Changes in the Electrical Characteristics of Perovskite Solar Cells with Aging Time
Abstract
1. Introduction
2. Results
3. Materials and Methods
3.1. Solar Cell Device Fabrication
3.2. Device Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron−hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef]
- Tavakoli, M.M.; Waleed, A.; Gu, L.; Zhang, D.; Tavakoli, R.; Lei, B.; Su, W.; Fang, F.; Fan, Z. A non−catalytic vapor growth regime for organohalide perovskite nanowires using anodic aluminum oxide templates. Nanoscale 2017, 9, 5828–5834. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Adinolfi, V.; Comin, R.; Yuan, M.; Alarousu, E.; Buin, A.; Chen, Y.; Hoogland, S.; Rothenberger, A.; Katsiev, K.; et al. Low trap−state density and long carrier diffusion in organolead trihalide perovskite single crystals. Science 2015, 347, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, M.M.; Dastjerdi, H.T.; Prochowicz, D.; Yadav, P.; Tavakoli, R.; Saliba, M.; Fan, Z. Highly efficient and stable inverted perovskite solar cells using down−shifting quantum dots as a light management layer and moisture−assisted film growth. J. Mater. Chem. A 2019, 7, 14753–14760. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhao, Y.; Zhang, X.; Yang, X.; Chen, Y.; Chu, Z.; Ye, Q.; Li, X.; Yin, Z.; You, J. Surface passivation of perovskite film for efficient solar cells. Nat. Photonics 2019, 13, 460–466. [Google Scholar] [CrossRef]
- Prochowicz, D.; Tavakoli, M.M.; Alanazi, A.Q.; Trivedi, S.; Tavakoli Dastjerdi, H.; Zakeeruddin, S.M.; Grätzel, M.; Yadav, P. Charge Accumulation, Recombination, and Their Associated Time Scale in Efficient (GUA)x(MA)1−xPbI3−Based Perovskite Solar Cells. ACS Omega 2019, 4, 16840–16846. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, A.; Prochowicz, D.; Tavakoli, M.M.; Trivedi, S.; Kumar, P.; Yadav, P. A review of aspects of additive engineering in perovskite solar cells. J. Mater. Chem. A 2020, 8, 27–54. [Google Scholar] [CrossRef]
