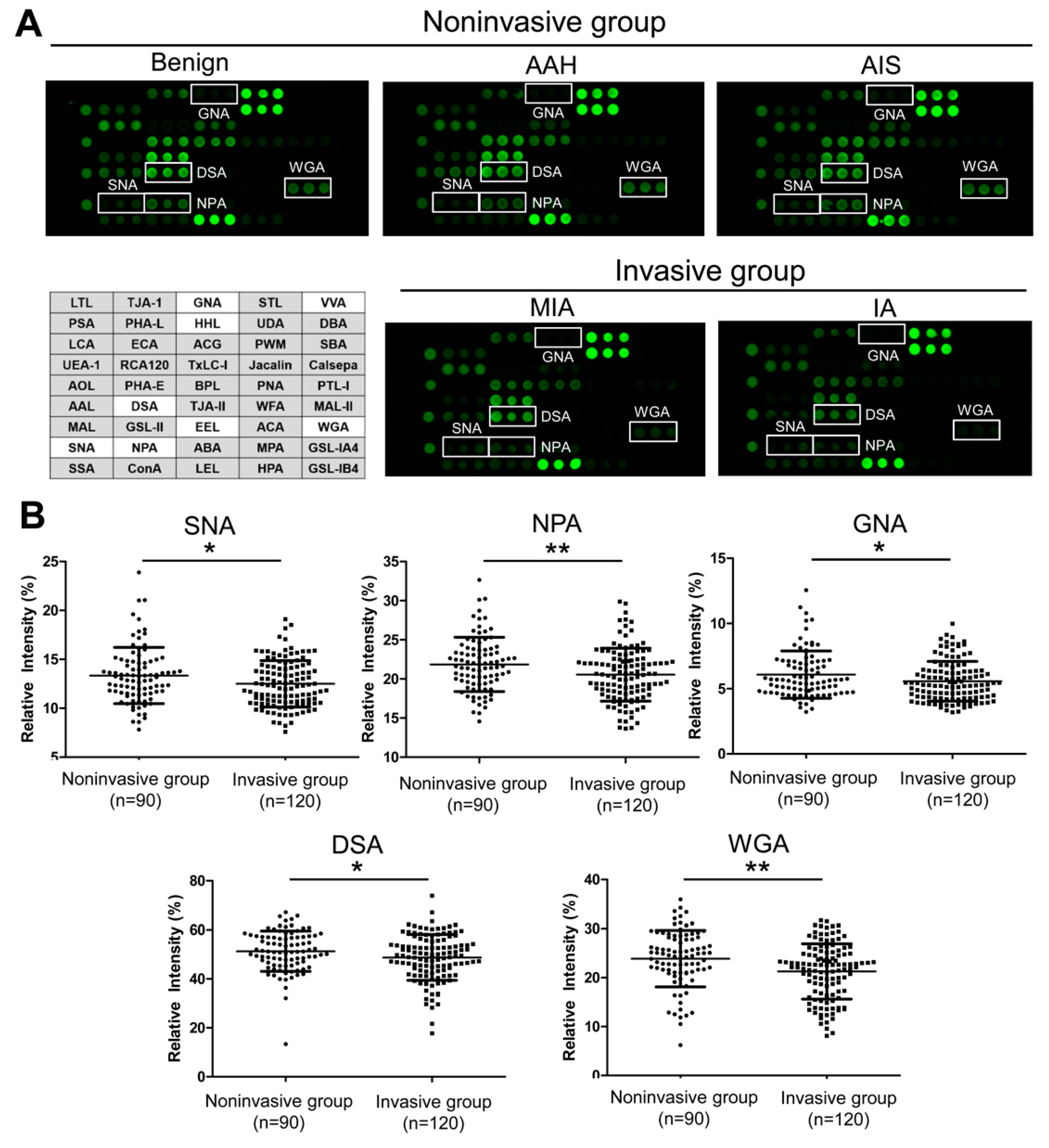
2.3. IgG Glycosylation Changes Detected by Lectin Microarray
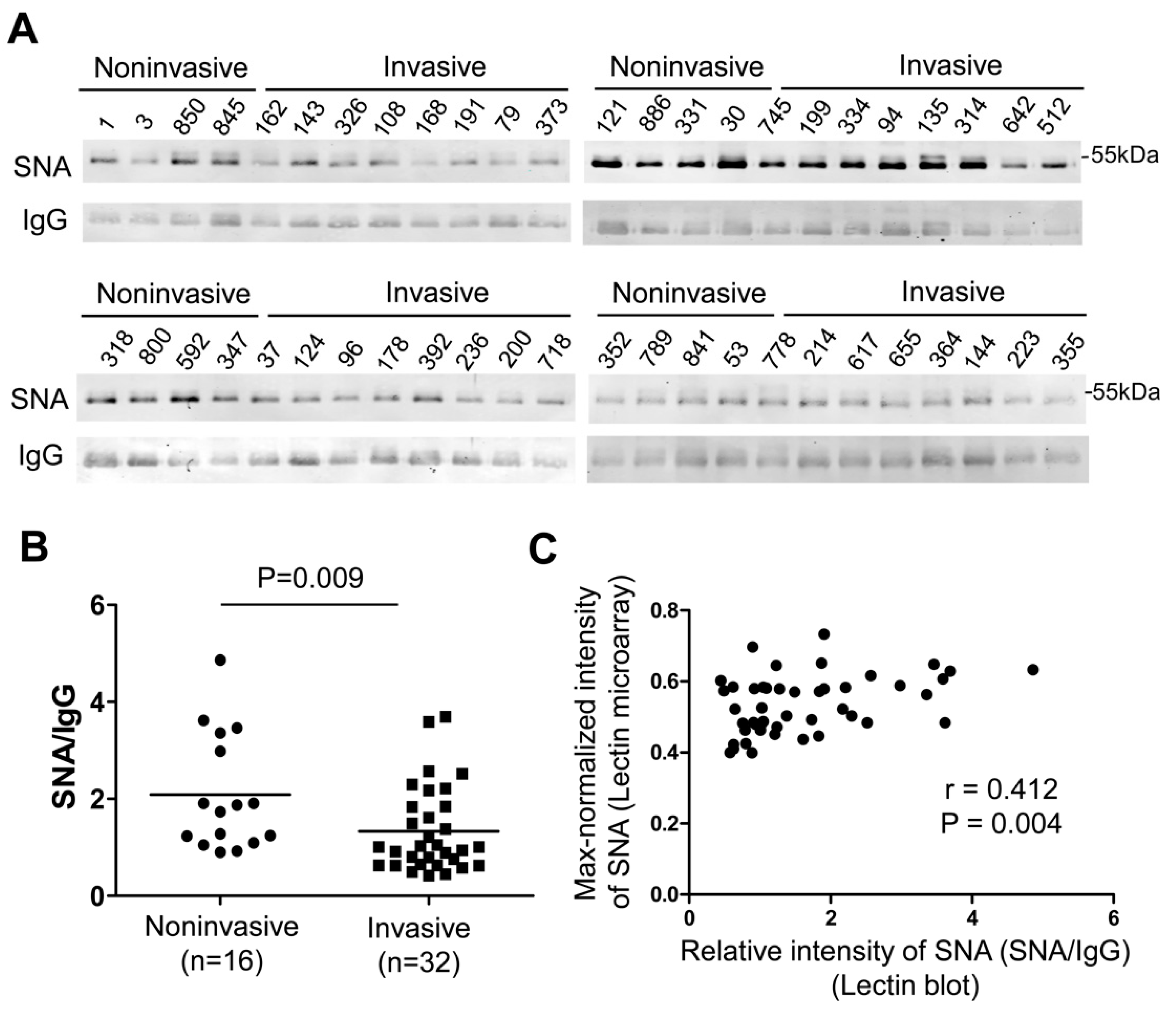
2.4. Validation of Glycosylation Changes of IgG by Lectin Blot
2.5. Evaluation of Multilectin Parameters to Assist CT Examination for the Detection of Invasive GGNs
3. Discussion
4. Materials and Methods
4.1. Patients and Study Design
4.2. CT Examination and Biochemical Data
4.3. IgG Isolation from Plasma
4.4. Lectin Microarray Analysis
4.5. Lectin Blot and Western Blot Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, S.J.; Wu, N. Early detection of lung cancer: Low-dose computed tomography screening in china. Thorac. Cancer 2015, 6, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Godoy, M.C.; Naidich, D.P. Overview and strategic management of subsolid pulmonary nodules. J. Thorac. Imaging 2012, 27, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Noguchi, M.; Nicholson, A.G.; Geisinger, K.R.; Yatabe, Y.; Beer, D.G.; Powell, C.A.; Riely, G.J.; Van Schil, P.E.; et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J. Thorac. Oncol. 2011, 6, 244–285. [Google Scholar] [CrossRef] [Green Version]
- Min, J.H.; Lee, H.Y.; Lee, K.S.; Han, J.; Park, K.; Ahn, M.J.; Lee, S.J. Stepwise evolution from a focal pure pulmonary ground-glass opacity nodule into an invasive lung adenocarcinoma: An observation for more than 10 years. Lung Cancer 2010, 69, 123–126. [Google Scholar] [CrossRef]
- Kakinuma, R.; Muramatsu, Y.; Kusumoto, M.; Tsuchida, T.; Tsuta, K.; Maeshima, A.M.; Asamura, H.; Moriyama, N. Solitary pure ground-glass nodules 5 mm or smaller: Frequency of growth. Radiology 2015, 276, 873–882. [Google Scholar] [CrossRef]
- Lee, S.M.; Park, C.M.; Goo, J.M.; Lee, H.J.; Wi, J.Y.; Kang, C.H. Invasive pulmonary adenocarcinomas versus preinvasive lesions appearing as ground-glass nodules: Differentiation by using ct features. Radiology 2013, 268, 265–273. [Google Scholar] [CrossRef]
- Chae, H.D.; Park, C.M.; Park, S.J.; Lee, S.M.; Kim, K.G.; Goo, J.M. Computerized texture analysis of persistent part-solid ground-glass nodules: Differentiation of preinvasive lesions from invasive pulmonary adenocarcinomas. Radiology 2014, 273, 285–293. [Google Scholar] [CrossRef]
- Kim, H.; Goo, J.M.; Park, C.M. A simple prediction model using size measures for discrimination of invasive adenocarcinomas among incidental pulmonary subsolid nodules considered for resection. Eur. Radiol. 2019, 29, 1674–1683. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Mitsudomi, T.; Sakao, Y.; Yatabe, Y. Genetic features of pulmonary adenocarcinoma presenting with ground-glass nodules: The differences between nodules with and without growth. Ann. Oncol. 2015, 26, 156–161. [Google Scholar] [CrossRef]
