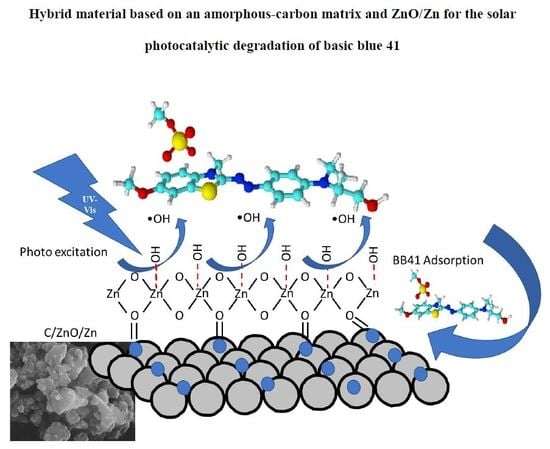
Hybrid Material Based on an Amorphous-Carbon Matrix and ZnO/Zn for the Solar Photocatalytic Degradation of Basic Blue 41
Abstract
1. Introduction
2. Results and Discussion
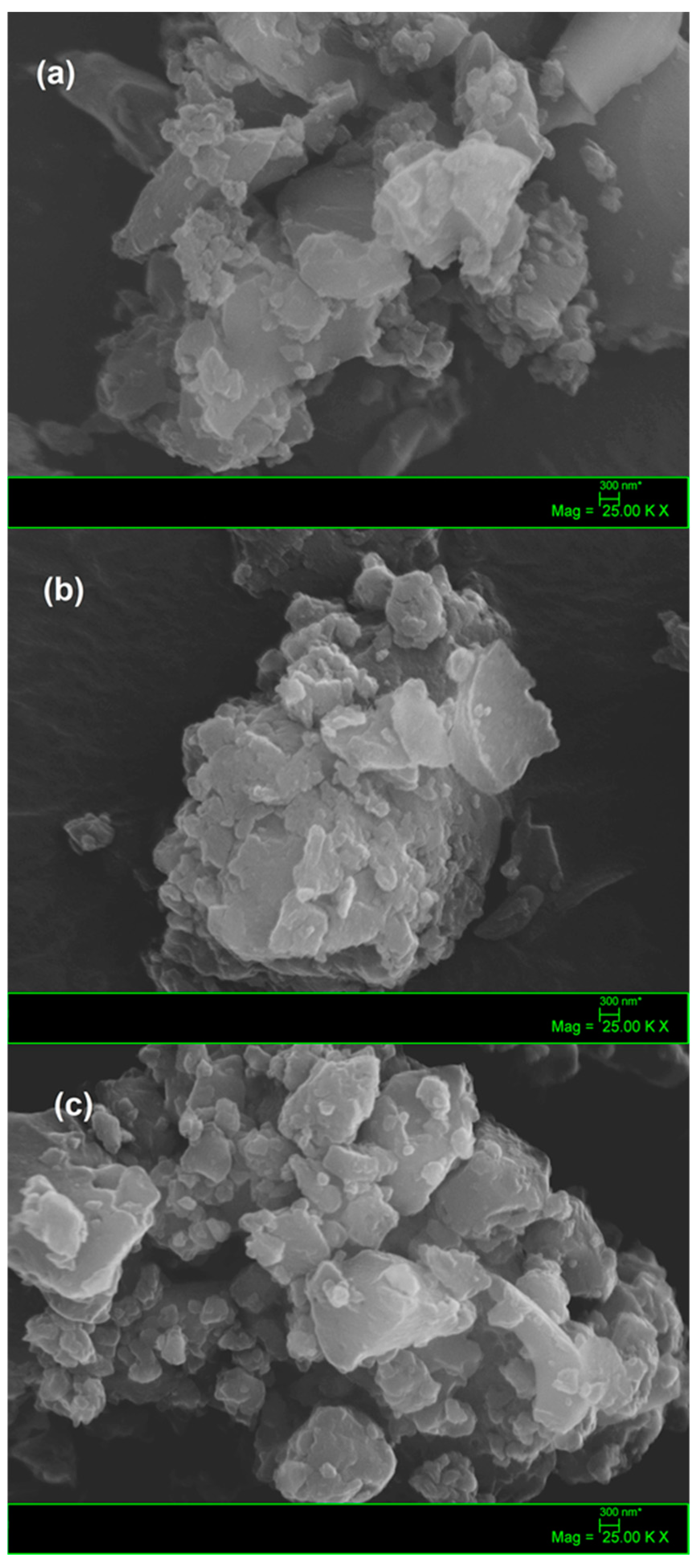
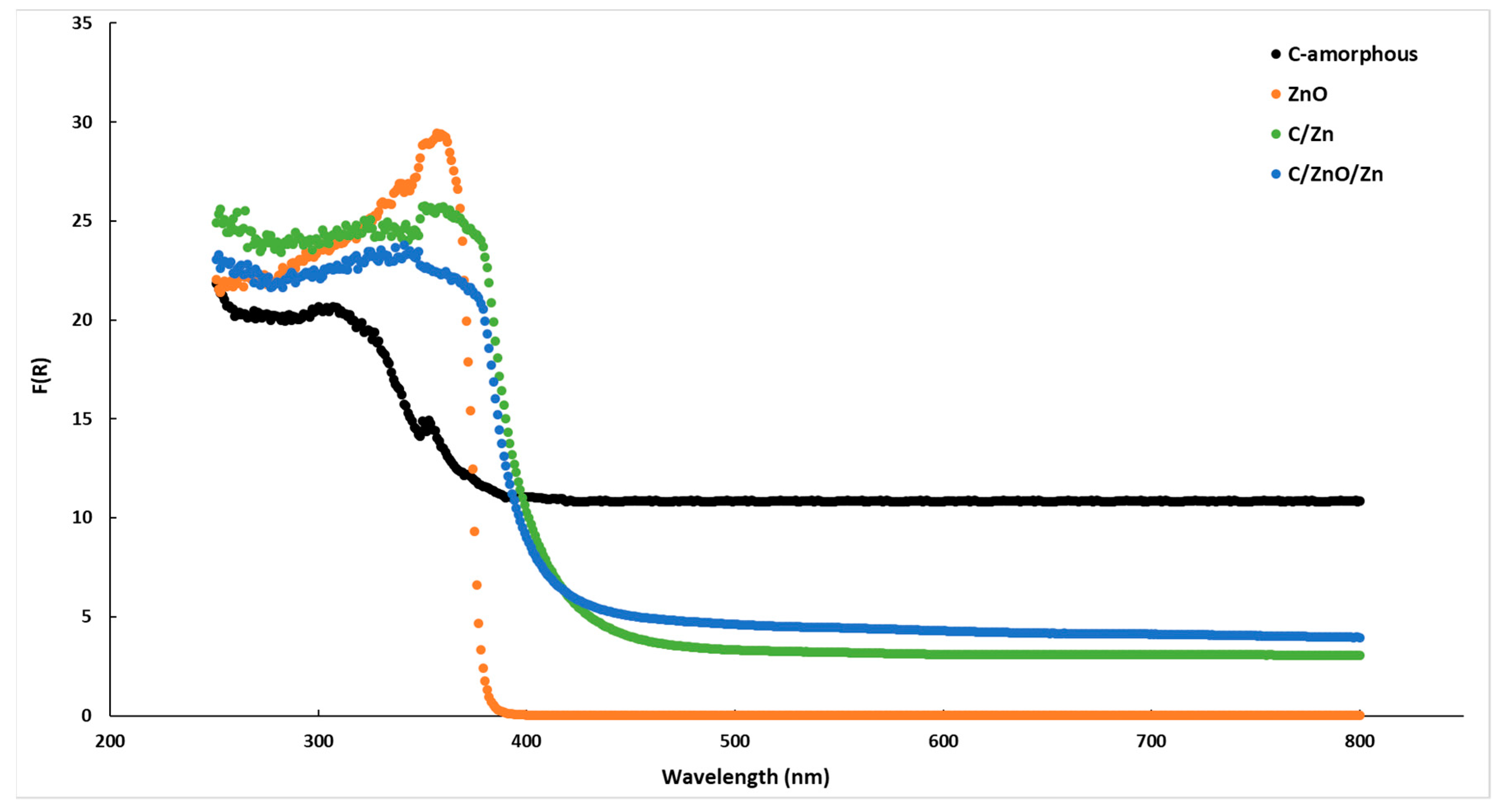
2.1. Characterization of Samples
2.2. Influence of Surface pH Upon the Kinetics of Adsorption in the Dark
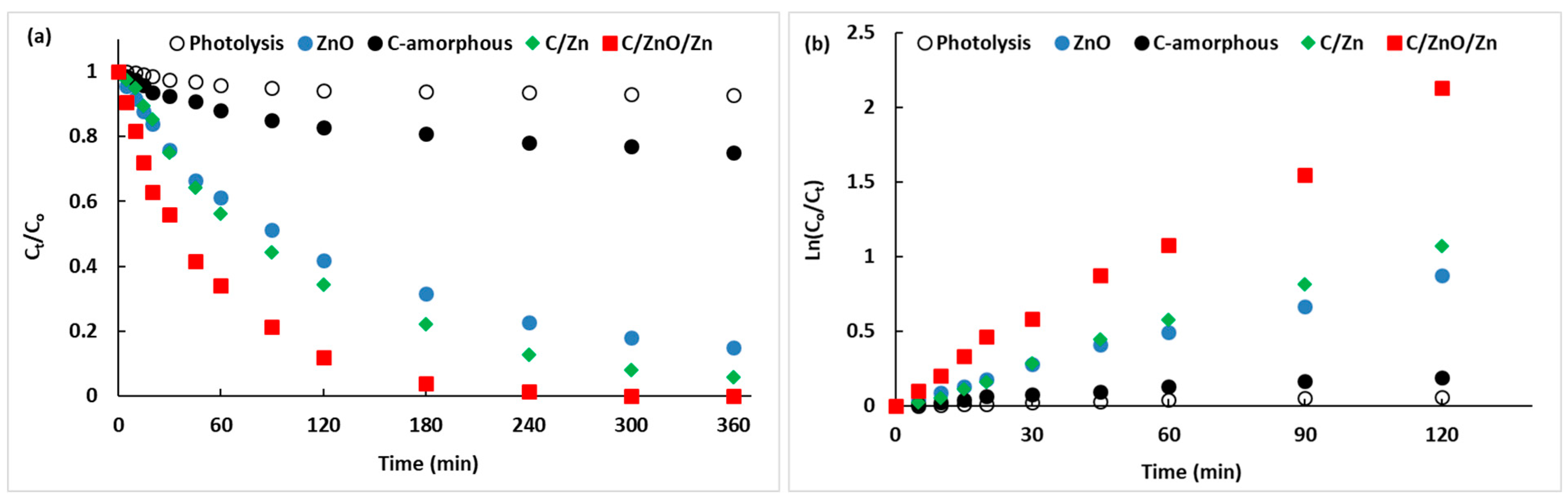
2.3. Photocatalytic Tests
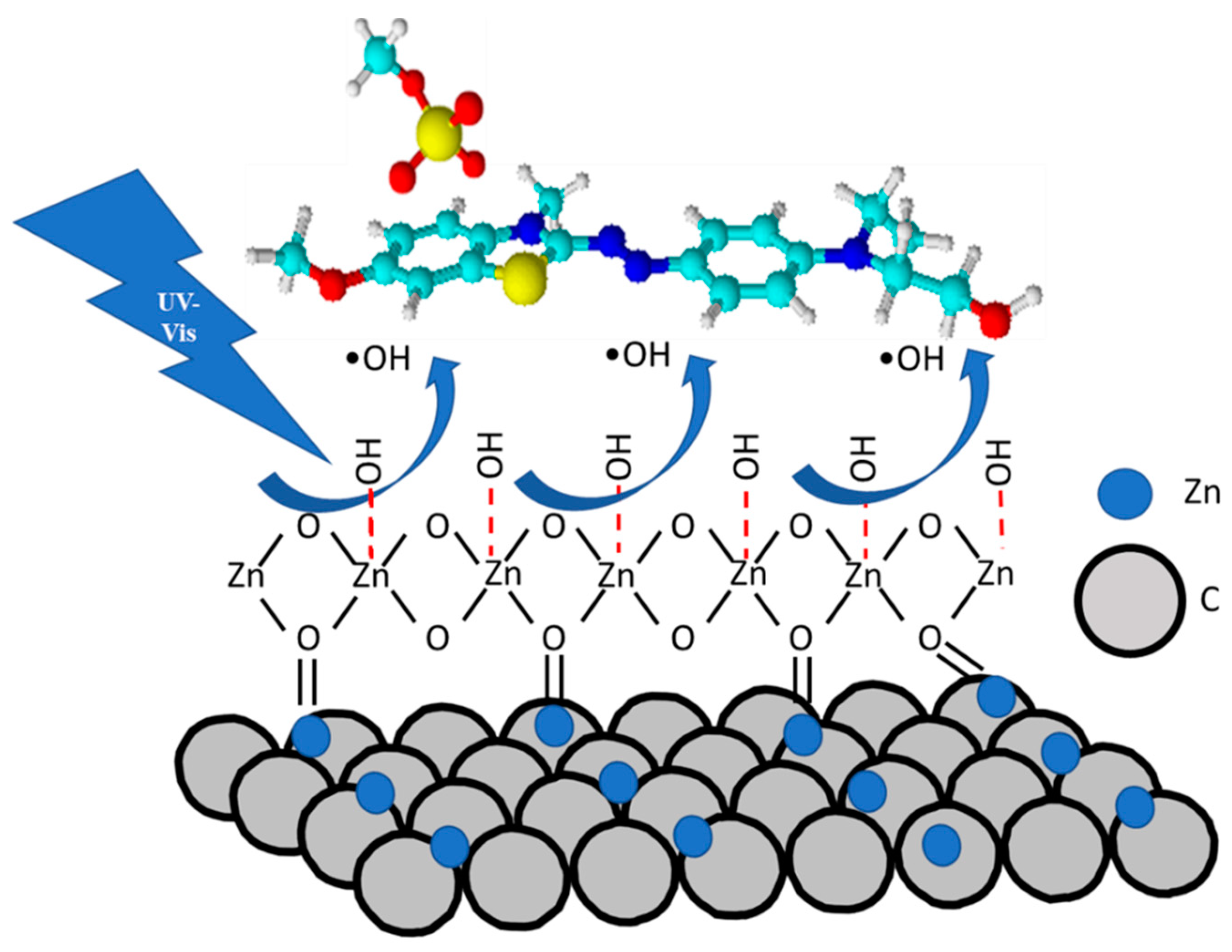
2.4. General Discussion
3. Experimental
3.1. Synthesis
3.2. Characterization
3.3. Adsorption in the Dark and Photocatalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Feng, Z.; Zhang, T.; Li, Y.; He, X.; Wang, R.; Xu, J.; Gao, G. The accumulation of microplastics in fish from an important fish farm and mariculture area, Haizhou Bay, China. Sci. Total Environ. 2019, 696, 133948. [Google Scholar] [CrossRef]
- Hou, X.; Liu, J.; Zhang, D.; Zhao, M.; Xia, C. Impact of urbanization on the eco-efficiency of cultivated land utilization: A case study on the Yangtze River Economic Belt, China. J. Clean. Prod. 2019, 238, 117916. [Google Scholar] [CrossRef]
- Margalef-Marti, R.; Carrey, R.; Soler, A.; Otero, N. Evaluating the potential use of a dairy industry residue to induce denitrification in polluted water bodies: A flow-through experiment. J. Environ. Manag. 2019, 245, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Alshabib, M.; Onaizi, S.A. A review on phenolic wastewater remediation using homogeneous and heterogeneous enzymatic processes: Current status and potential challenges. Sep. Purif. Technol. 2019, 219, 186–207. [Google Scholar] [CrossRef]
- Tan, G.; Sun, W.; Xu, Y.; Wang, H.; Xu, N. Sorption of mercury (II) and atrazine by biochar, modified biochars and biochar based activated carbon in aqueous solution. Bioresour. Technol. 2016, 211, 727–735. [Google Scholar] [CrossRef]
- Ioannidou, O.A.; Zabaniotou, A.A.; Stavropoulos, G.G.; Islam, M.A.; Albanis, T.A. Preparation of activated carbons from agricultural residues for pesticide adsorption. Chemosphere 2010, 80, 1328–1336. [Google Scholar] [CrossRef]
- Chan, H. Biodegradation of petroleum oil achieved by bacteria and nematodes in contaminated water. Sep. Purif. Technol. 2011, 80, 459–466. [Google Scholar] [CrossRef]
- García-Fernández, I.; Miralles-Cuevas, S.; Oller, I.; Fernández-Ibáñez, P.; Polo-López, M.I. Inactivation of E. coli and E. faecalis by solar photo-Fenton with EDDS complex at neutral pH in municipal wastewater effluents. J. Hazard. Mater. 2019, 372, 85–93. [Google Scholar] [CrossRef]
- Andronic, L.; Isac, L.; Miralles-Cuevas, S.; Visa, M.; Oller, I.; Duta, A.; Malato, S. Pilot-plant evaluation of TiO2 and TiO2-based hybrid photocatalysts for solar treatment of polluted water. J. Hazard. Mater. 2016, 320, 469–478. [Google Scholar] [CrossRef]