- Tavakoli, M.M.; Tavakoli, R.; Yadav, P.; Kong, J. A graphene/ZnO electron transfer layer together with perovskite passivation enables highly efficient and stable perovskite solar cells. J. Mater. Chem. A 2019, 7, 679–686. [Google Scholar] [CrossRef]
- Zhao, Z.; Gu, F.; Rao, H.; Ye, S.; Liu, Z.; Bian, Z.; Huang, C. Metal Halide Perovskite Materials for Solar Cells with Long−Term Stability. Adv. Energy Mater. 2019, 9, 1802671. [Google Scholar] [CrossRef]
- Park, B. wook; Seok, S. Il Intrinsic Instability of Inorganic–Organic Hybrid Halide Perovskite Materials. Adv. Mater. 2019, 31, 1805337. [Google Scholar] [CrossRef]
- Liu, P.; Wang, W.; Liu, S.; Yang, H.; Shao, Z. Fundamental Understanding of Photocurrent Hysteresis in Perovskite Solar Cells. Adv. Energy Mater. 2019, 9, 1803017. [Google Scholar] [CrossRef]
- Mingorance, A.; Xie, H.; Kim, H.; Wang, Z.; Balsells, M.; Morales−Melgares, A.; Domingo, N.; Kazuteru, N.; Tress, W.; Fraxedas, J.; et al. Interfacial Engineering of Metal Oxides for Highly Stable Halide Perovskite Solar Cells. Adv. Mater. Interfaces 2018, 5, 1800367. [Google Scholar] [CrossRef]
- Seok, S. Il; Grätzel, M.; Park, N.G. Methodologies toward Highly Efficient Perovskite Solar Cells. Small 2018, 14, 1704177. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Lu, B.; Niu, F.; Zeng, P.; Zhan, X. Electron−Transport Materials in Perovskite Solar Cells. Small Methods 2018, 2, 1800082. [Google Scholar] [CrossRef]
- Tavakoli, M.M.; Saliba, M.; Yadav, P.; Holzhey, P.; Hagfeldt, A.; Zakeeruddin, S.M.; Grätzel, M. Synergistic crystal and interface engineering for efficient and stable perovskite photovoltaics. Adv. Energy Mater. 2019, 9, 1802646. [Google Scholar] [CrossRef]
- Yang, M.; Zeng, Y.; Li, Z.; Kim, D.H.; Jiang, C.S.; Van De Lagemaat, J.; Zhu, K. Do grain boundaries dominate non−radiative recombination in CH3NH3PbI3 perovskite thin films? Phys. Chem. Chem. Phys. 2017, 19, 5043–5050. [Google Scholar] [CrossRef]