- Ishida, H.; Shimizu, Y.; Sakaguchi, H.; Nitanda, H.; Kaneko, K.; Yamazaki, N.; Yanagihara, A.; Taguchi, R.; Sakai, F.; Yasuda, M.; et al. Distinctive clinicopathological features of adenocarcinoma in situ and minimally invasive adenocarcinoma of the lung: A retrospective study. Lung Cancer 2019, 129, 16–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumagai, T.; Tomita, Y.; Nakatsuka, S.I.; Kimura, M.; Kunimasa, K.; Inoue, T.; Tamiya, M.; Nishino, K.; Susaki, Y.; Kusu, T.; et al. Her3 expression is enhanced during progression of lung adenocarcinoma without egfr mutation from stage 0 to ia1. Thorac. Cancer 2018, 9, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Hart, G.W.; Copeland, R.J. Glycomics hits the big time. Cell 2010, 143, 672–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Reviews. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Drake, P.M.; Cho, W.; Li, B.; Prakobphol, A.; Johansen, E.; Anderson, N.L.; Regnier, F.E.; Gibson, B.W.; Fisher, S.J. Sweetening the pot: Adding glycosylation to the biomarker discovery equation. Clin. Chem. 2010, 56, 223–236. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Mallory, T.; Satomura, S. Afp-l3: A new generation of tumor marker for hepatocellular carcinoma. Clin. Chim. Acta 2001, 313, 15–19. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Simurina, M.; de Haan, N.; Vuckovic, F.; Kennedy, N.A.; Stambuk, J.; Falck, D.; Trbojevic-Akmacic, I.; Clerc, F.; Razdorov, G.; Khon, A.; et al. Glycosylation of immunoglobulin g associates with clinical features of inflammatory bowel diseases. Gastroenterology 2018, 154, 1320–1333. [Google Scholar] [CrossRef]
- Wang, T.T.; Sewatanon, J.; Memoli, M.J.; Wrammert, J.; Bournazos, S.; Bhaumik, S.K.; Pinsky, B.A.; Chokephaibulkit, K.; Onlamoon, N.; Pattanapanyasat, K.; et al. Igg antibodies to dengue enhanced for fcgammariiia binding determine disease severity. Science 2017, 355, 395–398. [Google Scholar] [CrossRef] [Green Version]
- Ren, S.; Zhang, Z.; Xu, C.; Guo, L.; Lu, R.; Sun, Y.; Guo, J.; Qin, R.; Qin, W.; Gu, J. Distribution of igg galactosylation as a promising biomarker for cancer screening in multiple cancer types. Cell Res. 2016, 26, 963–966. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Wang, Y.; Qin, X.; Li, H.; Guo, Y.; Wang, Y.; Liu, H.; Wang, X.; Song, G.; Li, F.; et al. Change in igg1 fc n-linked glycosylation in human lung cancer: Age- and sex-related diagnostic potential. Electrophoresis 2013, 34, 2407–2416. [Google Scholar] [CrossRef] [PubMed]
