The Rise of Synaptic Density PET Imaging
Abstract
:1. Introduction
2. Radiochemistry of SV2A PET Radiotracers
3. Preclinical Developments of SV2A PET Radiotracers
3.1. Drug Metabolism and Pharmacokinetic (DMPK)
3.2. Preclinical PET Imaging with SV2A Radiotracers
4. Clinical Studies with SV2A PET Radiotracers
4.1. Quantification of SV2A PET Radiotracers Binding
4.1.1. UCB-H in Human Brain
4.1.2. UCB-J in Human Brain
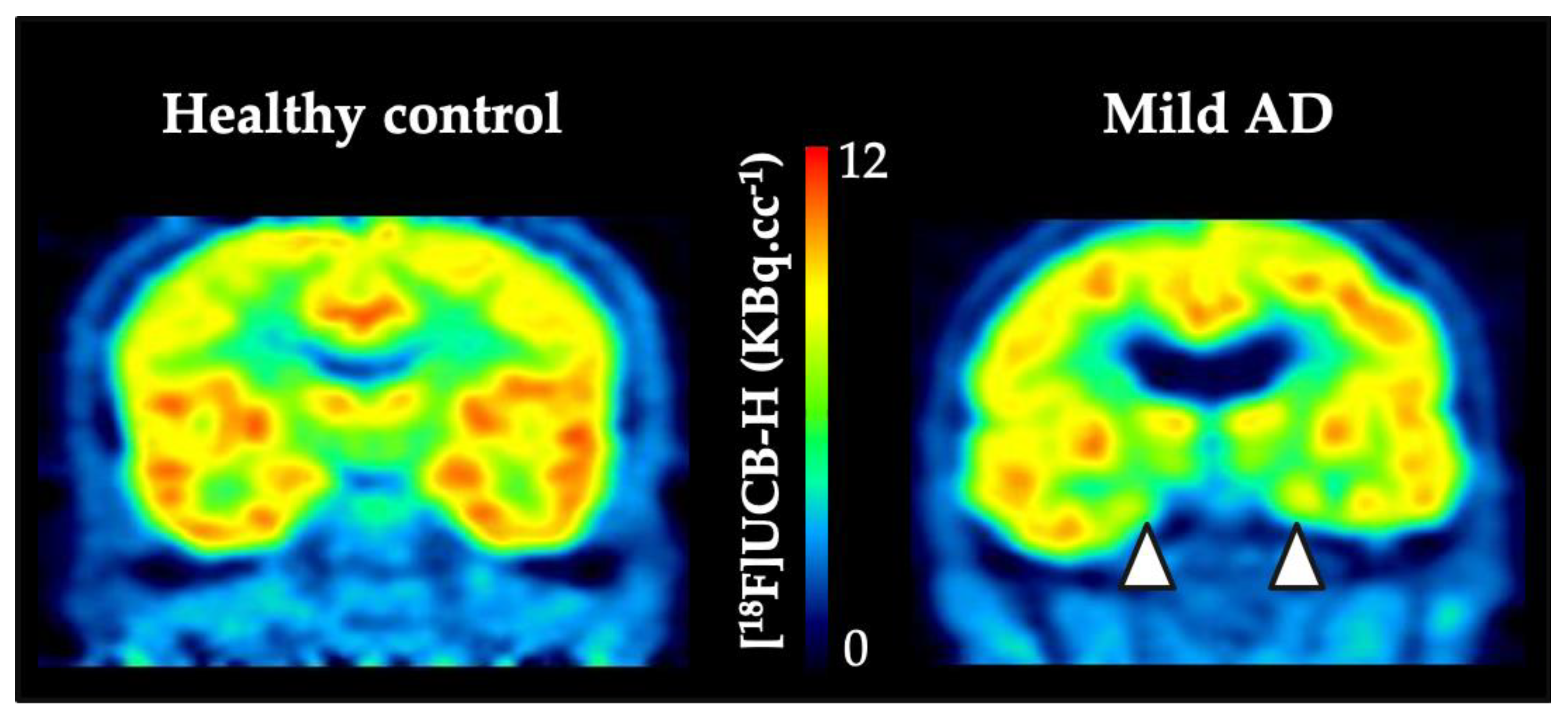
4.2. Clinical Outcomes with SV2A PET Imaging
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lynch, B.A.; Lambeng, N.; Nocka, K.; Kensel-Hammes, P.; Bajjalieh, S.M.; Matagne, A.; Fuks, B. The Synaptic Vesicle Protein Sv2a Is the Binding Site for the Antiepileptic Drug Levetiracetam. Proc. Natl. Acad. Sci. USA 2004, 101, 9861–9866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajjalieh, S.M.; Frantz, G.D.; Weimann, J.M.; McConnell, S.K.; Scheller, R.H. Differential Expression of Synaptic Vesicle Protein 2 (Sv2) Isoforms. J. Neurosci. 1994, 9, 5223–5235. [Google Scholar] [CrossRef] [Green Version]
- Crowder, K.M.; Gunther, J.M.; Jones, T.A.; Hale, B.D.; Zhang, H.Z.; Peterson, M.R.; Scheller, R.H.; Chavkin, C.; Bajjalieh, S.M. Abnormal Neurotransmission in Mice Lacking Synaptic Vesicle Protein 2a (Sv2a). Proc. Natl. Acad. Sci. USA 1999, 26, 15268–15273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartholome, O.; Van den Ackerveken, P.; Sánchez Gil, J.; de la Brassinne Bonardeaux, O.; Leprince, P.; Franzen, R.; Rogister, B. Puzzling out Synaptic Vesicle 2 Family Members Functions. Front. Mol. Neurosci. 2017, 148. [Google Scholar] [CrossRef]
- Bakker, A.; Krauss, G.L.; Albert, M.S.; Speck, C.L.; Jones, L.R.; Stark, C.E.; Yassa, M.A.; Bassett, S.S.; Shelton, A.L.; Gallagher, M. Reduction of Hippocampal Hyperactivity Improves Cognition in Amnestic Mild Cognitive Impairment. Neuron 2012, 3, 467–474. [Google Scholar] [CrossRef] [Green Version]
- Pascal, E.S.; Zhu, L.; Verret, L.; Vossel, K.A.; Orr, A.G.; Cirrito, J.R.; Devidze, N.; Ho, K.; Yu, G.-Q.; Palop, J.J.; et al. Levetiracetam Suppresses Neuronal Network Dysfunction and Reverses Synaptic and Cognitive Deficits in an Alzheimer’s Disease Model. Proc. Natl. Acad. Sci. USA 2012, 109, E2895–E2903. [Google Scholar]
- Terry, R.D.; Masliah, E.; Salmon, D.P.; Butters, N.; DeTeresa, R.; Hill, R.; Hansen, L.A.; Katzman, R. Physical Basis of Cognitive Alterations in Alzheimer’s Disease: Synapse Loss Is the Major Correlate of Cognitive Impairment. Ann. Neurol. 1991, 4, 572–580. [Google Scholar] [CrossRef]
