Antitumor Effect of a Novel Spiro-Acridine Compound is Associated with Up-Regulation of Th1-Type Responses and Antiangiogenic Action
Abstract
:1. Introduction
2. Results and Discussion
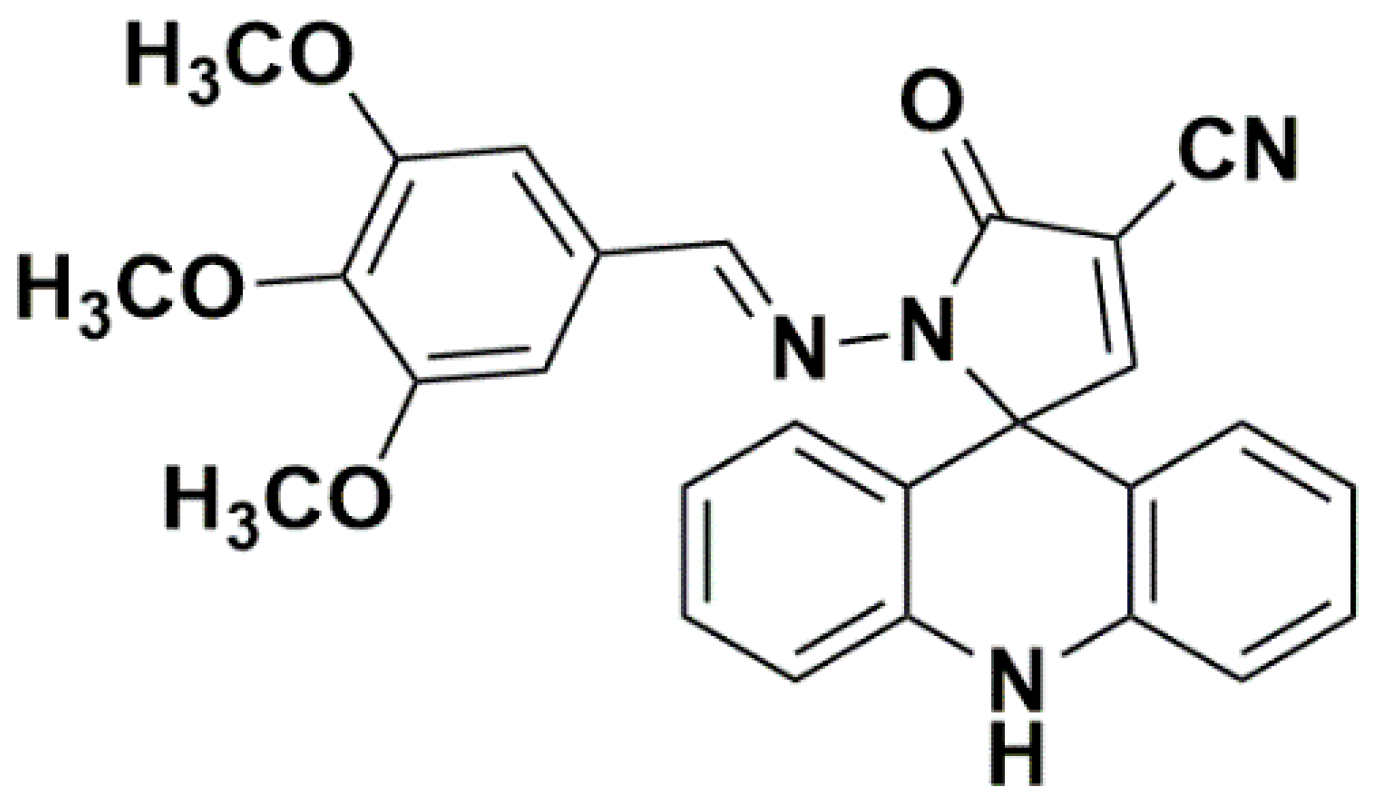
2.1. Chemistry
2.2. Biological Evaluation
3. Materials and Methods
3.1. Synthesis Methodology
3.2. Animals
3.3. Acute Non-Clinical Toxicity Assay
3.4. In Vivo Antitumor Activity
3.5. Peritoneal Angiogenesis
3.6. Cell Cycle Analyses
3.7. Quantification of Cytokines
3.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shalapour, S.; Karin, M. Immunity, inflammation, and cancer: An eternal fight between good and evil. J. Clin. Investig. 2015, 125, 3347–3355. [Google Scholar] [CrossRef] [Green Version]
- Bonelli, M.; Monica, S.L.; Fumarola, C.; Alfieri, R.; Bonelli, M.; Monica, S.L.; Fumarola, C.; Alfieri, R.; Alfieri, R. Multiple effects of CDK4/6 inhibition in cancer: From cell cycle arrest to immunomodulation. Biochem. Pharmacol. 2019, 170, 113676. [Google Scholar] [CrossRef] [PubMed]
- Al-abd, A.M.; Alamoudi, A.J.; Abdel-naim, A.B.; Neamatallah, T.A.; Ashour, O.M. Anti-angiogenic agents for the treatment of solid tumors: Potential pathways, therapy and current strategies—A review. J. Adv. Res. 2017, 8, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Johnson III, B.A.; Lutz, E.R.; Laheru, D.A.; Jaffee, E.M. Targeting neoantigens to augment antitumour immunity. Nat. Rev. Cancer 2017, 17, 209. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Zhang, J. Inflammasomes in Inflammation-Induced Cancer. Front. Immunol. 2017, 8, 271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, M.; Hellstrom, I.; Yip, Y.Y.; Sjögren, H.O.; Hellstrom, K.E. Tumor Regression and Cure Depends on Sustained Th1 Responses. J. Immunother. 2018, 41, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Vilková, M.; Prokaiová, M.; Imrich, J. Spontaneous cyclization of (acridin-9-ylmethyl)thioureas to spiro [dihydroacridine-9′(10′H),5-imidazolidine]-2-thiones, a novel type of acridine spirocycles. Tetrahedron 2014, 70, 944–961. [Google Scholar] [CrossRef]
- Almeida, S.M.V.d.; Lafayette, E.A.; Silva, W.L.; Serafim, V.d.L.; Menezes, T.M.; Neves, J.L.; Ruiz, A.L.T.G.; Carvalho, J.E.d.; Moura, R.O.d.; Beltrão, E.I.C.; et al. New spiro-acridines: DNA interaction, antiproliferative activity and inhibition of human DNA topoisomerases. Int. J. Biol. Macromol. 2016, 92, 467–475. [Google Scholar] [CrossRef]
- Gouveia, R.; Galdino, A.; Ângelo, M.; Pinheiro, S.; Ricardo, T.; Lima, C.D.; Mônica, S.; Almeida, V.D.; Olímpio, R.; Moura, D. Synthesis, DNA and protein interactions and human topoisomerase inhibition of novel Spiroacridine derivatives. Bioorganic Med. Chem. 2018, 26, 5911–5921. [Google Scholar] [CrossRef]