- Vilar, V.J.P.; Alfonso-Muniozguren, P.; Monteiro, J.P.; Lee, J.; Miranda, S.M.; Boaventura, R.A.R. Tube-in-tube membrane microreactor for photochemical UVC/H2O2 processes: A proof of concept. Chem. Eng. J. 2020, 379, 122341. [Google Scholar] [CrossRef]
- Marinho, B.A.; Cristóvão, R.O.; Djellabi, R.; Loureiro, J.M.; Boaventura, R.A.R.; Vilar, V.J.P. Photocatalytic reduction of Cr(VI) over TiO2-coated cellulose acetate monolithic structures using solar light. Appl. Catal. B Environ. 2017, 203, 18–30. [Google Scholar] [CrossRef]
- Bosio, M.; Satyro, S.; Bassin, J.P.; Saggioro, E.; Dezotti, M. Removal of pharmaceutically active compounds from synthetic and real aqueous mixtures and simultaneous disinfection by supported TiO2/UV-A, H2O2/UV-A, and TiO2/H2O2/UV-A processes. Environ. Sci. Pollut. Res. 2019, 26, 4288–4299. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.D.; Bassin, J.P.; Dezotti, M. Treatment of a simulated textile wastewater containing the Reactive Orange 16 azo dye by a combination of ozonation and moving-bed biofilm reactor: Evaluating the performance, toxicity, and oxidation by-products. Environ. Sci. Pollut. Res. 2017, 24, 6307–6316. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Chan, S.H.S.; Yeong Wu, T.; Juan, J.C.; Teh, C.Y. Recent developments of metal oxide semiconductors as photocatalysts in advanced oxidation processes (AOPs) for treatment of dye waste-water. J. Chem. Technol. Biotechnol. 2011, 86, 1130–1158. [Google Scholar] [CrossRef]
- Hernández-Ramirez, A.; Medina-Ramirez, I. Photocatalytic Semiconductors; Hernández-Ramírez, A., Medina-Ramirez, I., Eds.; Springer International Publisher: New York, NY, USA, 2015; ISBN 978-3-319-10998-5. [Google Scholar]
- Khan, M.M.; Ansari, S.A.; Pradhan, D.; Ansari, M.O.; Han, D.H.; Lee, J.; Cho, M.H. Band gap engineered TiO2 nanoparticles for visible light induced photoelectrochemical and photocatalytic studies. J. Mater. Chem. A 2014, 2, 637–644. [Google Scholar] [CrossRef]
- Matos, J.; García-López, E.; Palmisano, L.; García, A.; Marcì, G. Influence of activated carbon in TiO2 and ZnO mediated photo-assisted degradation of 2-propanol in gas–solid regime. Appl. Catal. B Environ. 2010, 99, 170–180. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Y.; Wu, H.; Guo, S. Control of the formation of rod-like ZnO mesocrystals and their photocatalytic properties. CrystEngComm 2013, 15, 2608–2615. [Google Scholar] [CrossRef]
- Nekouei, S.; Nekouei, F.; Kargarzadeh, H. Synthesis of ZnO photocatalyst modified with activated carbon for a perfect degradation of ciprofloxacin and its secondary pollutants. Appl. Organomet. Chem. 2018, 32, e4198. [Google Scholar] [CrossRef]
- Juabrum, S.; Chankhanittha, T.; Nanan, S. Hydrothermally grown SDS-capped ZnO photocatalyst for degradation of RR141 azo dye. Mater. Lett. 2019, 245, 1–5. [Google Scholar] [CrossRef]