- Scheff, S.W.; Price, D.A.; Schmitt, F.A.; Mufson, E.J. Hippocampal Synaptic Loss in Early Alzheimer’s Disease and Mild Cognitive Impairment. Neurobiol. Aging 2006, 10, 1372–1384. [Google Scholar] [CrossRef]
- Klitgaard, H.; Verdu, P. Levetiracetam: The First Sv2a Ligand for the Treatment of Epilepsy. Expert Opin. Drug Discov. 2007, 2, 11. [Google Scholar] [CrossRef]
- Gillard, M.; Fuks, B.; Michel, P.; Vertongen, P.; Massingham, R.; Chatelain, P. Binding Characteristics of [3h]Ucb 30889 to Levetiracetam Binding Sites in Rat Brain. Eur. J. Pharmacol. 2003, 478, 1–9. [Google Scholar] [CrossRef]
- Klitgaard, H.; Matagne, A.; Gobert, J.; Wulfert, E. Evidence for a Unique Profile of Levetiracetam in Rodent Models of Seizures and Epilepsy. Eur. J. Pharmacol. 1998, 353, 191–206. [Google Scholar] [CrossRef]
- Gillard, M.; Fuks, B.; Leclercq, K.; Matagne, A. Binding Characteristics of Brivaracetam, a Selective, High Affinity Sv2a Ligand in Rat, Mouse and Human Brain: Relationship to Anti-Convulsant Properties. Eur. J. Pharmacol. 2011, 664, 36–44. [Google Scholar] [CrossRef]
- Mercier, J.; Archen, L.; Bollu, V.; Carré, S.; Evrard, Y.; Jnoff, E.; Kenda, B.; Lallemand, B.; Michel, P.; Montel, F.; et al. Discovery of Heterocyclic Nonacetamide Synaptic Vesicle Protein 2a (Sv2a) Ligands with Single-Digit Nanomolar Potency: Opening Avenues Towards the First Sv2a Positron Emission Tomography (Pet) Ligands. Chem. Med. Chem. 2014, 9, 693–698. [Google Scholar] [CrossRef]
- Zhang, L.; Villalobos, A. Strategies to Facilitate the Discovery of Novel Cns Pet Ligands. EJNMMI Radiopharm. Chem. 2017, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Need, A.; Kant, N.; Jesudason, C.; Barth, V. Approaches for the Discovery of Novel Positron Emission Tomography Radiotracers for Brain Imaging. Clin. Transl. Imaging 2017, 5, 265–274. [Google Scholar] [CrossRef]
- Pike, V.W. Considerations in the Development of Reversibly Binding Pet Radioligands for Brain Imaging. Curr. Med. Chem. 2016, 18, 1818–1869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Villalobos, A.; Beck, E.M.; Bocan, T.; Chappie, T.A.; Chen, L.; Grimwood, S.; Heck, S.D.; Helal, C.J.; Hou, X.; et al. Design and Selection Parameters to Accelerate the Discovery of Novel Central Nervous System Positron Emission Tomography (Pet) Ligands and Their Application in the Development of a Novel Phosphodiesterase 2a Pet Ligand. J. Med. Chem. 2013, 56, 4568–4579. [Google Scholar] [CrossRef]
