Calpurnia aurea (Aiton) Benth Extracts Reduce Quorum Sensing Controlled Virulence Factors in Pseudomonas aeruginosa
Abstract
:1. Introduction
2. Results
2.1. Antibacterial Activities
2.1.1. In Vitro Antibacterial Activities
2.1.2. Minimum Inhibitory Concentrations (MIC)
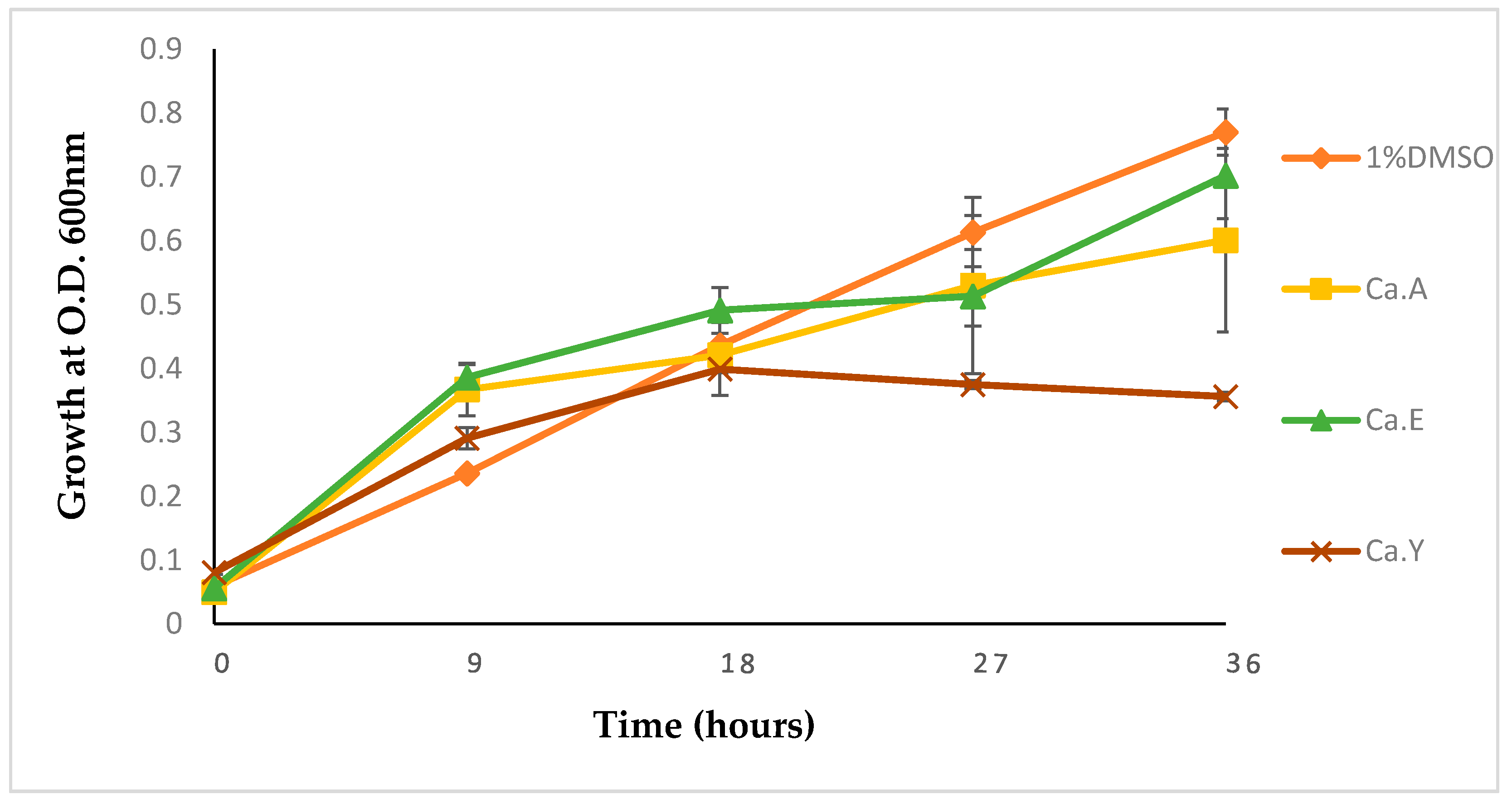
2.1.3. Growth Kinetics of Pseudomonas Aeruginosa Treated with Calpurnia Aurea
2.2. Anti-Quorum Sensing (AQS) Activity of Plant Extracts
2.2.1. Qualitative AQS Activity
2.2.2. Quantitative AQS Activity
2.3. GC-MS Analysis of Calpurnia Aurea Extract
2.4. In Silico Molecular Docking of Compounds Identified by GC-MS
2.5. Effect of Plant Extracts on Antiadhesion and Biofilm Eradication
Confocal Laser Scanning Microscopy (CLSM) Observation of Biofilm Inhibition
2.6. Effect of C. aurea Extracts on Swarming and Swimming Motility
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Extractions
4.2. Culturing and Maintenance of Microorganisms
4.3. Antibacterial Activity
4.3.1. Minimum Inhibitory Concentration (MIC)
4.3.2. Growth kinetics of Pseudomonas aeruginosa
4.4. Anti-Quorum Sensing (AQS) Activity
4.4.1. Qualitative AQS Activity
4.4.2. Quantitative AQS Activity
4.5. GC MS Analysis
4.6. Molecular Docking of Selected Phytochemical Compounds
4.7. Antivirulence Activity of Plant Extracts Against P. aeruginosa
4.7.1. Inhibition of Cell Attachment
4.7.2. Inhibition of Preformed Biofilm
4.7.3. Confocal Laser Scanning Microscopy
4.7.4. Swarming Motility Assay
4.7.5. Swimming Motility Assay
4.7.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 3-oxo-C12 HSL | N-(3-oxo-dodecanoyl)-L-Homoserine lactone |
| AHL | acylhomoserine lactone |
| AQS | anti-quorum sensing |
| ATCC | American Type Culture Collection |
| c-di-GMP | cyclic dimeric guanosine monophosphate |
| cAMP | cyclic adenosine monophosphate |
| C4-HSL | N-butanoyl-L-homoserine lactone |
| DCM | dichloromethane |
| DMSO | dimethyl sulfoxide |
| EtAC | Ethyl acetate |
| HPTLC | high-performance thin layer chromatography |
| INT | p-iodonitrotetrazolium violet |
| LB | Luria Bertani |
| MIC | minimum inhibitory concentration |
| GC-MS | gas chromatography-mass spectrometry |
| QS | quorum sensing |
| QSI | quorum sensing inhibition |
| R | resistant |
| LPS | liposaccharides |
| MH | Muller Hinton |
| MS | mass spectrometry |
| NaCl | sodium chloride |
| CV | Chromobacterium violaceum |
References
- Gellatly, S.L.; Hancock, R.E. Psuedomonas aeruginosa: New insights into pathogenesis and host defenses. Path. Dis. 2013, 67, 159–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Driscoll, J.A.; Brody, S.L.; Kollef, M.H. The epidemiology, pathogenesis and treatment of Pseudomonas aeruginosa infections. Drugs 2007, 67, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.G.; Ansari, M.A.; Khan, H.M.; Jalal, M.; Mahdi, A.A.; Cameotra, S.S. Crataeva nurvala nanoparticles inhibit virulence factors and biofilm formation in clinical isolates of Psuedomonas aeruginosa. JBM 2016, 57, 193–203. [Google Scholar]