- Wang, H.; Rogach, A.L. Hierarchical SnO2 Nanostructures: Recent Advances in Design, Synthesis, and Applications. Chem. Mater. 2014, 26, 123–133. [Google Scholar] [CrossRef]
- Sun, C.; Li, H.; Chen, L. Nanostructured ceria-based materials: Synthesis, properties and applications. Energy Environ. Sci. 2012, 5, 8475–8505. [Google Scholar] [CrossRef]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Matos, J.; Ocares-Riquelme, J.; Poon, P.S.; Montaña, R.; García, X.; Campos, K.; Hernández-Garrido, J.C.; Titirici, M.M. C-doped anatase TiO2: Adsorption kinetics and photocatalytic degradation of methylene blue and phenol, and correlations with DFT estimations. J. Colloid Interface Sci. 2019, 547, 14–29. [Google Scholar] [CrossRef]
- European Scientist. Available online: https://www.europeanscientist.com/fr/sante/la-france-va-interdire-le-dioxyde-de-titane-a-compter-de-2020/ (accessed on 22 April 2019).
- Matos, J.; Montaña, R.; Rivero, E.; Velasco, J.; Ledezma, G. Photocatalytic activity of ZnO-Biochar hybrid composites. Eurasian Chem. Technol. J. 2014, 16, 293–297. [Google Scholar] [CrossRef]
- Lanfredi, S.; Silveira, G.S.; Potensa, B.S.; Nobre, M.A.L. ZnO/Zn/Amorphous Carbon Matrix Nanostructured Composite Powder: A New Photocatalyst for Dye. MRS Adv. 2016, 1, 1327–1332. [Google Scholar] [CrossRef]
- Gandelman, H.; da Silva, A.L.; Caliman, L.B.; Gouvêa, D. Surface and grain boundary excess of ZnO-doped TiO2 anatase nanopowders. Ceram. Int. 2018, 44, 11390–11396. [Google Scholar] [CrossRef]
- Tisseraud, C.; Comminges, C.; Habrioux, A.; Pronier, S.; Pouilloux, Y.; Le Valant, A. Cu-ZnO catalysts for CO2 hydrogenation to methanol: Morphology change induced by ZnO lixiviation and its impact on the active phase formation. Mol. Catal. 2018, 446, 98–105. [Google Scholar] [CrossRef]
- Rangel-Mendez, J.R.; Matos, J.; Cházaro-Ruiz, L.F.; González-Castillo, A.C.; Barrios-Yáñez, G. Microwave-assisted synthesis of C-doped TiO2 and ZnO hybrid nanostructured materials as quantum-dots sensitized solar cells. Appl. Surf. Sci. 2018, 434, 744–755. [Google Scholar] [CrossRef]
- Matos, J.; Díaz, K.; García, V.; Cordero, T.C.; Brito, J.L. Methane Transformation in Presence of Carbon Dioxide on Activated Carbon Supported Nickel-calcium Catalysts. Catal. Lett. 2006, 109, 163–169. [Google Scholar] [CrossRef]
- Matos, J.; Rosales, M.; Demir-Cakan, R.; Titirici, M.M. Methane conversion on Pt–Ru nanoparticles alloy supported on hydrothermal carbon. Appl. Catal. A Gen. 2010, 386, 140–146. [Google Scholar] [CrossRef]
- Geng, D.; Chen, Y.; Chen, Y.; Li, Y.; Li, R.; Sun, X.; Ye, S.; Knights, S. High oxygen-reduction activity and durability of nitrogen-doped graphene. Energy Environ. Sci. 2011, 4, 760–764. [Google Scholar] [CrossRef]
- Guo, D.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Leite, E.R.; Nobre, M.A.L.; Longo, E.; Varela, J.A. Microstructural development of ZnO varistor during reactive liquid phase sintering. J. Mater. Sci. 1996, 31, 5391–5398. [Google Scholar] [CrossRef]