- Hancheng, C.; Mangner, T.J.; Muzik, O.; Wang, M.-W.; Chugani, D.C.; Chugani, H.T. Radiosynthesis of (11)C-Levetiracetam: A Potential Marker for Pet Imaging of Sv2a Expression. ACS Med. Chem. Lett. 2014, 10, 1152–1155. [Google Scholar]
- Warnock, G.I.; Aerts, J.; Bahri, M.A.; Bretin, F.; Lemaire, C.; Giacomelli, F.; Mievis, F.; Mestdagh, N.; Buchanan, T.; Valade, A.; et al. Evaluation of 18f-Ucb-H as a Novel Pet Tracer for Synaptic Vesicle Protein 2a in the Brain. J. Nucl. Med. 2014, 8, 1336–1341. [Google Scholar] [CrossRef] [Green Version]
- Aerts, J.; Otabashi, M.; Giacomelli, F.; Warnock, G.; Bahri, M.; Bretin, F.; Sauvage, X.; Thielen, C.; Lemaire, C.; Salmon, E.; et al. Radiosynthesis and First Small Animal Micropet Imaging of [18f]Ucb-H, a New Fluorine-18 Labelled Tracer Targeting Synaptic Vesicle Protein 2a (Sv2a). EANM Abstr. 2013, 40, S158. [Google Scholar]
- Warnier, C.; Lemaire, C.; Becker, G.; Zaragoza, G.; Giacomelli, F.; Aerts, J.; Otabashi, M.; Bahri, M.A.; Mercier, J.; Plenevaux, A.; et al. Enabling Efficient Positron Emission Tomography (Pet) Imaging of Synaptic Vesicle Glycoprotein 2a (Sv2a) with a Robust and One-Step Radiosynthesis of a Highly Potent 18f-Labeled Ligand ([18f]Ucb-H). J. Med. Chem. 2016, 59, 8955–8966. [Google Scholar] [CrossRef]
- Pike, V.W.; Aigbirhio, F.I. Reactions of Cyclotron-Produced [18f] Fluoride with Diaryliodonium Salts—A Novel Single-Step Route to No-Carrier-Added [18] Fluoroarenes. J. Chem. Soc. Chem. Commun. 1995, 21, 2215–2216. [Google Scholar] [CrossRef]
- Estrada, S.; Lubberink, M.; Thibblin, A.; Sprycha, M.; Buchanan, T.; Mestdagh, N.; Kenda, B.; Mercier, J.; Provins, L.; Gillard, M.; et al. [11c]Ucb-a, a Novel Pet Tracer for Synaptic Vesicle Protein 2a. Nucl. Med. Biol. 2016, 43, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Nabulsi, N.B.; Mercier, J.; Holden, D.; Carre, S.; Najafzadeh, S.; Vandergeten, M.C.; Lin, S.F.; Deo, A.; Price, N.; Wood, M.; et al. Synthesis and Preclinical Evaluation of 11c-Ucb-J as a Pet Tracer for Imaging the Synaptic Vesicle Glycoprotein 2a in the Brain. J. Nucl. Med. 2016, 57, 777–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rokka, J.; Schlein, E.; Eriksson, J. Improved synthesis of SV2A targeting radiotracer [11C]UCB-J. EJNMMI Radiopharm. Chem. 