- Kanoh, Y.; Ohara, T.; Tadano, T.; Kanoh, M.; Akahoshi, T. Changes to n-linked oligosaccharide chains of human serum immunoglobulin g and matrix metalloproteinase-2 with cancer progression. Anticancer Res. 2008, 28, 715–720. [Google Scholar] [PubMed]
- Ruhaak, L.R.; Nguyen, U.T.; Stroble, C.; Taylor, S.L.; Taguchi, A.; Hanash, S.M.; Lebrilla, C.B.; Kim, K.; Miyamoto, S. Enrichment strategies in glycomics-based lung cancer biomarker development. Proteomics. Clin. Appl. 2013, 7, 664–676. [Google Scholar] [CrossRef] [Green Version]
- Kuno, A.; Itakura, Y.; Toyoda, M.; Takahashi, Y.; Yamada, M.; Umezawa, A.; Hirabayashi, J. Development of a data-mining system for differential profiling of cell glycoproteins based on lectin microarray. J Proteom. Bioinform 2008, 1, 68–72. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.H.; Fan, Y.G.; Bu, H.; Wang, Y.; Wu, N.; Huang, Y.C.; Wang, G.; Wang, X.Y.; Qiao, Y.L. China national lung cancer screening guideline with low-dose computed tomography (2015 version). Thorac. Cancer 2015, 6, 812–818. [Google Scholar] [CrossRef]
- Hirabayashi, J.; Yamada, M.; Kuno, A.; Tateno, H. Lectin microarrays: Concept, principle and applications. Chem. Soc. Rev. 2013, 42, 4443–4458. [Google Scholar] [CrossRef]
- Togayachi, A.; Iwaki, J.; Kaji, H.; Matsuzaki, H.; Kuno, A.; Hirao, Y.; Nomura, M.; Noguchi, M.; Ikehara, Y.; Narimatsu, H. Glycobiomarker, fucosylated short-form secretogranin iii levels are increased in serum of patients with small cell lung carcinoma. J. Proteome Res. 2017, 16, 4495–4505. [Google Scholar] [CrossRef]
- Kanoh, Y.; Ohara, T.; Mashiko, T.; Abe, T.; Masuda, N.; Akahoshi, T. Relationship between n-linked oligosaccharide chains of human serum immunoglobulin g and serum tumor markers with non-small cell lung cancer progression. Anticancer Res. 2006, 26, 4293–4297. [Google Scholar]
- Ohmi, Y.; Ise, W.; Harazono, A.; Takakura, D.; Fukuyama, H.; Baba, Y.; Narazaki, M.; Shoda, H.; Takahashi, N.; Ohkawa, Y.; et al. Sialylation converts arthritogenic igg into inhibitors of collagen-induced arthritis. Nat. Commun. 2016, 7, 11205. [Google Scholar] [CrossRef] [Green Version]
- Vuckovic, F.; Theodoratou, E.; Thaci, K.; Timofeeva, M.; Vojta, A.; Stambuk, J.; Pucic-Bakovic, M.; Rudd, P.M.; Derek, L.; Servis, D.; et al. Igg glycome in colorectal cancer. Clin. Cancer Res. 2016, 22, 3078–3086. [Google Scholar] [CrossRef] [Green Version]