- Abrarov, S.M.; Yuldashev, S.U.; Lee, S.B.; Kang, T.W. Suppression of the green photoluminescence band in ZnO embedded into porous opal by spray pyrolysis. J. Lumin. 2004, 109, 25–29. [Google Scholar] [CrossRef]
- Wayu, M.B.; Spidle, R.T.; Devkota, T.; Deb, A.K.; Delong, R.K.; Ghosh, K.C.; Wanekaya, A.K.; Chusuei, C.C. Morphology of hydrothermally synthesized ZnO nanoparticles tethered to carbon nanotubes affects electrocatalytic activity for H2O2 detection. Electrochim. Acta 2013, 97, 99–104. [Google Scholar] [CrossRef]
- Wang, X.; Dong, L.H.; Ma, X.L.; Zheng, Y.F. Microstructure, mechanical property and corrosion behaviors of interpenetrating C/Mg-Zn-Mn composite fabricated by suction casting. Mater. Sci. Eng. C 2013, 33, 618–625. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Tran, M.L.; Tran, T.T.; Juang, R.-S. Enhanced removal of various dyes from aqueous solutions by UV and simulated solar photocatalysis over TiO2/ZnO/rGO composites. Sep. Purif. Technol. 2020, 232, 115962. [Google Scholar] [CrossRef]
- Srinivasan, N.; Anbuchezhiyan, M.; Harish, S.; Ponnusamy, S. Hydrothermal synthesis of C doped ZnO nanoparticles coupled with BiVO4 and their photocatalytic performance under the visible light irradiation. Appl. Surf. Sci. 2019, 494, 771–782. [Google Scholar] [CrossRef]
- Pechini, M.P. Method of Preparing Lead and Alkaline Earth Titanates and Niobates and Coating Method Using the Same to Form a Capacitor 1967. U.S. Patent 3,330,697, 11 July 1967. [Google Scholar]
- Benvenuti, J.; Fisch, A.; dos Santos, J.H.Z.; Gutterres, M. Silica-based adsorbent material with grape bagasse encapsulated by the sol-gel method for the adsorption of Basic Blue 41 dye. J. Environ. Chem. Eng. 2019, 7, 103342. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Abdi, J. Nanoporous metal-organic framework (MOF-199): Synthesis, characterization and photocatalytic degradation of Basic Blue 41. Microchem. J. 2019, 144, 436–442. [Google Scholar] [CrossRef]
- Sharma, D.; Jha, R. Analysis of structural, optical and magnetic properties of Fe/Co co-doped ZnO nanocrystals. Ceram. Int. 2017, 43, 8488–8496. [Google Scholar] [CrossRef]
- Puziy, A.; Poddubnaya, O.; Martínez-Alonso, A.; Suárez-García, F.; Tascón, J.M. Synthetic carbons activated with phosphoric acid: I. Surface chemistry and ion binding properties. Carbon 2002, 40, 1493–1505. [Google Scholar] [CrossRef]
- Hasanpour, A.; Niyaifar, M.; Asan, M.; Amighian, J. Synthesis and characterization of Fe3O4 and ZnO nanocomposites by the sol–gel method. J. Magn. Magn. Mater. 2013, 334, 41–44. [Google Scholar] [CrossRef]
- Lanfredi, S.; Nobre, M.A.L.; Moraes, P.G.P.; Matos, J. Photodegradation of phenol red on a Ni-doped niobate/carbon composite. Ceram. Int. 2014, 40, 9525–9534. [Google Scholar] [CrossRef]