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Sephton, S.M.; Miklovicz, T.; Russell, J.J.; Doke, A.; Li, L.; Boros, I.; Aigbirhio, F.I. Automated radiosynthesis of [11C]UCB-J for imaging synaptic density by positron emission tomography. J. Label. Compd. Radiopharm. 2020, 63, 151–158. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Cai, Z.; Zhang, W.; Holden, D.; Lin, S.-F.; Finnema, S.J.; Shirali, A.; Ropchan, J.; Carre, S.; Mercier, J.; et al. Synthesis and in vivo evaluation of [18F]UCB-J for PET imaging of synaptic vesicle glycoprotein 2A (SV2A). Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1952–1965. [Google Scholar] [CrossRef]
- Li, S.; Cai, Z.; Wu, X.; Holden, D.; Pracitto, R.; Kapinos, M.; Gao, H.; Labaree, D.C.; Nabulsi, N.; Carson, R.E.; et al. Synthesis and in Vivo Evaluation of a Novel PET Radiotracer for Imaging of Synaptic Vesicle Glycoprotein 2A (SV2A) in Nonhuman Primates. ACS Chem. Neurosci. 2018, 10, 1544–1554. [Google Scholar] [CrossRef]
- Constantinescu, C.C.; Tresse, C.; Zheng, M.; Gouasmat, A.; Carroll, V.M.; Mistico, L.; Alagille, D.; Sandiego, C.M.; Papin, C.; Marek, K.; et al. Development and In Vivo Preclinical Imaging of Fluorine-18-Labeled Synaptic Vesicle Protein 2A (SV2A) PET Tracers. Mol. Imaging Boil. 2018, 21, 509–518. [Google Scholar] [CrossRef]
- Cai, Z.; Li, S.; Finnema, S.; Lin, S.; Zhang, W.; Holden, D.; Carson, R.; Huang, Y. Imaging Synaptic Density with Novel 18f-Labeled Radioligands for Synaptic Vesicle Protein-2a (Sv2a): Synthesis and Evaluation in Nonhuman Primates. J. Nucl. Med. 2017, 58 (Suppl. S1), 547. [Google Scholar]
- Cai, Z.; Li, S.; Zhang, W.; Pracitto, R.; Wu, X.; Baum, E.; Finnema, S.J.; Holden, D.; Toyonaga, T.; Lin, S.-F.; et al. Synthesis and Preclinical Evaluation of an 18F-Labeled Synaptic Vesicle Glycoprotein 2A PET Imaging Probe: [18F]SynVesT-2. ACS Chem. Neurosci. 2020, 11, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Knight, A.; Krause, S.; Teceno, T.; Tresse, C.; Li, S.; Cai, Z.; Gouasmat, A.; Carroll, V.M.; Barret, O.; et al. Preclinical In Vitro and In Vivo Characterization of Synaptic Vesicle 2A-Targeting Compounds Amenable to F-18 Labeling as Potential PET Radioligands for Imaging of Synapse Integrity. Mol. Imaging Biol. 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Trump, L.; Lemos, A.; Lallemand, B.; Pasau, P.; Mercier, J.; Lemaire, C.; Luxen, A.; Genicot, C. Late-Stage 18f-Difluoromethyl Labeling of N-Heteroaromatics with High Molar Activity for Pet Imaging. Angew. Chem. Int. Ed. 2019, 131, 13283–13288. [Google Scholar] [CrossRef] [Green Version]