- Prochowicz, D.; Tavakoli, M.M.; Solanki, A.; Goh, T.W.; Pandey, K.; Sum, T.C.; Saliba, M.; Yadav, P. Understanding the effect of chlorobenzene and isopropanol anti−solvent treatments on the recombination and interfacial charge accumulation in efficient planar perovskite solar cells. J. Mater. Chem. A 2018, 6, 14307–14314. [Google Scholar] [CrossRef]
- Yadav, P.; Turren−Cruz, S.H.; Prochowicz, D.; Tavakoli, M.M.; Pandey, K.; Zakeeruddin, S.M.; Grätzel, M.; Hagfeldt, A.; Saliba, M. Elucidation of charge recombination and accumulation mechanism in mixed perovskite solar cells. J. Phys. Chem. C 2018, 122, 15149–15154. [Google Scholar] [CrossRef]
- Wang, H.; Guerrero, A.; Bou, A.; Al−Mayouf, A.M.; Bisquert, J. Kinetic and material properties of interfaces governing slow response and long timescale phenomena in perovskite solar cells. Energy Environ. Sci. 2019, 12, 2054–2079. [Google Scholar] [CrossRef]
- Deng, Y.; Xiao, Z.; Huang, J. Light−Induced Self−Poling Effect on Organometal Trihalide Perovskite Solar Cells for Increased Device Efficiency and Stability. Adv. Energy Mater. 2015, 5, 1500721. [Google Scholar] [CrossRef]
- Nie, W.; Blancon, J.C.; Neukirch, A.J.; Appavoo, K.; Tsai, H.; Chhowalla, M.; Alam, M.A.; Sfeir, M.Y.; Katan, C.; Even, J.; et al. Light−activated photocurrent degradation and self−healing in perovskite solar cells. Nat. Commun. 2016, 7, 11574. [Google Scholar] [CrossRef] [PubMed]
- Prochowicz, D.; Tavakoli, M.M.; Turren−Cruz, S.H.; Pandey, K.; Saliba, M.; Yadav, P. Blue and red wavelength resolved impedance response of efficient perovskite solar cells. Sustain. Energy Fuels 2018, 2, 2407–2411. [Google Scholar] [CrossRef]
- Liu, C.; Fan, J.; Zhang, X.; Shen, Y.; Yang, L.; Mai, Y. Hysteretic behavior upon light soaking in perovskite solar cells prepared via modified vapor−assisted solution process. ACS Appl. Mater. Interfaces 2015, 7, 9066–9071. [Google Scholar] [CrossRef] [PubMed]