- Jennings, L.K.; Storek, K.M.; Ledvina, H.E.; Coulon, C.; Marmont, L.S.; Sadovskaya, I.; Secor, P.R.; Tseng, B.S.; Scian, M.; Filloux, A. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc. Natl. Acad. Sci. USA 2015, 112, 11353–11358. [Google Scholar] [CrossRef] [Green Version]
- Fazli, M.; Almblad, H.; Rybtke, M.L.; Givskov, M.; Eberl, L.; Tolker-Nielsen, T. Regulation of biofilm formation in Pseudomonas and Burkholderia species. Environ. Microbiol. 2014, 16, 1961–1981. [Google Scholar] [CrossRef]
- Richards, J.J.; Melander, C. Controlling bacterial biofilms. Chem. Biochem. 2009, 10, 2287–2294. [Google Scholar] [CrossRef]
- Lee, J.; Zhang, L. The hierarchy quorum-sensing network in Pseudomonas aeruginosa. Prot. Cell 2015, 6, 26–41. [Google Scholar] [CrossRef] [Green Version]
- Blakes, J. Infobiotics: Computer-Aided Synthetic Systems Biology. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2013. [Google Scholar]
- Ochsner, U.A.; Koch, A.K.; Fiechter, A.; Reiser, J. Isolation and characterization of a regulatory gene affecting rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. J. Bacteriol. 1994, 176, 2044–2054. [Google Scholar] [CrossRef] [Green Version]
- McDonald, D.; Rice, S.A.; Barraud, N.; Steinberg, P.D.; Kjelleberg, S. Should we stay or should we go: Mechanisms and ecological consequences for biofilm dispersal. Nat. Rev. Microbiol. 2012, 55, 165–199. [Google Scholar]
- Dusane, D.H.; Zinjarde, S.S.; Venugopalan, V.P.; Mclean, R.J.; Weber, M.M.; Rahman, P.K. Quorum sensing: Implications on rhamnolipid biosurfactant production. Biotechnol. Genet. Eng. Rev. 2010, 27, 159–184. [Google Scholar] [CrossRef] [Green Version]
- Kearns, D.B. A field guide to bacterial swarming motility. Nat. Rev. Microbiol. 2010, 8, 634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, D.G.; Kuchma, S.L.; O’Toole, G.A. Plate-based assay for swimming motility in Pseudomonas aeruginosa. In Pseudomonas Methods and Protocols; Humana Press: New York, NY, USA, 2014; Volume 1148, pp. 59–65. [Google Scholar]
- Cosa, S.; Chaudhary, S.K.; Chen, W.; Combrinck, S.; Viljoen, A.M. Exploring common culinary herbs and spices as potential anti-quorum sensing agents. Nutrients 2019, 11, 739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.S.; Zahin, M.; Hasan, S.; Husain, F.M.; Ahmad, I. Inhibition of quorum sensing regulated bacterial functions by plant essential oils with special reference to clove oil. Lett. Appl. Microbiol. 2009, 49, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Louw, C.; Regnier, T.; Korsten, L. Medicinal bulbous plants of South Africa and their traditional relevance in the control of infectious diseases. J. Ethnopharmacol. 2002, 82, 147–154. [Google Scholar] [CrossRef]