- Menezes, T.M.; Almeida, S.M.V.d.; Neves, J.L.; Moura, R.O.D.; Seabra, G.; Carmo, M. Spiro-acridine inhibiting tyrosinase enzyme: Kinetic, proteinligand interaction and molecular docking studies. Int. J. Biol. Macromol. 2018, 122, 289–297. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development (OECD). Guideline for Testing of Chemicals n. 423: Acute Oral Toxicity. Available online: https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecd_gl423.pdf (accessed on 25 November 2019).
- Mangueira, V.; Batista, T.; Brito, M.; Abrantes, R.; Sousa, T.; Cavalcanti, R.; Almeida, I.; Medeiros, D.; Karla, K.; Medeiros, D.P.; et al. A new acridine derivative induces cell cycle arrest and antiangiogenic effect on Ehrlich ascites carcinoma model. Biomed. Pharmacother. 2017, 90, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Kukowska, M. Amino acid or peptide conjugates of acridine/acridone and quinoline/quinolone-containing drugs. A critical examination of their clinical effectiveness within a twenty-year timeframe in antitumor chemotherapy and treatment of infectious diseases. Eur. J. Pharm. Sci. 2017, 109, 587–615. [Google Scholar] [CrossRef] [PubMed]
- Jernei, T.; Duró, C.; Dembo, A.; Lajkó, E.; Takács, A.; Kohidai, L.; Schlosser, G.; Csámpai, A. Synthesis, Structure and In Vitro Cytotoxic Activity of Novel Cinchona — Chalcone Hybrids with 1,4-Disubstituted- and 1,5-Disubstituted 1,2,3-Triazole Linkers. Molecules 2019, 24, 4077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somma, S.D.; Amato, J.; Iaccarino, N.; Pagano, B.; Randazzo, A.; Portella, G.; Malfitano, A.M. G-Quadruplex Binders Induce Immunogenic Cell Death Markers in Aggressive Breast Cancer Cells. Cancers 2019, 11, 1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olszewska, P.; Mikiciuk-Olasik, E.; Błaszczak-Swiatkiewicz, K.; Szyman’, J.; Ski, P.S. Novel tetrahydroacridine derivatives inhibit human lung adenocarcinoma cell growth by inducing G1 phase cell cycle arrest and apoptosis. Biomed. Pharmacother. 2014, 68, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro Franco, P.I.; Rodrigues, A.P.; Menezes, L.B.d.; Miguel, M.P. Tumor microenvironment components: Allies of cancer progression. Pathol. - Res. Pract. 2019, in press. [Google Scholar] [CrossRef]
- Hinshaw, D.C.; Shevde, L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef] [Green Version]