- Pockett, A.; Eperon, G.E.; Sakai, N.; Snaith, H.J.; Peter, L.M.; Cameron, P.J. Microseconds, milliseconds and seconds: Deconvoluting the dynamic behaviour of planar perovskite solar cells. Phys. Chem. Chem. Phys. 2017, 19, 5959–5970. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, W.; Van Reenen, S.; Sutton, R.J.; Fan, J.; Haghighirad, A.A.; Johnston, M.B.; Wang, L.; Snaith, H.J. Enhanced UV−light stability of planar heterojunction perovskite solar cells with caesium bromide interface modification. Energy Environ. Sci. 2016, 9, 490–498. [Google Scholar] [CrossRef]
- Deng, X.; Wen, X.; Zheng, J.; Young, T.; Lau, C.F.J.; Kim, J.; Green, M.; Huang, S.; Ho−Baillie, A. Dynamic study of the light soaking effect on perovskite solar cells by in−situ photoluminescence microscopy. Nano Energy 2018, 46, 356–364. [Google Scholar] [CrossRef]
- Yadav, P.; Prochowicz, D.; Alharbi, E.A.; Zakeeruddin, S.M.; Grätzel, M. Intrinsic and interfacial kinetics of perovskite solar cells under photo and bias−induced degradation and recovery. J. Mater. Chem. C 2017, 5, 7799–7805. [Google Scholar] [CrossRef]
- Wetzelaer, G.A.H.; Kuik, M.; Lenes, M.; Blom, P.W.M. Origin of the dark−current ideality factor in polymer: Fullerene bulk heterojunction solar cells. Appl. Phys. Lett. 2011, 99, 153506. [Google Scholar] [CrossRef]
- Abdi−Jalebi, M.; Dar, M.I.; Senanayak, S.P.; Sadhanala, A.; Andaji−Garmaroudi, Z.; Pazos−Outón, L.M.; Richter, J.M.; Pearson, A.J.; Sirringhaus, H.; Grätzel, M.; et al. Charge extraction via graded doping of hole transport layers gives highly luminescent and stable metal halide perovskite devices. Sci. Adv. 2019, 5, eaav2012. [Google Scholar] [CrossRef]