- Van Wyk, B.; Gericke, N. People’s Plants. A Guide to Useful Plants of Southern Africa; Briza Publications: Pretoria, South Africa, 2000; p. 352. [Google Scholar]
- Vergara-Jimenez, M.; Almatrafi, M.M.; Fernandez, M.L. Bioactive components in Moringa oleifera leaves protect against chronic disease. Antioxidants 2017, 16, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazimba, O. Leonotis leonurus: A herbal medicine review. J. Pharmacogn. Phytochem. 2015, 3, 74–82. [Google Scholar]
- Zorloni, A.; Penzhorn, B.L.; Eloff, J.N. Extract of Calpurnia aurea leaves from Southern Ethiopia attract and immobilise or killed ticks. Vet. Parasitol. 2010, 168, 160–164. [Google Scholar] [CrossRef] [Green Version]
- Koh, C.-L.; Sam, C.-K.; Yin, W.-F.; Tan, L.Y.; Krishnan, T.; Chong, Y.M.; Chan, K.-G. Plant-derived natural products as sources of anti-quorum sensing compounds. Sensors 2013, 13, 6217–6228. [Google Scholar] [CrossRef] [Green Version]
- Damte, D.; Gebru, E.; Lee, S.; Suh, J.; Park, S. Evaluation of anti-quorum sensing activity of 97 indigenous plant extracts from Korea through bioreporter bacterial strains Chromobacterium violaceum and Pseudomonas aeruginosa. J. Microbiol. Biochem. Technol. 2013, 5, 42–46. [Google Scholar] [CrossRef]
- Wendakoon, C.; Peter, C.; Daniel, G. Evaluation of selected medicinal plants extracted in different ethanol concentrations for antibacterial activity against human pathogens. J. Med. Act. Plants 2012, 2, 60–68. [Google Scholar]
- Lister, P.D.; Wolter, D.J.; Hanson, N.D. Antibacterial-resistant Pseudomonas aeruginosa: Clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin. Microbiol. Rev. 2009, 22, 582–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oyedeji, O.A.; Afolayan, A.J.; Eloff, J.N. Comparative study of the essential oil composition and antimicrobial activity of Leonotis leonurus and L. ocymifolia in the Eastern Cape, South Africa. S. Afr. J. Bot. 2005, 71, 114–116. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, D.M.; Melo, F.G.; Balogun, S.O.; Flach, A.; de Souza, E.C.; de Souza, G.P.; Rocha Ido, N.; da Costa, L.A.; Soares, I.M.; da Silva, L.I.; et al. Antibacterial mode of action of the hydroethanolic extract of Leonotis nepetifolia (L.) R. Br. involves bacterial membrane perturbations. J. Ethnopharmacol. 2015, 172, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Moyo, B.; Masika, P.J.; Muchenje, V. Antimicrobial activities of Moringa oleifera Lam leaf extracts. Afr. J. Biotechnol. 2012, 11, 2797–2802. [Google Scholar] [CrossRef]