- Liu, C.; Chen, H.; Dai, K.; Xue, A.; Chen, H.; Huang, Q. Synthesis, characterization, and its photocatalytic activity of double-walled carbon nanotubes-TiO2 hybrid. Mater. Res. Bull. 2013, 48, 1499–1505. [Google Scholar] [CrossRef]
- Martınez, M.T.; Callejas, M.A.; Benito, A.M.; Cochet, M.; Seeger, T.; Ansón, A.; Schreiber, J.; Gordon, C.; Marhic, C.; Chauvet, O.; et al. Sensitivity of single wall carbon nanotubes to oxidative processing: Structural modification, intercalation and functionalisation. Carbon 2003, 41, 2247–2256. [Google Scholar] [CrossRef]
- Nyquist, R.A.; Kagel, R.O. Infrared Spectra of Inorganic Compounds. In Handbook of Infrared and Raman Spectra of Inorganic Compounds and Organic Salts; Nyquist, R.A., Kagel, R.O., Eds.; Academic Press: San Diego, CA, USA, 1971; pp. 1–18. ISBN 978-0-12-523450-4. [Google Scholar]
- Steiner, T.D. Semiconductor Nanostructures for Optoelectronic Applications. In Artech House Semiconductor Materials and Devices Library; Artech House: Norwood, MA, USA, 2004; ISBN 9781580537513. [Google Scholar]
- Nakamoto, K. Infrared and Raman spectra of inorganic and coordination compounds. In Infrared & Raman Spectra of Inorganic & Coordination Chemistry; Wiley-Interscience: New York, NY, USA, 1986; ISBN 9780471010661. [Google Scholar]
- Liu, G.; Wu, H.; Gupta, R.P.; Lucas, J.A.; Tate, A.G.; Wall, T.F. Modeling the fragmentation of non-uniform porous char particles during pulverized coal combustion. Fuel 2000, 79, 627–633. [Google Scholar] [CrossRef]
- Chae, O.B.; Park, S.; Ryub, J.H.; Oh, S.M. Performance Improvement of Nano-Sized Zinc Oxide Electrode by Embedding in Carbon Matrix for Lithium-Ion Batteries. J. Electrochem. Soc. 2013, 160, A11–A14. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Jagiello, J.; Olivier, J.P. 2D-NLDFT adsorption models for carbon slit-shaped pores with surface energetical heterogeneity and geometrical corrugation. Carbon 2013, 55, 70–80. [Google Scholar] [CrossRef]
- Jagiello, J.; Thommes, M. Comparison of DFT characterization methods based on N2, Ar, CO2, and H2 adsorption applied to carbons with various pore size distributions. Carbon 2004, 42, 1227–1232. [Google Scholar] [CrossRef]
- Kooli, F.; Yan, L.; Al-Faze, R.; Al-Sehimi, A. Removal enhancement of basic blue 41 by brick waste from an aqueous solution. Arab. J. Chem. 2015, 8, 333–342. [Google Scholar] [CrossRef]
- Humelnicu, I.; Băiceanu, A.; Ignat, M.-E.; Dulman, V. The removal of Basic Blue 41 textile dye from aqueous solution by adsorption onto natural zeolitic tuff: Kinetics and thermodynamics. Process Saf. Environ. Prot. 2017, 105, 274–287. [Google Scholar] [CrossRef]
- Afshin, S.; Mokhtari, S.A.; Vosoughi, M.; Sadeghi, H.; Rashtbari, Y. Data of adsorption of Basic Blue 41 dye from aqueous solutions by activated carbon prepared from filamentous algae. Data Br. 2018, 21, 1008–1013. [Google Scholar] [CrossRef]