- Prochowicz, D.; Runjhun, R.; Tavakoli, M.M.; Yadav, P.; Saski, M.; Alanazi, A.Q.; Kubicki, D.J.; Kaszkur, Z.; Zakeeruddin, S.M.; Lewiński, J.; et al. Engineering of Perovskite Materials Based on Formamidinium and Cesium Hybridization for High−Efficiency Solar Cells. Chem. Mater. 2019, 31, 1620–1627. [Google Scholar] [CrossRef]
- Sandberg, O.J.; Sundqvist, A.; Nyman, M.; Österbacka, R. Relating Charge Transport, Contact Properties, and Recombination to Open−Circuit Voltage in Sandwich−Type Thin−Film Solar Cells. Phys. Rev. Appl. 2016, 5, 044005. [Google Scholar] [CrossRef]
- Koster, L.J.A.; Mihailetchi, V.D.; Xie, H.; Blom, P.W.M. Origin of the light intensity dependence of the short−circuit current of polymer/fullerene solar cells. Appl. Phys. Lett. 2005, 87, 203502. [Google Scholar] [CrossRef]
- Yadav, P.; Prochowicz, D.; Saliba, M.; Boix, P.; Zakeeruddin, S.; Grätzel, M. Interfacial Kinetics of Efficient Perovskite Solar Cells. Crystals 2017, 7, 252. [Google Scholar] [CrossRef]
- Guerrero, A.; Garcia−Belmonte, G.; Mora−Sero, I.; Bisquert, J.; Kang, Y.S.; Jacobsson, T.J.; Correa−Baena, J.P.; Hagfeldt, A. Properties of Contact and Bulk Impedances in Hybrid Lead Halide Perovskite Solar Cells Including Inductive Loop Elements. J. Phys. Chem. C 2016, 120, 8023–8032. [Google Scholar] [CrossRef]
- Prochowicz, D.; Tavakoli, M.M.; Solanki, A.; Goh, T.W.; Sum, T.C.; Yadav, P. Correlation of recombination and open circuit voltage in planar heterojunction perovskite solar cells. J. Mater. Chem. C 2019, 7, 1273–1279. [Google Scholar] [CrossRef]
- Yadav, P.; Alotaibi, M.H.; Arora, N.; Dar, M.I.; Zakeeruddin, S.M.; Grätzel, M. Influence of the Nature of A Cation on Dynamics of Charge Transfer Processes in Perovskite Solar Cells. Adv. Funct. Mater. 2018, 28, 1706073. [Google Scholar] [CrossRef]
- Almora, O.; Zarazua, I.; Mas−Marza, E.; Mora−Sero, I.; Bisquert, J.; Garcia−Belmonte, G. Capacitive dark currents, hysteresis, and electrode polarization in lead halide perovskite solar cells. J. Phys. Chem. Lett. 2015, 6, 1645–1652. [Google Scholar] [CrossRef]