- Trump, L.; Lemos, A.; Jacq, J.; Pasau, P.; Lallemand, B.; Mercier, J.; Genicot, C.; Luxen, A.; Lemaire, C. Development of a General Automated Flow Photoredox 18F-Difluoromethylation of N-Heteroaromatics in an AllinOne Synthesizer. Org. Process. Res. Dev. 2020. [Google Scholar] [CrossRef]
- Serrano, M.E.; Becker, G.; Bahri, M.A.; Seret, A.; Mestdagh, N.; Mercier, J.; Mievis, F.; Giacomelli, F.; Lemaire, C.; Salmon, E.; et al. Evaluating the In Vivo Specificity of [18F]UCB-H for the SV2A Protein, Compared with SV2B and SV2C in Rats Using microPET. Molecules 2019, 24, 1705. [Google Scholar] [CrossRef] [Green Version]
- Bretin, F.; Warnock, G.; Bahri, M.A.; Aerts, J.; Mestdagh, N.; Buchanan, T.; Valade, A.; Mievis, F.; Giacomelli, F.; Lemaire, C.; et al. Preclinical radiation dosimetry for the novel SV2A radiotracer [18F]UCB-H. EJNMMI Res. 2013, 3, 35. [Google Scholar] [CrossRef] [Green Version]
- Becker, G.; Warnier, C.; Serrano, M.E.; Bahri, M.A.; Mercier, J.; Lemaire, C.; Salmon, E.; Luxen, A.; Plenevaux, A. Pharmacokinetic Characterization of [18F]UCB-H PET Radiopharmaceutical in the Rat Brain. Mol. Pharm. 2017, 14, 2719–2725. [Google Scholar] [CrossRef]
- Serrano, M.E.; Bahri, M.A.; Becker, G.; Seret, A.; Mievis, F.; Giacomelli, F.; Lemaire, C.; Salmon, E.; Luxen, A.; Plenevaux, A. Quantification of [18F]UCB-H Binding in the Rat Brain: From Kinetic Modelling to Standardised Uptake Value. Mol. Imaging Boil. 2018, 21, 888–897. [Google Scholar] [CrossRef]
- Serrano, M.E.; Bahri, M.A.; Becker, G.; Seret, A.; Germonpré, C.; Lemaire, C.; Giacomelli, F.; Mievis, F.; Luxen, A.; Salmon, E.; et al. Exploring with [18F]UCB-H the in vivo Variations in SV2A Expression through the Kainic Acid Rat Model of Temporal Lobe Epilepsy. Mol. Imaging Boil. 2020, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Finnema, S.J.; Nabulsi, N.B.; Eid, T.; Detyniecki, K.; Lin, S.-F.; Chen, M.-K.; Dhaher, R.; Matuskey, D.; Baum, E.; Holden, D.; et al. Imaging synaptic density in the living human brain. Sci. Transl. Med. 2016, 8, 348ra96. [Google Scholar] [CrossRef] [Green Version]