- Anthony, R.M.; Kobayashi, T.; Wermeling, F.; Ravetch, J.V. Intravenous gammaglobulin suppresses inflammation through a novel t(h)2 pathway. Nature 2011, 475, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Quast, I.; Keller, C.W.; Maurer, M.A.; Giddens, J.P.; Tackenberg, B.; Wang, L.X.; Munz, C.; Nimmerjahn, F.; Dalakas, M.C.; Lunemann, J.D. Sialylation of igg fc domain impairs complement-dependent cytotoxicity. J. Clin. Investig. 2015, 125, 4160–4170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasseur, J.A.; Goetz, J.A.; Alley, W.R., Jr.; Novotny, M.V. Smoking and lung cancer-induced changes in n-glycosylation of blood serum proteins. Glycobiology 2012, 22, 1684–1708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruhaak, L.R.; Taylor, S.L.; Stroble, C.; Nguyen, U.T.; Parker, E.A.; Song, T.; Lebrilla, C.B.; Rom, W.N.; Pass, H.; Kim, K.; et al. Differential n-glycosylation patterns in lung adenocarcinoma tissue. J. Proteome Res. 2015, 14, 4538–4549. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.B.; Oswald, D.M.; Joshi, S.; Whiteheart, S.W.; Orlando, R.; Cobb, B.A. B-cell-independent sialylation of igg. Proc. Natl. Acad. Sci. USA 2016, 113, 7207–7212. [Google Scholar] [CrossRef] [Green Version]
- Goetze, A.M.; Liu, Y.D.; Zhang, Z.; Shah, B.; Lee, E.; Bondarenko, P.V.; Flynn, G.C. High-mannose glycans on the fc region of therapeutic igg antibodies increase serum clearance in humans. Glycobiology 2011, 21, 949–959. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.R.; Gao, W.N.; Grimm, R.; Jiang, S.; Liang, Y.; Ye, H.; Li, Z.G.; Yau, L.F.; Huang, H.; Liu, J.; et al. A method to identify trace sulfated igg n-glycans as biomarkers for rheumatoid arthritis. Nat. Commun. 2017, 8, 631. [Google Scholar] [CrossRef]
- Bones, J.; Byrne, J.C.; O’Donoghue, N.; McManus, C.; Scaife, C.; Boissin, H.; Nastase, A.; Rudd, P.M. Glycomic and glycoproteomic analysis of serum from patients with stomach cancer reveals potential markers arising from host defense response mechanisms. J. Proteome Res. 2011, 10, 1246–1265. [Google Scholar] [CrossRef]
- Keusch, J.; Lydyard, P.M.; Delves, P.J. The effect on igg glycosylation of altering beta1, 4-galactosyltransferase-1 activity in b cells. Glycobiology 1998, 8, 1215–1220. [Google Scholar] [CrossRef] [Green Version]
- Lauc, G.; Huffman, J.E.; Pucic, M.; Zgaga, L.; Adamczyk, B.; Muzinic, A.; Novokmet, M.; Polasek, O.; Gornik, O.; Kristic, J.; et al. Loci associated with n-glycosylation of human immunoglobulin g show pleiotropy with autoimmune diseases and haematological cancers. Plos Genet. 2013, 9, e1003225. [Google Scholar] [CrossRef]