- Abalaka, M.E.; Daniyan, S.Y.; Oyeleke, S.B.; Adeyemo, S.O. The antibacterial evaluation of Moringa oleifera leaf extracts on selected bacterial pathogens. J. Microbiol. Res. 2012, 2, 1–4. [Google Scholar] [CrossRef]
- Van Vuuren, S.F. Antimicrobial activity of South African medicinal plants. J. Ethnopharmacol. 2008, 119, 462–472. [Google Scholar] [CrossRef]
- Umer, S.; Tekewe, A.; Kebede, N. Antidiarrhoeal and antimicrobial activity of Calpurnia aurea leaf extract. BMC Complement. Altern. Med. 2013, 13, 21. [Google Scholar] [CrossRef] [Green Version]
- Adedapo, A.A.; Jimoh, F.O.; Koduru, S.; Afolayan, A.J.; Masika, P.J. Antibacterial and antioxidant properties of the methanol extracts of the leaves and stems of Calpurnia aurea. BMC Complement. Altern. Med. 2008, 8, 53. [Google Scholar] [CrossRef] [Green Version]
- Schweizer, P.H. Efflux as a mechanism of resistance to antimicrobials in Psuedomonas aeruginosa and related bacteria: Unanswered questions. Genet. Mol. Res. 2003, 2, 28–62. [Google Scholar]
- Al-Yousef, H.M.; Ahmed, A.; Al-Shabib, N.A.; Laeeq, S.; Khan, R.A.; Rehman, M.T.; Alsalme, A.; Al-Ajmi, M.F.; Khan, M.S.; Husain, F.M. Onion peel ethyl acetate fraction and its derived constituent quercetin 4-O-D glucopyranoside attenuates quorum sensing regulated virulence and biofilm formation. Front. Microbiol. 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Al-Hussaini, R.; Mahasneh, A.M. Microbial growth and quorum sensing antagonist activities of herbal extracts. Molecules 2009, 14, 3425–3435. [Google Scholar] [CrossRef] [PubMed]
- Abraham Isaac, S.V.P.; Palani, A.; Ramaswamy, B.R.; Shunmugiah, K.P.; Arumgam, V.R. Antiquorum sensing and antibiofilm potential of Capparis spinose. Arch. Med. Res. 2011, 42, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Vasavi, H.S.; Arun, A.B.; Rekha, P.D. Anti-quorum sensing activity of flavonoid-rich fraction from Centella asiatica L. against Pseudomonas aeruginosa PAO1. J. Microbiol. Immunol. Inf. 2016, 49, 8–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emran, T.B.; Rahman, M.A.; Nasir Uddin, M.M.; Dash, R.; Hossen, M.F.; Mohiuddin, M.; Alam, M.R. Molecular docking and inhibition studies on the interactions of Bacopa monnieri’s potent phytochemicals against pathogenic Staphylococcus aureus. DARU J. Pharm. Sci. 2015, 23, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annapoorani, A.; Kalpana, B.; Musthafa, K.S.; Pandian, S.K.; Ravi, A.V. Antipathogenic potential of Rhizophora spp. against the quorum sensing mediated virulence factors production in drug resistant Pseudomonas aeruginosa. Phytomedicine 2013, 20, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Mishra, A.; Jha, B. Anti-quorum sensing and anti-biofilm activity of Delftia tsuruhatensis extract by attenuating the quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa. Front. Cel. Infec. Microbiol. 2017, 7, 337. [Google Scholar]