- Toyonaga, T.; Smith, L.M.; Finnema, S.J.; Gallezot, J.-D.; Naganawa, M.; Bini, J.; Mulnix, T.; Cai, Z.; Ropchan, J.; Huang, Y.; et al. In Vivo Synaptic Density Imaging with 11C-UCB-J Detects Treatment Effects of Saracatinib in a Mouse Model of Alzheimer Disease. J. Nucl. Med. 2019, 60, 1780–1786. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.-M.; Hannestad, J.; Holden, D.; Kervyn, S.; Nabulsi, N.; Tytgat, D.; Huang, Y.; Chanteux, H.; Staelens, L.; Matagne, A.; et al. Brivaracetam, a selective high-affinity synaptic vesicle protein 2A (SV2A) ligand with preclinical evidence of high brain permeability and fast onset of action. Epilepsia 2015, 57, 201–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahri, M.A.; Plenevaux, A.; Aerts, J.; Bastin, C.; Becker, G.; Mercier, J.; Valade, A.; Buchanan, T.; Mestdagh, N.; LeDoux, D.; et al. Measuring brain synaptic vesicle protein 2A with positron emission tomography and [18 F]UCB-H. Alzheimer’s Dementia: Transl. Res. Clin. Interv. 2017, 3, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Bastin, C.; Bahri, M.A.; Meyer, F.; Manard, M.; Delhaye, E.; Plenevaux, A.; Becker, G.; Seret, A.; Mella, C.; Giacomelli, F.; et al. In vivo imaging of synaptic loss in Alzheimer’s disease with [18F]UCB-H positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging 2019, 47, 390–402. [Google Scholar] [CrossRef]
- Salinas, C.A.; Searle, G.E.; Gunn, R.N. The simplified reference tissue model: Model assumption violations and their impact on binding potential. Br. J. Pharmacol. 2014, 35, 304–311. [Google Scholar] [CrossRef]
- Wahlund, L.O.; Barkhof, F.; Fazekas, F.; Bronge, L.; Augustin, M.; Sjögren, M.; Wallin, A.; Ader, H.; Leys, D.; Pantoni, L.; et al. A New Rating Scale for Age-Related White Matter Changes Applicable to MRI and CT. Stroke 2001, 32, 1318–1322. [Google Scholar] [CrossRef]
- Schain, M.; Benjaminsson, S.; Varnäs, K.; Forsberg, A.; Halldin, C.; Lansner, A.; Farde, L.; Varrone, A. Arterial input function derived from pairwise correlations between PET-image voxels. Br. J. Pharmacol. 2013, 33, 1058–1065. [Google Scholar] [CrossRef]
- Rousset, O.G.; Ma, Y.; Evans, A.C. Correction for partial volume effects in PET: Principle and validation. J. Nucl. Med. 1998, 39, 904–911. [Google Scholar]
- Erlandsson, K.; Buvat, I.; Pretorius, P.H.; Thomas, B.A.; Hutton, B.F. A review of partial volume correction techniques for emission tomography and their applications in neurology, cardiology and oncology. Phys. Med. Boil. 2012, 57, R119–R159. [Google Scholar] [CrossRef] [Green Version]