- Sharapov, S.Z.; Tsepilov, Y.A.; Klaric, L.; Mangino, M.; Thareja, G.; Shadrina, A.S.; Simurina, M.; Dagostino, C.; Dmitrieva, J.; Vilaj, M.; et al. Defining the genetic control of human blood plasma n-glycome using genome-wide association study. Hum. Mol. Genet. 2019, 28, 2062–2077. [Google Scholar] [CrossRef] [PubMed]
- Zoldos, V.; Novokmet, M.; Beceheli, I.; Lauc, G. Genomics and epigenomics of the human glycome. Glycoconj. J. 2013, 30, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Inouye, Y.; Okano, T.; Taniguchi, A. Regulation of sialyl-lewis x epitope expression by tnf-alpha and egf in an airway carcinoma cell line. Glycoconj. J. 2005, 22, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Balog, C.I.; Stavenhagen, K.; Koeleman, C.A.; Scherer, H.U.; Selman, M.H.; Deelder, A.M.; Huizinga, T.W.; Toes, R.E.; Wuhrer, M. Fc-glycosylation of igg1 is modulated by b-cell stimuli. Mol. Cell. Proteom. 2011, 10, M110 004655. [Google Scholar] [CrossRef] [Green Version]
- Pfeifle, R.; Rothe, T.; Ipseiz, N.; Scherer, H.U.; Culemann, S.; Harre, U.; Ackermann, J.A.; Seefried, M.; Kleyer, A.; Uderhardt, S.; et al. Regulation of autoantibody activity by the il-23-th17 axis determines the onset of autoimmune disease. Nat. Immunol. 2017, 18, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Axford, J.S.; Sumar, N.; Alavi, A.; Isenberg, D.A.; Young, A.; Bodman, K.B.; Roitt, I.M. Changes in normal glycosylation mechanisms in autoimmune rheumatic disease. J. Clin. Investig. 1992, 89, 1021–1031. [Google Scholar] [CrossRef] [Green Version]
- Shade, K.C.; Anthony, R.M. Antibody glycosylation and inflammation. Antibodies 2013, 2, 392–414. [Google Scholar] [CrossRef] [Green Version]
- Russell, A.; Adua, E.; Ugrina, I.; Laws, S.; Wang, W. Unravelling immunoglobulin g fc n-glycosylation: A dynamic marker potentiating predictive, preventive and personalised medicine. Int. J. Mol. Sci. 2018, 19, 390. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, M. Stepwise progression of pulmonary adenocarcinoma--clinical and molecular implications. Cancer Metastasis Rev. 2010, 29, 15–21. [Google Scholar] [CrossRef]
- Nakamura, H.; Koizumi, H.; Kimura, H.; Marushima, H.; Saji, H.; Takagi, M. Epidermal growth factor receptor mutations in adenocarcinoma in situ and minimally invasive adenocarcinoma detected using mutation-specific monoclonal antibodies. Lung Cancer 2016, 99, 143–147. [Google Scholar] [CrossRef]