- Zarezadeh-Mehrizi, M.; Badiei, A. Highly efficient removal of basic blue 41 with nanoporous silica. Water Resour. Ind. 2014, 5, 49–57. [Google Scholar] [CrossRef]
- Lee, C.-K.; Liu, S.-S.; Juang, L.-C.; Wang, C.-C.; Lin, K.-S.; Lyu, M.-D. Application of MCM-41 for dyes removal from wastewater. J. Hazard. Mater. 2007, 147, 997–1005. [Google Scholar] [CrossRef]
- Matos, J.; Hofman, M.; Pietrzak, R. Synergy effect in the photocatalytic degradation of methylene blue on a suspended mixture of TiO2 and N-containing carbons. Carbon 2013, 54, 460–471. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Matos, J.; Seredych, M.; Islam, M.S.Z.; Alfano, R. Photoactivity of S-doped nanoporous activated carbons: A new perspective for harvesting solar energy on carbon-based semiconductors. Appl. Catal. A Gen. 2012, 445–446, 159–165. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Policicchio, A.; Florent, M.; Li, W.; Poon, P.S.; Matos, J. Solar light-driven photocatalytic degradation of phenol on S-doped nanoporous carbons: The role of functional groups in governing activity and selectivity. Carbon 2020, 156, 10–23. [Google Scholar] [CrossRef]
- Velasco, L.F.; Fonseca, I.M.; Parra, J.B.; Lima, J.C.; Ania, C.O. Photochemical behaviour of activated carbons under UV irradiation. Carbon 2012, 50, 249–258. [Google Scholar] [CrossRef]
- Velasco, L.F.; Carmona, R.J.; Matos, J.; Ania, C.O. Performance of activated carbons in consecutive phenol photooxidation cycles. Carbon 2014, 73, 206–215. [Google Scholar] [CrossRef]
- Ania, C.O.; Seredych, M.; Rodríguez-Castellón, E.; Bandosz, T.J. Visible light driven photoelectrochemical water splitting on metal free nanoporous carbon promoted by chromophoric functional groups. Carbon 2014, 79, 432–441. [Google Scholar] [CrossRef]
- Gomis-Berenguer, A.; Seredych, M.; Iniesta, J.; Lima, J.C.; Bandosz, T.J.; Ania, C.O. Sulfur-mediated photochemical energy harvesting in nanoporous carbons. Carbon 2016, 104, 253–259. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Ania, C.O. Origin and Perspectives of the Photochemical Activity of Nanoporous Carbons. Adv. Sci. 2018, 5, 1800293. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Tejeda, L.; Palomares, F.J.; Cabal, B.; López-Píriz, R.; Fernández, A.; Sevillano, D.; Alou, L.; Torrecillas, R.; Moya, J.S. Effect of the medium composition on the Zn2+ lixiviation and the antifouling properties of a glass with a high ZnO content. Materials 2017, 10, 167. [Google Scholar] [CrossRef] [PubMed]
- Gouvêa, D.; do Rosário, D.C.C.; Caliman, L.B. Surface and grain-boundary excess of ZnO-doped SnO2 nanopowders by the selective lixiviation method. J. Am. Ceram. Soc. 2017, 100, 4331–4340. [Google Scholar] [CrossRef]
- Boumaza, S.; Kaouah, F.; Omeiri, S.; Trari, M.; Bendjama, Z. Removal of dyes by an integrated process coupling adsorption and photocatalysis in batch mode. Res. Chem. Intermed. 2015, 41, 2353–2375. [Google Scholar] [CrossRef]