- Majnooni, M.B.; Fakhri, S.; Smeriglio, A.; Trombetta, D.; Croley, C.R.; Bhattacharyya, P.; Sobarzo-s, E.; Farzaei, M.H.; Bishayee, A. Antiangiogenic Effects of Coumarins against Cancer: From Chemistry to Medicine. Molecules 2019, 24, 4278. [Google Scholar] [CrossRef] [Green Version]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2019, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Tugues, S.; Burkhard, S.H.; Ohs, I.; Vrohlings, M.; Nussbaum, K.; Berg, J.; Kulig, P.; Becher, B. New insights into IL-12-mediated tumor suppression. Cell Death Differ. 2014, 22, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.; Brito, M.; Ferreira, R.; Moura, A.P.; Sousa, T.; Batista, T.; Mangueira, V.; Leite, F.; Cruz, R.; Vieira, G.; et al. Th1-Biased Immunomodulation and In Vivo Antitumor Effect of a Novel Piperine Analogue. Int. J. Mol. Sci. 2018, 19, 2594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H. Th1 cytokine-based immunotherapy for cancer. Hepatobiliary Pancreat. Dis. Int. 2014, 13, 482–494. [Google Scholar] [CrossRef]
- Petty, A.J.; Yang, Y. Tumor-associated macrophages: Implications in cancer immunotherapy. Immunotherapy 2017, 9, 289–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moura, E.C.R.; Leal, P.d.C.; Serra, I.C.P.B.; Ribeiro, B.d.P.; do Nascimento, J.R.; Nascimento, F.R.F.d.; Sakata, R.K. Tumor growth activity of duloxetine in Ehrlich carcinoma in mice. BMC Res. Notes 2018, 11, 525. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.S.; Saraswati, S.; Mathur, R.; Pandey, M. Cytotoxic and antitumor effects of brucine on Ehrlich ascites tumor and human cancer cell line. Life Sci. 2011, 89, 147–158. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compound are available from the authors. |




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, D.K.F.; Duarte, S.S.; Lisboa, T.M.H.; Ferreira, R.C.; Lopes, A.L.d.O.; Carvalho, D.C.M.; Rodrigues-Mascarenhas, S.; da Silva, P.M.; Segundo, M.A.S.P.; Moura, R.O.d.; et al. Antitumor Effect of a Novel Spiro-Acridine Compound is Associated with Up-Regulation of Th1-Type Responses and Antiangiogenic Action. Molecules 2020, 25, 29. https://doi.org/10.3390/molecules25010029
Silva DKF, Duarte SS, Lisboa TMH, Ferreira RC, Lopes ALdO, Carvalho DCM, Rodrigues-Mascarenhas S, da Silva PM, Segundo MASP, Moura ROd, et al. Antitumor Effect of a Novel Spiro-Acridine Compound is Associated with Up-Regulation of Th1-Type Responses and Antiangiogenic Action. Molecules. 2020; 25(1):29. https://doi.org/10.3390/molecules25010029
Chicago/Turabian StyleSilva, Daiana K. Frade, Sâmia S. Duarte, Thaís M. H. Lisboa, Rafael C. Ferreira, Ana Luíza de O. Lopes, Deyse C. M. Carvalho, Sandra Rodrigues-Mascarenhas, Patricia Mirella da Silva, Miguel A. S. Pinheiro Segundo, Ricardo O. de Moura, and et al. 2020. "Antitumor Effect of a Novel Spiro-Acridine Compound is Associated with Up-Regulation of Th1-Type Responses and Antiangiogenic Action" Molecules 25, no. 1: 29. https://doi.org/10.3390/molecules25010029