- Izumchenko, E.; Chang, X.; Brait, M.; Fertig, E.; Kagohara, L.T.; Bedi, A.; Marchionni, L.; Agrawal, N.; Ravi, R.; Jones, S.; et al. Targeted sequencing reveals clonal genetic changes in the progression of early lung neoplasms and paired circulating DNA. Nat. Commun. 2015, 6, 8258. [Google Scholar] [CrossRef] [PubMed]
- Vinayanuwattikun, C.; Le Calvez-Kelm, F.; Abedi-Ardekani, B.; Zaridze, D.; Mukeria, A.; Voegele, C.; Vallee, M.; Purnomosari, D.; Forey, N.; Durand, G.; et al. Elucidating genomic characteristics of lung cancer progression from in situ to invasive adenocarcinoma. Sci. Rep. 2016, 6, 31628. [Google Scholar] [CrossRef] [PubMed]
- Banat, G.A.; Tretyn, A.; Pullamsetti, S.S.; Wilhelm, J.; Weigert, A.; Olesch, C.; Ebel, K.; Stiewe, T.; Grimminger, F.; Seeger, W.; et al. Immune and inflammatory cell composition of human lung cancer stroma. PloS ONE 2015, 10, e0139073. [Google Scholar] [CrossRef] [PubMed]
- Caetano, M.S.; Zhang, H.; Cumpian, A.M.; Gong, L.; Unver, N.; Ostrin, E.J.; Daliri, S.; Chang, S.H.; Ochoa, C.E.; Hanash, S.; et al. Il6 blockade reprograms the lung tumor microenvironment to limit the development and progression of k-ras-mutant lung cancer. Cancer Res. 2016, 76, 3189–3199. [Google Scholar] [CrossRef] [Green Version]
- Miranda, S.; Canellada, A.; Gentile, T.; Margni, R. Interleukin-6 and dexamethasone modulate in vitro asymmetric antibody synthesis and udp-glc glycoprotein glycosyltransferase activity. J. Reprod. Immunol. 2005, 66, 141–150. [Google Scholar] [CrossRef]
- Radhakrishnan, P.; Chachadi, V.; Lin, M.F.; Singh, R.; Kannagi, R.; Cheng, P.W. Tnfalpha enhances the motility and invasiveness of prostatic cancer cells by stimulating the expression of selective glycosyl- and sulfotransferase genes involved in the synthesis of selectin ligands. Biochem. Biophys. Res. Commun. 2011, 409, 436–441. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Wan, J.; Liu, J.; Xie, W.; Diao, X.; Xu, J.; Zhu, B.; Chen, Z. Increased il-17-producing cells correlate with poor survival and lymphangiogenesis in nsclc patients. Lung Cancer 2010, 69, 348–354. [Google Scholar] [CrossRef]
- Li, Q.; Han, Y.; Fei, G.; Guo, Z.; Ren, T.; Liu, Z. Il-17 promoted metastasis of non-small-cell lung cancer cells. Immunol. Lett. 2012, 148, 144–150. [Google Scholar] [CrossRef]
- Bao, Z.; Lu, G.; Cui, D.; Yao, Y.; Yang, G.; Zhou, J. Il-17a-producing t cells are associated with the progression of lung adenocarcinoma. Oncol. Rep. 2016, 36, 641–650. [Google Scholar] [CrossRef] [Green Version]