- Eloff, J.N. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998, 64, 711–713. [Google Scholar] [CrossRef] [Green Version]
- Choo, J.; Rukayadi, Y.; Hwang, J.K. Inhibition of bacterial quorum sensing by vanilla extract. Lett. Appl. Microbiol. 2006, 42, 637–641. [Google Scholar] [CrossRef]
- Quecan, B.X.V.; Santos, J.T.C.; Rivera, M.L.C.; Hassimotto, N.M.A.; Almeida, F.A.; Pinto, U.M. Effect of quercetin rich onion extracts on bacterial quorum sensing. Front. Microbiol. 2019, 10, 867. [Google Scholar] [CrossRef]
- Sandasi, M.; Leonard, C.; Van Vuuren, S.; Viljoen, A. Peppermint (Mentha Piperita) inhibits microbial biofilms in vitro. SAJB 2011, 77, 80–85. [Google Scholar] [CrossRef] [Green Version]
- Quave, C.L.; Estévez-Carmona, M.; Compadre, C.M.; Hobby, G.; Hendrickson, H.; Beenken, K.E.; Smeltzer, M.S. Ellagic acid derivatives from Rubus ulmifolius inhibit Staphylococcus aureus biofilm formation and improve response to antibiotics. PLoS ONE 2012, 7, e28737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, T.S.; Ledizet, M.; Kazmierczak, B.I. Swarming motility, secretion of type 3 effectors and biofilm formation phenotypes exhibited within a large cohort of Pseudomonas aeruginosa clinical isolates. J. Med. Microbiol. 2010, 59, 511–520. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the plant material are available from the authors. |







| Plant Species | Extracting Solvents Ethanol Acetone Ethyl Acetate | ||
|---|---|---|---|
| Calpurnia aurea | 1.56 | 1.56 | 6.25 |
| Leonotis ocymifolia | 6.25 | 6.25 | 6.25 |
| Moringa oleifera | 6.25 | 6.25 | 6.25 |
| Positive and negative controls | |||
| Ciprofloxacin | 0.06 | ||
| 1% DMSO | ≥6.25 | ||
| Zone Diameters (mm) and Associated Susceptibility Phenotypes | |||
|---|---|---|---|
| Chromobacterium violaceum ATCC 31532 | |||
| Concentration (mg/mL) | Ca.A | Ca.E | Ca.Y |
| 0.5 | 0.00 (R) | 0.00 (R) | 0.00 (R) |
| 1 | 8.00 (R) | 8.00 (R) | 0.00 (R) |
| 1.5 | 9.00 (R) | 8.25 (R) | 0.00 (R) |
| 2 | 10.00 (R) | 11.00 (I) | 0.00 (R) |
| 2.5 | 11.00 (I) | 12.00 (S) | 9.00 (R) |
| Positive and Negative Controls | |||
| Ciprofloxacin (5 µg/mL) | 29.30 (S) | ||
| DMSO (1%) | 0.00 (R) | ||
| Peak | Ret. Time | Name | MS Fragmentation | Chemical Class | Area% |
|---|---|---|---|---|---|
| 1 | 8.878 | n-Teteradecane | 198, 169, 141, 113, 99 | Hydrocarbon | 0.75 |
| 2 | 11.303 | 1,2-Benzenedicarboxylic acid | 293, 167, 149, 127 | Organic acid | 2.76 |
| 3 | 11.921 | 1,4-Benzenedicarboxylic acid diethyl ester | 222, 194, 177, 149 | Organic acid ester | 20.49 |
| 4 | 12.141 | Methyl mannose | 194, 177, 145, 115, 87 | Sugar | 15.76 |
| 5 | 13.301 | n-Nonadecane | 268, 196, 169, 141, 131, 99 | Hydrocarbon | 1.12 |