- Bouafia-Chergui, S.; Oturan, N.; Khalaf, H.; Oturan, M.A. Parametric study on the effect of the ratios [H2O2]/[Fe3+] and [H2O2]/[substrate] on the photo-Fenton degradation of cationic azo dye Basic Blue 41. J. Environ. Sci. Health Part. A Toxic Hazar. Subs. Environ. Eng. 2010, 45, 622–629. [Google Scholar] [CrossRef]
- Stathatos, E.; Papoulis, D.; Aggelopoulos, C.A.; Panagiotaras, D.; Nikolopoulou, A. TiO2/palygorskite composite nanocrystalline films prepared by surfactant templating route: Synergistic effect to the photocatalytic degradation of an azo-dye in water. J. Hazard. Mater. 2012, 211–212, 68–76. [Google Scholar] [CrossRef]
- Limsapapkasiphon, S.; Sirisaksoontorn, W.; Songsasen, A. Inhibition of photocatalytic activity of basic blue-41 by ZnO modified surface with amino silane. IOP Conf. Ser. Mater. Sci. Eng. 2018, 317, 012014. [Google Scholar] [CrossRef]
- Behpour, M.; Shirazi, P.; Rahbar, M. Immobilization of the Fe2O3/TiO2 photocatalyst on carbon fiber cloth for the degradation of a textile dye under visible light irradiation. Reac. Kinet. Mechan. Catal. 2019, 127, 1073–1085. [Google Scholar] [CrossRef]
- Galceran, M.; Pujol, M.C.; Aguiló, M.; Díaz, F. Sol-gel modified Pechini method for obtaining nanocrystalline KRE (WO4)2 (RE = Gd and Yb). J. Sol Gel Sci. Technol. 2007, 42, 79–88. [Google Scholar] [CrossRef]
- Rouquerol, J.; Llewellyn, P.; Rouquerol, F. Is the bet equation applicable to microporous adsorbents? Characterization of porous solids VII. Stud. Surf. Sci. Catal. 2007, 160, 49–56. [Google Scholar]
- Ho, Y.S.; McKay, G. A Comparison of Chemisorption Kinetic Models Applied to Pollutant Removal on Various Sorbents. Process Saf. Environ. Prot. 1998, 76, 332–340. [Google Scholar] [CrossRef]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Kinetic modeling of liquid-phase adsorption of reactive dyes and metal ions on chitosan. Water Res. 2001, 35, 613–618. [Google Scholar] [CrossRef]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem. Eng. J. 2009, 153, 1–8. [Google Scholar] [CrossRef]
- McKay, G.; Otterburn, M.S.; Sweeney, A.G. The removal of colour from effluent using various adsorbents—III. Silica: Rate processes. Water Res. 1980, 14, 15–20. [Google Scholar] [CrossRef]
- Matos, J.; Lanfredi, S.; Montaña, R.; Nobre, M.A.L.; de Córdoba, M.C.F.; Ania, C.O. Photochemical reactivity of apical oxygen in KSr2Nb5O15 materials for environmental remediation under UV irradiation. J. Colloid Interface Sci. 2017, 496, 211–221. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

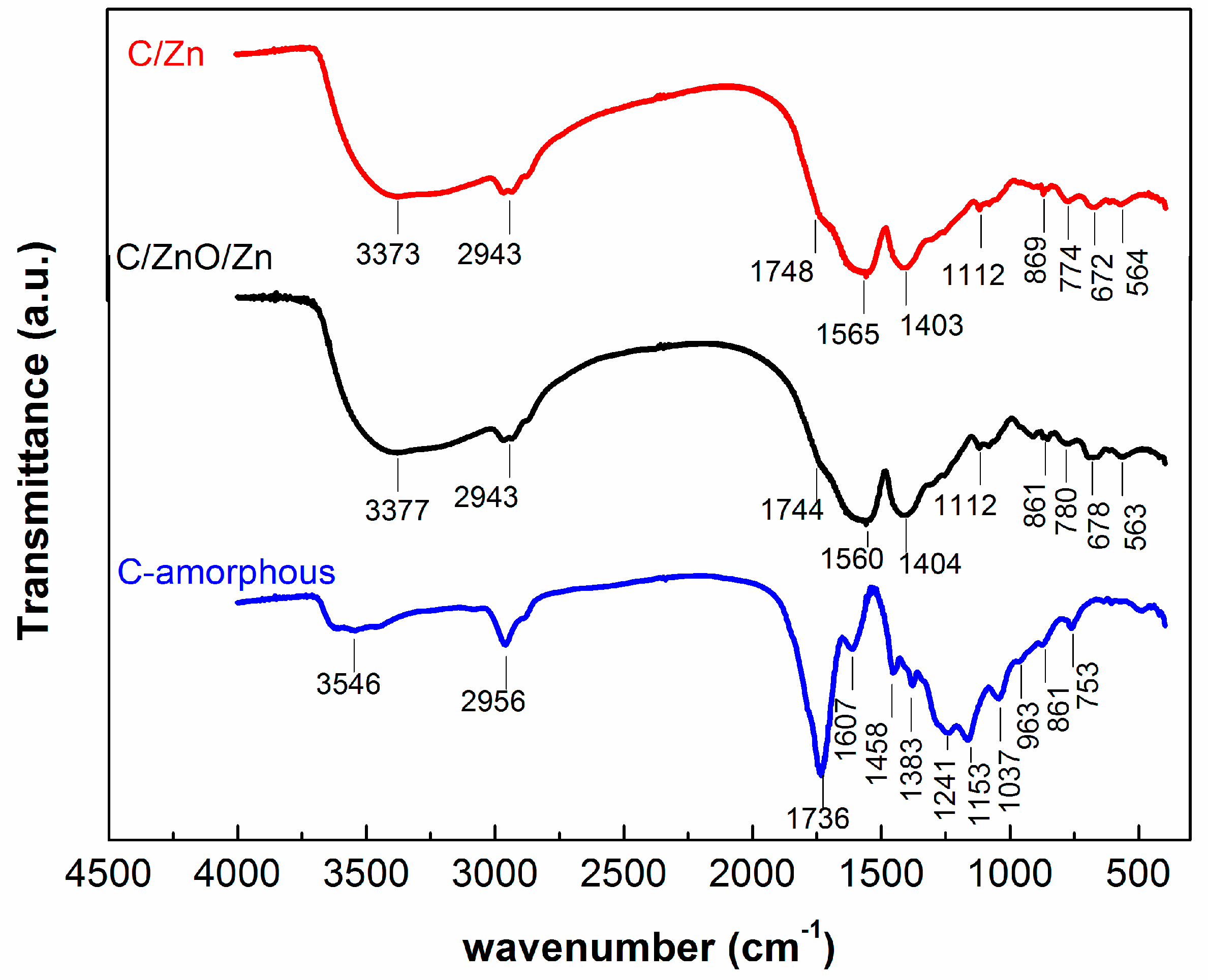


| Assignments | Wavenumber (cm−1) | References |
|---|---|---|
| νsim (OH) water | 3546–3373 | [46,47] |
| νsim (C–H) | 2943 | [27,28] |
| νsim (C=O) ester | 1748–1736 | [29,49,50] |
| δ O–H, δass C–H | 1607–1403 | [28,48] |
| ν (C–O) ester, δsim C–H | 1383–1037 | [29,49,50] |