- Bi, Y.; Cao, J.; Jin, S.; Lv, L.; Qi, L.; Liu, F.; Geng, J.; Yu, Y. Interleukin-22 promotes lung cancer cell proliferation and migration via the il-22r1/stat3 and il-22r1/akt signaling pathways. Mol. Cell. Biochem. 2016, 415, 1–11. [Google Scholar] [CrossRef]
- Jones, M.B.; Nasirikenari, M.; Lugade, A.A.; Thanavala, Y.; Lau, J.T. Anti-inflammatory igg production requires functional p1 promoter in beta-galactoside alpha2,6-sialyltransferase 1 (st6gal-1) gene. J. Biol. Chem. 2012, 287, 15365–15370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, V.H.; Pipinikas, C.P.; Pennycuick, A.; Lee-Six, H.; Chandrasekharan, D.; Beane, J.; Morris, T.J.; Karpathakis, A.; Feber, A.; Breeze, C.E.; et al. Deciphering the genomic, epigenomic, and transcriptomic landscapes of pre-invasive lung cancer lesions. Nat. Med. 2019, 25, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Yoshida, M.; Nagai-Okatani, C.; Iwaki, J.; Matsuda, A.; Tan, B.; Hagiwara, K.; Sato, T.; Itakura, Y.; Noro, E.; et al. A standardized method for lectin microarray-based tissue glycome mapping. Sci. Rep. 2017, 7, 43560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuno, A.; Matsuda, A.; Unno, S.; Tan, B.; Hirabayashi, J.; Narimatsu, H. Differential glycan analysis of an endogenous glycoprotein: Toward clinical implementation--from sample pretreatment to data standardization. Methods Mol. Biol. 2014, 1200, 265–285. [Google Scholar]
- Yan, X.; Lu, J.; Zou, X.; Zhang, S.; Cui, Y.; Zhou, L.; Liu, F.; Shan, A.; Lu, J.; Zheng, M.; et al. The polypeptide n-acetylgalactosaminyltransferase 4 exhibits stage-dependent expression in colorectal cancer and affects tumorigenesis, invasion and differentiation. FEBS J. 2018, 285, 3041–3055. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Xu, K.; Xu, Z.; de Las Rivas, M.; Wang, C.; Li, X.; Lu, J.; Zhou, Y.; Delso, I.; Merino, P.; et al. The small molecule luteolin inhibits n-acetyl-alpha-galactosaminyltransferases and reduces mucin-type o-glycosylation of amyloid precursor protein. J. Biol. Chem. 2017, 292, 21304–21319. [Google Scholar] [CrossRef] [Green Version]


| Discovery Set (n = 92) | Test Set (n = 210) | |||||
|---|---|---|---|---|---|---|
| Noninvasive Group (n = 37) | Invasive Group (n = 55) | p3 | Noninvasive Group (n = 90) | Invasive Group (n = 120) | p3 | |
| Age (year) 1 | 51.19 ± 10.98 | 54.69 ± 9.72 | 0.176 | 51.50 ± 10.77 | 52.08 ± 11.19 | 0.639 |
| Gender (male/female) | 8/29 | 12/43 | 0.982 | 19/71 | 29/91 | 0.602 |