| 6 | 13.713 | Phthalic acid undecene-undecyl ester | 321, 304, 271, 167, 149, 132 | Organic acid ester | 3.79 |
| 7 | 14.115 | Tetramethyl-2-hexadece-nol | 296, 193, 138, 123, 96, 81 | Alcohol | 0.83 |
| 8 | 14.500 | Beta-H-pregna | 288, 165, 151, 125, 113, 125, 97, 57 | Steroid | 0.41 |
| 9 | 16.159 | Terephthalic acid, ethyl isobutyl ester | 250, 233, 195, 177, 149, 121, 84 | Organic acid ester | 3.96 |
| 10 | 16.243 | Pentadecanol | 210, 182, 168, 154, 140, 125, 111, 97, 83 | Alcohol | 14.54 |
| 11 | 18.322 | Hexadecanol | 224, 16, 168, 154, 139, 125, 111, 97 | Alcohol | 0.63 |
| 12 | 19.132 | Octadecanol | 252, 224, 210, 196, 182,168, 153, 139,125, 111, 97 | Alcohol | 1.70 |
| 13 | 20.055 | 2-(1H-indol-3-yl)acetic acid | 334, 277, 253, 213, 199, 183 | Organic acid | 1.06 |
| 14 | 22.170 | Octadecanal | 268, 250, 224, 222, 208, 194,182 | Aldehyde | 1.01 |
| 15 | 25.310 | Eicosanol | 298, 280, 253, 167, 139, 125 | Alcohol | 12.12 |
| 16 | 27.085 | Stigmasterol | 412, 351, 300, 271, 255, 159 | Steroid | 0.65 |
| 17 | 28.973 | Tetracosanal | 352, 334, 306, 278, 250, 208 | Aldehyde | 3.94 |
| Compound | Total Energy | Docking Score | Glide Energy | H Bond |
|---|---|---|---|---|
| Pentadecanol | 3.860 | −3.758 | −32.825 | −0.358 |
| Dimethyl terephthalate | 6.895 | −5.486 | −34.901 | −0.237 |
| Phthalic acid | - | - | - | - |
| Eicosanol | - | - | - | - |
| Tetracosanal | - | - | - | - |
| Terephthalic acid | 24.218 | −6.186 | −26.550 | −0.385 |
| Didecyl phthalate | - | - | - | - |
| Methyl mannose | 9.681 | −7.000 | −37.276 | −2.598 |
| Vanillin | 14.223 | −4.880 | −25.901 | −0.872 |
| Quercetin | 25.683 | −10.613 | −44.877 | −2.643 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosa, S.; Rakoma, J.R.; Yusuf, A.A.; Tshikalange, T.E. Calpurnia aurea (Aiton) Benth Extracts Reduce Quorum Sensing Controlled Virulence Factors in Pseudomonas aeruginosa. Molecules 2020, 25, 2283. https://doi.org/10.3390/molecules25102283
Cosa S, Rakoma JR, Yusuf AA, Tshikalange TE. Calpurnia aurea (Aiton) Benth Extracts Reduce Quorum Sensing Controlled Virulence Factors in Pseudomonas aeruginosa. Molecules. 2020; 25(10):2283. https://doi.org/10.3390/molecules25102283
Chicago/Turabian StyleCosa, Sekelwa, Jostina R. Rakoma, Abdullahi A. Yusuf, and Thilivhali E. Tshikalange. 2020. "Calpurnia aurea (Aiton) Benth Extracts Reduce Quorum Sensing Controlled Virulence Factors in Pseudomonas aeruginosa" Molecules 25, no. 10: 2283. https://doi.org/10.3390/molecules25102283
APA StyleCosa, S., Rakoma, J. R., Yusuf, A. A., & Tshikalange, T. E. (2020). Calpurnia aurea (Aiton) Benth Extracts Reduce Quorum Sensing Controlled Virulence Factors in Pseudomonas aeruginosa. Molecules, 25(10), 2283. https://doi.org/10.3390/molecules25102283