- Almora, O.; Aranda, C.; Mas−Marzá, E.; Garcia−Belmonte, G. On Mott−Schottky analysis interpretation of capacitance measurements in organometal perovskite solar cells. Appl. Phys. Lett. 2016, 109, 173903. [Google Scholar] [CrossRef]
- Jacobs, D.A.; Shen, H.; Pfeffer, F.; Peng, J.; White, T.P.; Beck, F.J.; Catchpole, K.R. The Two Faces of Capacitance: New Interpretations for Electrical Impedance Measurements of Perovskite Solar Cells and Their Relation to Hysteresis. J. Appl. Phys. 2018, 124, 225702. [Google Scholar] [CrossRef]
- Lin, S.; Yang, B.; Qiu, X.; Yan, J.; Shi, J.; Yuan, Y.; Tan, W.; Liu, X.; Huang, H.; Gao, Y.; et al. Efficient and stable planar hole−transport−material−free perovskite solar cells using low temperature processed SnO2 as electron transport material. Org. Electron. 2018, 53, 235–241. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Phuyal, D.; Du, J.; Tian, L.; Öberg, V.A.; Johansson, M.B.; Cappel, U.B.; Karis, O.; Liu, J.; et al. Inorganic CsPbI3 Perovskite Coating on PbS Quantum Dot for Highly Efficient and Stable Infrared Light Converting Solar Cells. Adv. Energy Mater. 2018, 8, 1702049. [Google Scholar] [CrossRef]
- Sandberg, O.J.; Kurpiers, J.; Stolterfoht, M.; Neher, D.; Meredith, P.; Shoaee, S.; Armin, A. On the Question of the Need for a Built−In Potential in Perovskite Solar Cells. Adv. Mater. Interfaces 2020. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y. Electrical characterization of TiO2/CH3NH3PbI3 heterojunction solar cells. J. Mater. Chem. A 2014, 2, 10244–10249. [Google Scholar] [CrossRef]
- Tavakoli, M.M.; Yadav, P.; Tavakoli, R.; Kong, J. Surface Engineering of TiO2 ETL for Highly Efficient and Hysteresis−Less Planar Perovskite Solar Cell (21.4%) with Enhanced Open−Circuit Voltage and Stability. Adv. Energy Mater. 2018, 8, 1800794. [Google Scholar] [CrossRef]