| νsim (C–C) | 963–753 | [27,28] |
| Vibrations Zn–O | 672–563 | [45,51,52] |
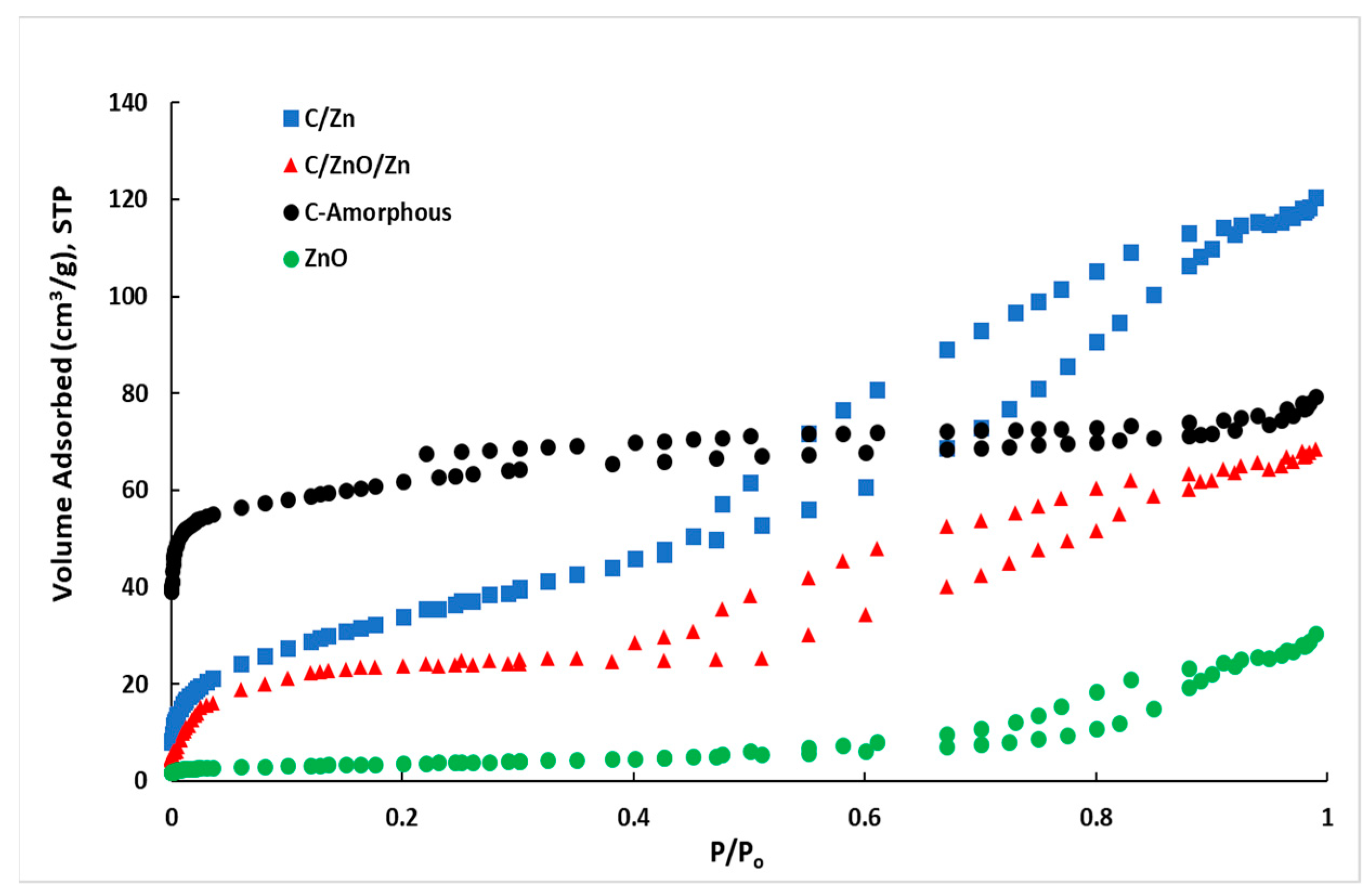
| Sample | SBET a (m2 g−1) | VDR b (cm3 g−1) | V0.99 c (cm3 g−1) | Vmeso d (cm3 g−1) | V<1 nm-DFT e (cm3 g−1) |
|---|---|---|---|---|---|
| ZnO | 13 | 0.001 | 0.048 | 0.047 | 0.001 |
| C-amorphous | 196 | 0.230 | 0.298 | 0.066 | 0.094 |
| C/Zn | 125 | 0.050 | 0.190 | 0.140 | 0.013 |
| C/ZnO/Zn | 75 | 0.022 | 0.114 | 0.092 | 0.006 |
| Sample | BB41ads-eq a (μmol) | k1 b (min−1) | Rk1 c | k2 d (μmol−1 min−1) | Rk2 e | kp f (μmol min−0.5) | C g (μmol) | Rkp h | pHPZC i |
|---|---|---|---|---|---|---|---|---|---|
| ZnO | 1.95 | 0.049 | 0.911 | 0.216 | 0.951 | 0.056 | 1.51 | 0.973 | 8.9 |
| C-amorphous | 0.54 | 0.048 | 0.958 | 1.491 | 0.907 | 0.031 | 0.30 | 0.714 | 5.4 |
| C/Zn | 0.87 | 0.037 | 0.946 | 0.746 | 0.940 | 0.025 | 0.68 | 0.704 | 6.6 |
| C/ZnO/Zn | 1.46 | 0.060 | 0.957 | 0.686 | 0.949 | 0.047 | 1.10 | 0.737 | 7.9 |
| Sample | BB41ads-eq a (μmol) | kapp × 10−3 (min−1) b | Rkapp c | Φrel-ZnO d | BB41Conv-3h e (%) | BB41Conv-6h f (%) |
|---|---|---|---|---|---|---|
| Photolysis | - | 0.5 | 0.960 | 0.07 | 6 | 7 |
| ZnO | 1.95 | 7.3 | 0.991 | 1.0 | 69 | 85 |
| C-amorphous | 0.54 | 1.6 | 0.958 | 0.2 | 19 | 25 |
| C/Zn | 0.87 | 9.2 | 0.996 | 1.3 | 78 | 94 |
| C/ZnO/Zn | 1.46 | 17.2 | 0.996 | 2.4 | 96 | 100 |
| Sample | C a (%) | H a (%) | O a (%) | Zn + O b (%) |
|---|---|---|---|---|
| ZnO | - | - | - | 100 |
| C-amorphous | 93 | 0.5 | 6.5 | -- |
| C/Zn | 59 | 0.3 | 3.7 | 37 |
| C/ZnO/Zn | 52 | 0.3 | 3.7 | 44 |
| Photocatalytic Run | kapp × 10−3 (min−1) a | R2 b | Δkapp c (%) | Zn d (g L−1) | Leached Zn e (%) |
|---|---|---|---|---|---|
| 1 | 17.2 | 0.996 | 0 | <LOD f | 0 |
| 2 | 16.8 | 0.992 | 2 | 0.0005 | 1.4 |
| 3 | 15.9 | 0.988 | 7 | 0.0008 | 2.2 |
| 4 | 14.7 | 0.990 | 14 | 0.0012 | 3.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanfredi, S.; Nobre, M.A.L.; Poon, P.S.; Matos, J. Hybrid Material Based on an Amorphous-Carbon Matrix and ZnO/Zn for the Solar Photocatalytic Degradation of Basic Blue 41. Molecules 2020, 25, 96. https://doi.org/10.3390/molecules25010096
Lanfredi S, Nobre MAL, Poon PS, Matos J. Hybrid Material Based on an Amorphous-Carbon Matrix and ZnO/Zn for the Solar Photocatalytic Degradation of Basic Blue 41. Molecules. 2020; 25(1):96. https://doi.org/10.3390/molecules25010096
Chicago/Turabian StyleLanfredi, Silvania, Marcos A. L. Nobre, Po S. Poon, and Juan Matos. 2020. "Hybrid Material Based on an Amorphous-Carbon Matrix and ZnO/Zn for the Solar Photocatalytic Degradation of Basic Blue 41" Molecules 25, no. 1: 96. https://doi.org/10.3390/molecules25010096
APA StyleLanfredi, S., Nobre, M. A. L., Poon, P. S., & Matos, J. (2020). Hybrid Material Based on an Amorphous-Carbon Matrix and ZnO/Zn for the Solar Photocatalytic Degradation of Basic Blue 41. Molecules, 25(1), 96. https://doi.org/10.3390/molecules25010096