- Thomas, B.A.; Cuplov, V.; Bousse, A.; Mendes, A.; Thielemans, K.; Hutton, B.F.; Erlandsson, K. PETPVC: A toolbox for performing partial volume correction techniques in positron emission tomography. Phys. Med. Boil. 2016, 61, 7975–7993. [Google Scholar] [CrossRef]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Mazoyer, B.; Joliot, M. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. NeuroImage 2002, 15, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Carson, R.E. Noise Reduction in the Simplified Reference Tissue Model for Neuroreceptor Functional Imaging. J. Cereb. Blood. Flow Metab. 2002, 22, 1440–1452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossano, S.; Toyonaga, T.; Finnema, S.J.; Naganawa, M.; Lu, Y.; Nabulsi, N.; Ropchan, J.; De Bruyn, S.; Otoul, C.; Stockis, A.; et al. Assessment of a white matter reference region for 11C-UCB-J PET quantification. Br. J. Pharmacol. 2019, 271678 19879230. [Google Scholar] [CrossRef]
- Koole, M.; Van Aalst, J.; Devrome, M.; Mertens, N.; Serdons, K.; Lacroix, B.; Mercier, J.; Sciberras, D.; Maguire, R.P.; Van Laere, K. Quantifying SV2A density and drug occupancy in the human brain using [11C]UCB-J PET imaging and subcortical white matter as reference tissue. Eur. J. Nucl. Med. Mol. Imaging 2018, 46, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Varnäs, K.; Stepanov, V.; Halldin, C. Autoradiographic mapping of synaptic vesicle glycoprotein 2A in non-human primate and human brain. Synapse 2020. [Google Scholar] [CrossRef] [Green Version]
- Bretin, F.; Bahri, M.A.; Bernard, C.; Warnock, G.; Aerts, J.; Mestdagh, N.; Buchanan, T.; Otoul, C.; Koestler, F.; Mievis, F.; et al. Biodistribution and Radiation Dosimetry for the Novel SV2A Radiotracer [18F]UCB-H: First-in-Human Study. Mol. Imaging Boil. 2015, 17, 557–564. [Google Scholar] [CrossRef]
- Chen, M.-K.; Mecca, A.P.; Naganawa, M.; Finnema, S.J.; Toyonaga, T.; Lin, S.-F.; Najafzadeh, S.; Ropchan, J.; Lu, Y.; McDonald, J.W.; et al. Assessing Synaptic Density in Alzheimer Disease With Synaptic Vesicle Glycoprotein 2A Positron Emission Tomographic Imaging. JAMA Neurol. 2018, 75, 1215–1224. [Google Scholar] [CrossRef]
- Vanhaute, C.H.R.J.; Ceccarini, J.; Michiels, L.; Sunaert, S.; Lemmens, R.; Emsell, L.; Vandenbulcke, M.; Van Laere, K. Changes in Synaptic Density in Relation to Tau Deposition in Prodromal Alzheimer’s Disease: A Dual Protocol Pet-Mr Study. In Proceedings of the European Association of Nuclear Medicine 2019, EANM, Barcelona, Spain, 12–16 October 2019; Volume 46, pp. S177–S178. [Google Scholar]