- Tavakoli, M.M.; Yadav, P.; Prochowicz, D.; Sponseller, M.; Osherov, A.; Bulović, V.; Kong, J. Controllable perovskite crystallization via antisolvent technique using chloride additives for highly efficient planar perovskite solar cells. Adv. Energy Mater. 2019, 9, 1803587. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
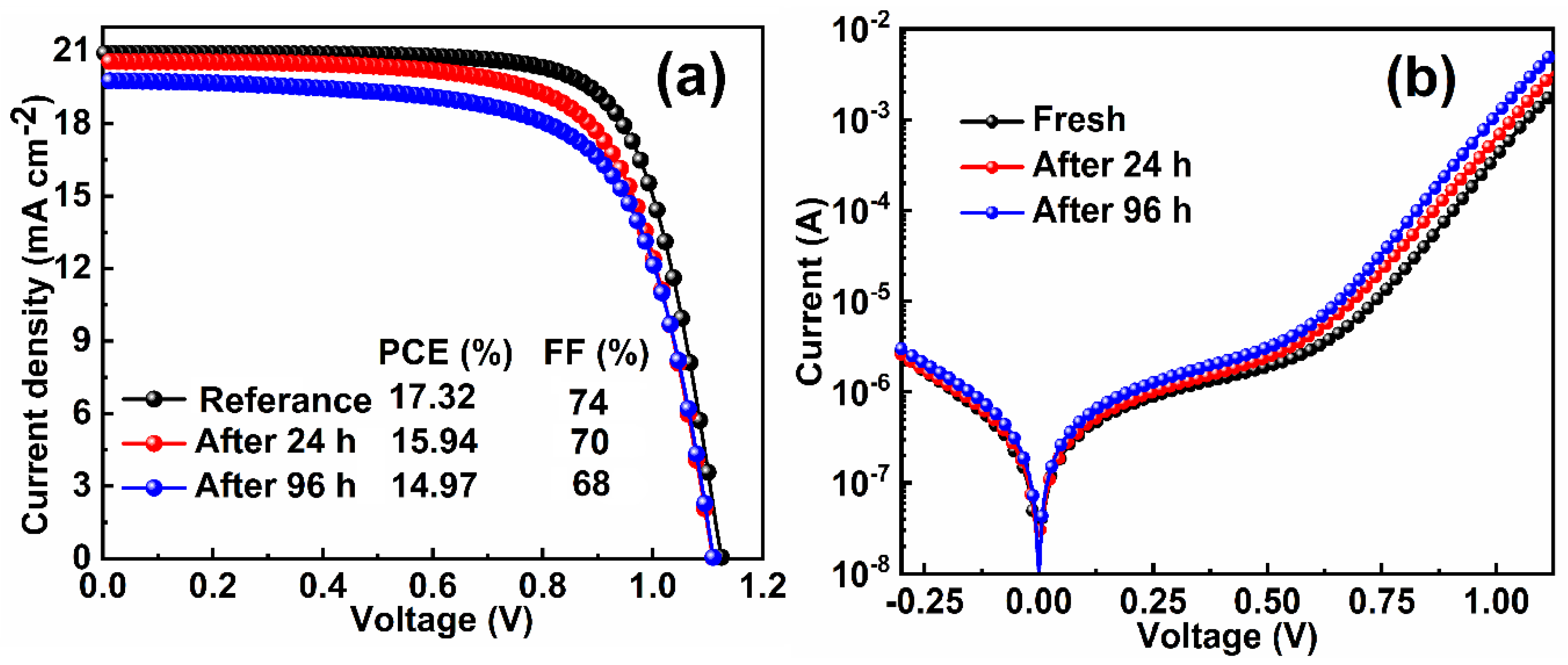
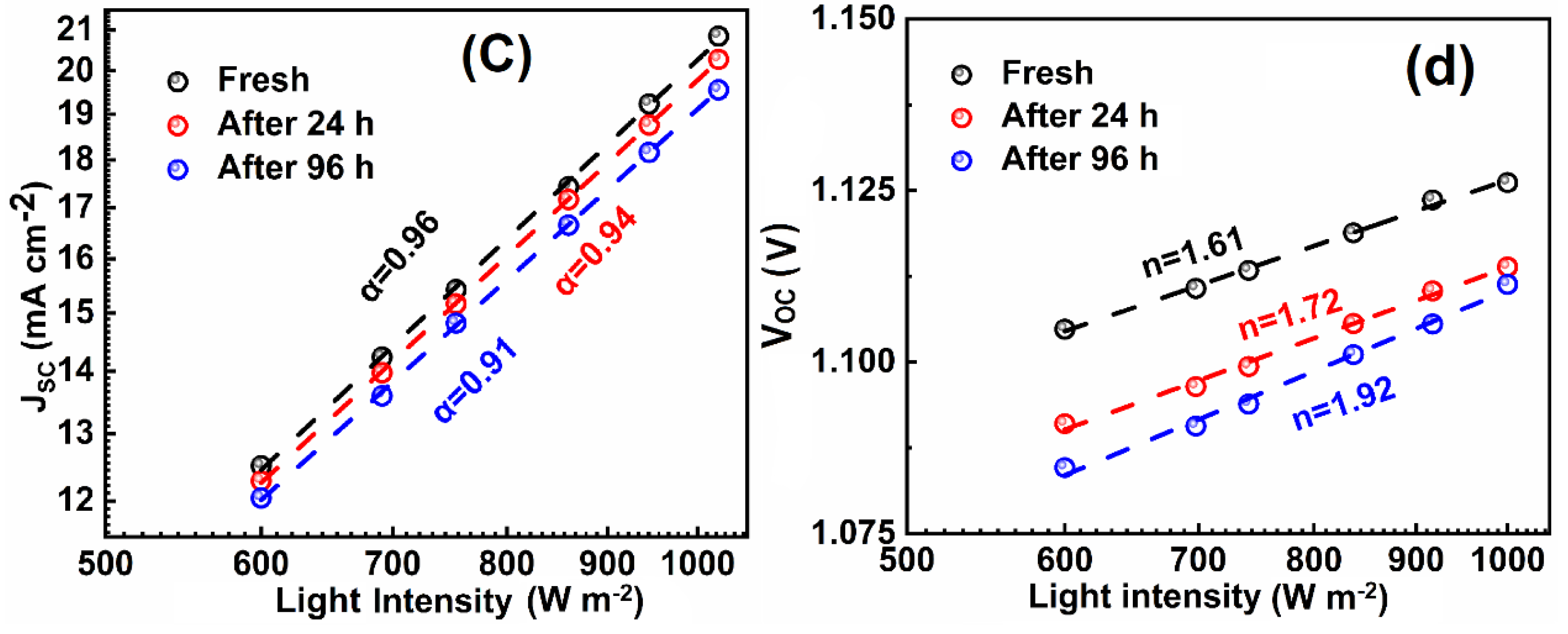
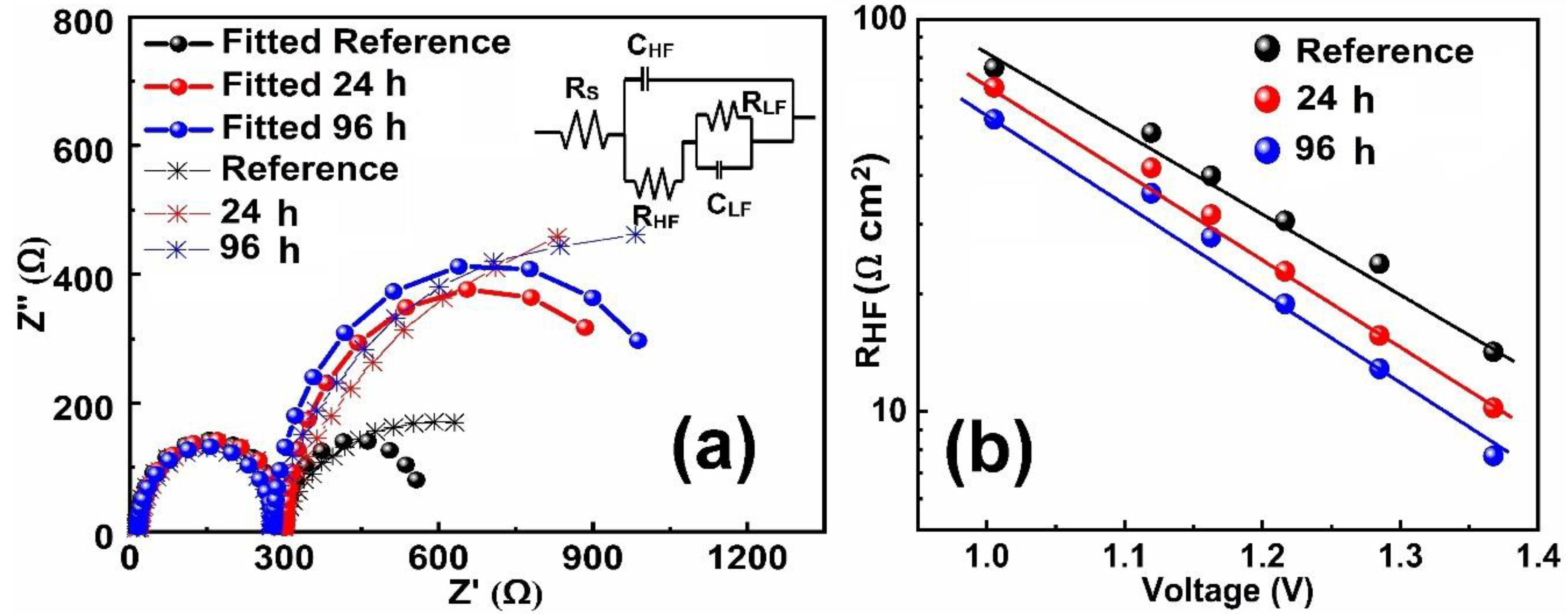
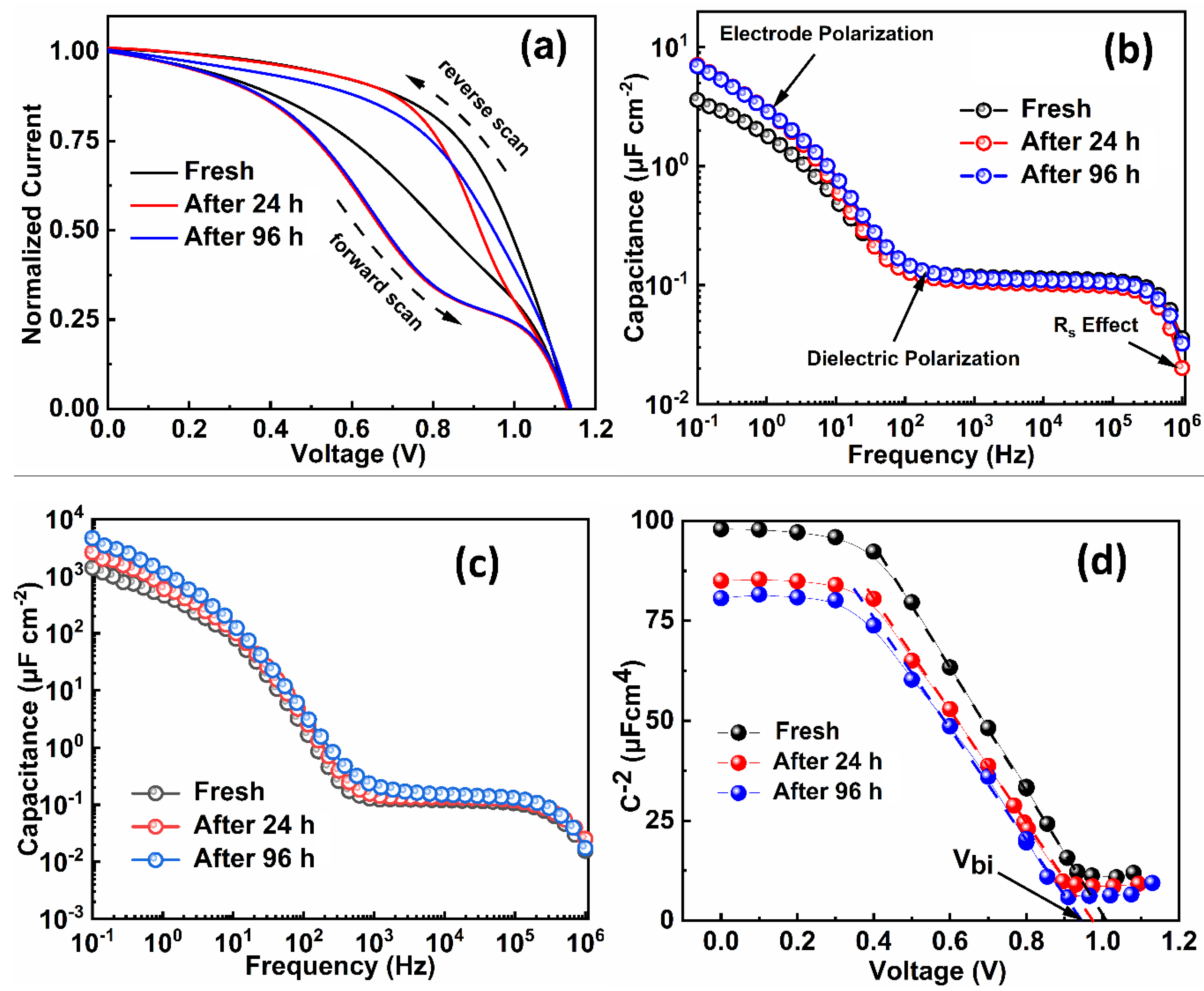
| Index | VOC (V) | JSC (mAcm−2) | FF (%) | PCE (%) | Ideality Factor (n) (Dark) | Ideality Factor (n) (Light) |
|---|---|---|---|---|---|---|
| 0 min | 1.128 | 20.91 | 74 | 17.32 | 2.69 | 1.64 |
| 24 h | 1.110 | 20.57 | 70 | 15.94 | 2.73 | 1.72 |
| 96 h | 1.111 | 19.78 | 68 | 14.97 | 2.84 | 1.92 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahapatra, A.; Parikh, N.; Kumar, P.; Kumar, M.; Prochowicz, D.; Kalam, A.; Tavakoli, M.M.; Yadav, P. Changes in the Electrical Characteristics of Perovskite Solar Cells with Aging Time. Molecules 2020, 25, 2299. https://doi.org/10.3390/molecules25102299
Mahapatra A, Parikh N, Kumar P, Kumar M, Prochowicz D, Kalam A, Tavakoli MM, Yadav P. Changes in the Electrical Characteristics of Perovskite Solar Cells with Aging Time. Molecules. 2020; 25(10):2299. https://doi.org/10.3390/molecules25102299
Chicago/Turabian StyleMahapatra, Apurba, Nishi Parikh, Pawan Kumar, Manoj Kumar, Daniel Prochowicz, Abul Kalam, Mohammad Mahdi Tavakoli, and Pankaj Yadav. 2020. "Changes in the Electrical Characteristics of Perovskite Solar Cells with Aging Time" Molecules 25, no. 10: 2299. https://doi.org/10.3390/molecules25102299
APA StyleMahapatra, A., Parikh, N., Kumar, P., Kumar, M., Prochowicz, D., Kalam, A., Tavakoli, M. M., & Yadav, P. (2020). Changes in the Electrical Characteristics of Perovskite Solar Cells with Aging Time. Molecules, 25(10), 2299. https://doi.org/10.3390/molecules25102299