| Nodule diameter (mm) 1 | 8.7 ± 2.5 | 9.3 ± 2.4 | 0.240 | 7.7 ± 1.4 | 8.2 ± 1.5 | 0.009 |
| Pathology 2 | / | / | ||||
| Benign | 4 (10.8%) | / | 10 (4.8%) | / | ||
| AAH | 5 (13.5%) | / | 4 (1.9%) | / | ||
| AIS | 28 (75.7%) | / | 76 (36.2%) | / | ||
| MIA | / | 34 (61.8%) | / | 84 (40.0%) | ||
| IA | / | 21 (38.2%) | / | 36 (17.1%) | ||
| Clinical parameters 1 | ||||||
| CEA (ng/mL) | 1.55 ± 0.60 | 1.95 ± 0.94 | 0.059 | 1.39 ± 0.90 | 1.67 ± 1.31 | 0.099 |
| Cyfra21-1 (ng/mL) | 1.44 ± 0.67 | 1.48 ± 0.99 | 0.647 | 1.39± 0.71 | 1.34 ± 0.64 | 0.793 |
| SCC-Ag (ng/mL) | 0.85 ± 0.43 | 0.80 ± 0.34 | 0.978 | 1.20 ± 3.16 | 0.92 ± 0.93 | 0.660 |
| NSE (ng/mL) | 11.64 ± 4.46 | 11.38 ± 3.27 | 0.845 | 13.87 ± 5.05 | 13.55 ± 5.12 | 0.671 |
| CA125 (ng/mL) | 16.19 ± 16.39 | 11.57 ± 5.71 | 0.454 | 12.67 ± 11.57 | 11.73 ± 6.70 | 0.638 |
| CT value (HU) | −542.28 ± 132.68 | −433.24 ± 179.68 | 0.007 | −515.50 ± 207.82 | −421.25 ± 202.35 | 0.001 |
| Binding Specificity | Test Set 1 | ||||
|---|---|---|---|---|---|
| Noninvasive Group (n = 90) | Invasive Group (n = 120) | p2 | |||
| SNA | Siaα2-6Gal/GalNAc | 13.35 ± 2.86 | 12.50 ± 2.37 | 0.046 | <0.001 |
| DSA | (GlcNAc)n, polyLacNAc and branched LacNAc | 51.33 ± 8.20 | 48.78 ± 9.32 | 0.046 | 0.001 |
| NPA | Non-substituted α1-6Man | 21.85 ± 3.44 | 20.55 ± 3.37 | 0.009 | |
| GNA | Non-substituted α1-6Man | 6.08 ± 1.80 | 5.57 ± 1.52 | 0.045 | 0.001 |
| HHL | Non-substituted α1-6Man | 0.66 ± 0.22 | 0.60 ± 0.18 | 0.025 | |
| EEL | Galα1-3(Fuc α1-2)Gal | 0.15 ± 0.06 | 0.12 ± 0.05 | 0.002 | <0.001 |
| VVA | terminal GalNAc | 0.03 ± 0.04 | 0.04 ± 0.03 | 0.009 | 0.001 |
| WGA | (GlcNAc)n and multivalent Sia | 23.88 ± 5.72 | 21.27 ± 5.62 | 0.001 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, X.; Yao, F.; Yang, F.; Zhang, F.; Xu, Z.; Shi, J.; Kuno, A.; Zhao, H.; Zhang, Y. Glycomic Signatures of Plasma IgG Improve Preoperative Prediction of the Invasiveness of Small Lung Nodules. Molecules 2020, 25, 28. https://doi.org/10.3390/molecules25010028
Zou X, Yao F, Yang F, Zhang F, Xu Z, Shi J, Kuno A, Zhao H, Zhang Y. Glycomic Signatures of Plasma IgG Improve Preoperative Prediction of the Invasiveness of Small Lung Nodules. Molecules. 2020; 25(1):28. https://doi.org/10.3390/molecules25010028
Chicago/Turabian StyleZou, Xia, Feng Yao, Fang Yang, Fang Zhang, Zhijue Xu, Jingjing Shi, Atsushi Kuno, Heng Zhao, and Yan Zhang. 2020. "Glycomic Signatures of Plasma IgG Improve Preoperative Prediction of the Invasiveness of Small Lung Nodules" Molecules 25, no. 1: 28. https://doi.org/10.3390/molecules25010028