- Finnema, S.J.; Rossano, S.; Naganawa, M.; Henry, S.; Gao, H.; Pracitto, R.; Maguire, R.P.; Mercier, J.; Kervyn, S.; Nicolas, J.; et al. A single-center, open-label positron emission tomography study to evaluate brivaracetam and levetiracetam synaptic vesicle glycoprotein 2A binding in healthy volunteers. Epilepsia 2019, 60, 958–967. [Google Scholar] [CrossRef] [Green Version]
- Matuskey, D.; Tinaz, S.; Wilcox, K.C.; Naganawa, M.; Toyonaga, T.; Dias, M.; Henry, S.; Pittman, B.; Ropchan, J.; Nabulsi, N.; et al. Synaptic Changes in Parkinson Disease Assessed with in vivo Imaging. Ann. Neurol. 2020, 87, 329–338. [Google Scholar] [CrossRef] [Green Version]
- Bellucci, A.; Mercuri, N.B.; Venneri, A.; Faustini, G.; Longhena, F.; Pizzi, M.; Missale, C.; Spano, P. Review: Parkinson’s disease: From synaptic loss to connectome dysfunction. Neuropathol. Appl. Neurobiol. 2016, 42, 77–94. [Google Scholar] [CrossRef] [Green Version]
- Onwordi, E.C.; Halff, E.F.; Whitehurst, T.; Mansur, A.; Cotel, M.C.; Wells, L.; Creeney, H.; Bonsall, D.; Rogdaki, M.; Shatalina, E.; et al. Synaptic Density Marker Sv2a Is Reduced in Schizophrenia Patients and Unaffected by Antipsychotics in Rats. Nat Commun. 2020, 11, 246. [Google Scholar] [CrossRef] [PubMed]
- Holmes, S.E.; Scheinost, D.; Finnema, S.J.; Naganawa, M.; Davis, M.T.; DellaGioia, N.; Nabulsi, N.; Matuskey, D.; Angarita, G.A.; Pietrzak, R.H.; et al. Lower Synaptic Density Is Associated with Depression Severity and Network Alterations. Nat. Commun. 2019, 10, 1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds......are available from the authors. |
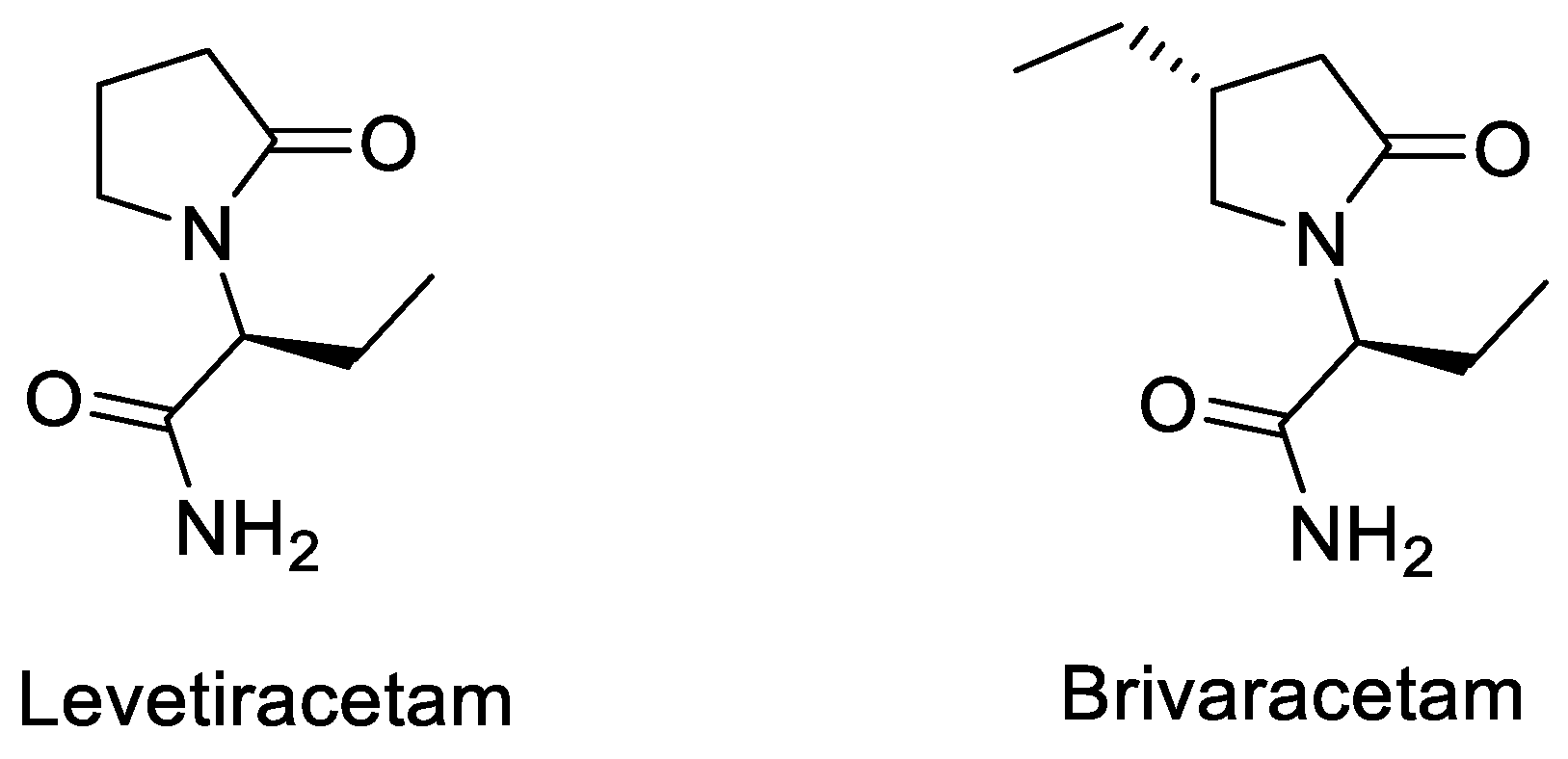
| Entry | Tracer | Ref | Synthesis of the Radiotracer | pIC50 for Human SV2A | Ki (nM) for Human SV2A | Molar Activity (GBq. µmol−1) | RCY (%) |
|---|---|---|---|---|---|---|---|
| 1 | [11C]Levetir-acetam | [18] |  | 5.7 [24] | 2500 | 17 | 8.3 (dc) |
| 2 | [18F]UCB-H | [19] |  | 7.8 | 9.0 | 518 | 15 (ndc) |
| 3 | [21] |  | 815 ± 185 | 35 (ndc) | |||
| 4 | [18F]UCB-A | [23] | 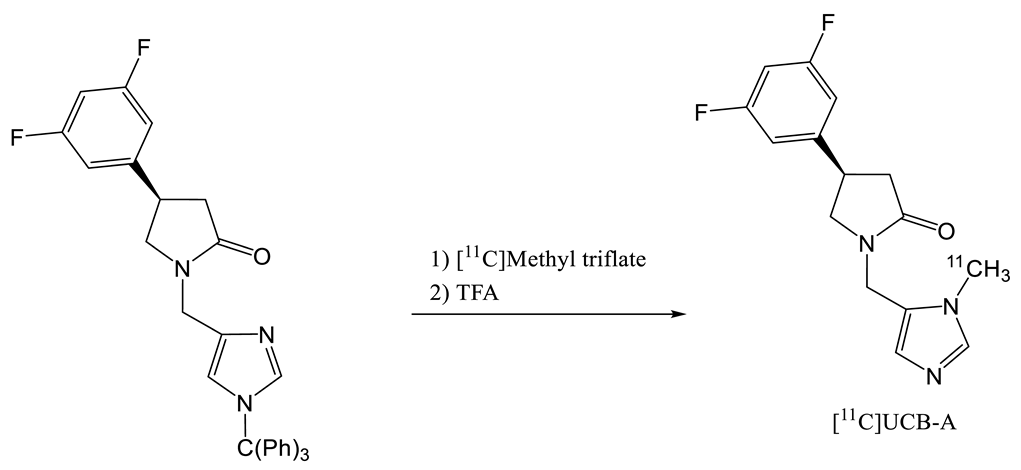 | 7.9 [24] | ND a | 65 | 14 (dc) |
| 5 | [11C]UCB-J | [24] | 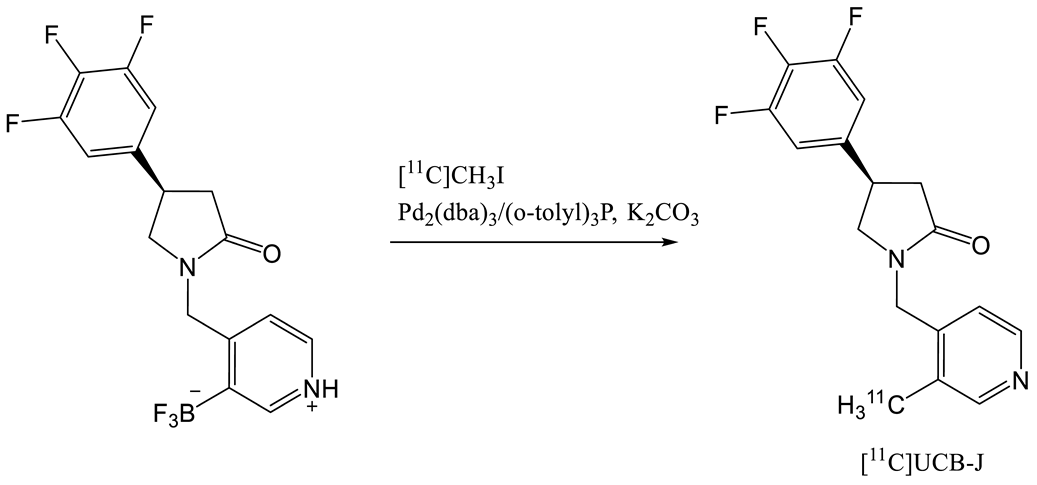 | 8.2 | 1.5 | 215 | 35 (dc) |
| 6 | [18F]UCB-J | [27] | 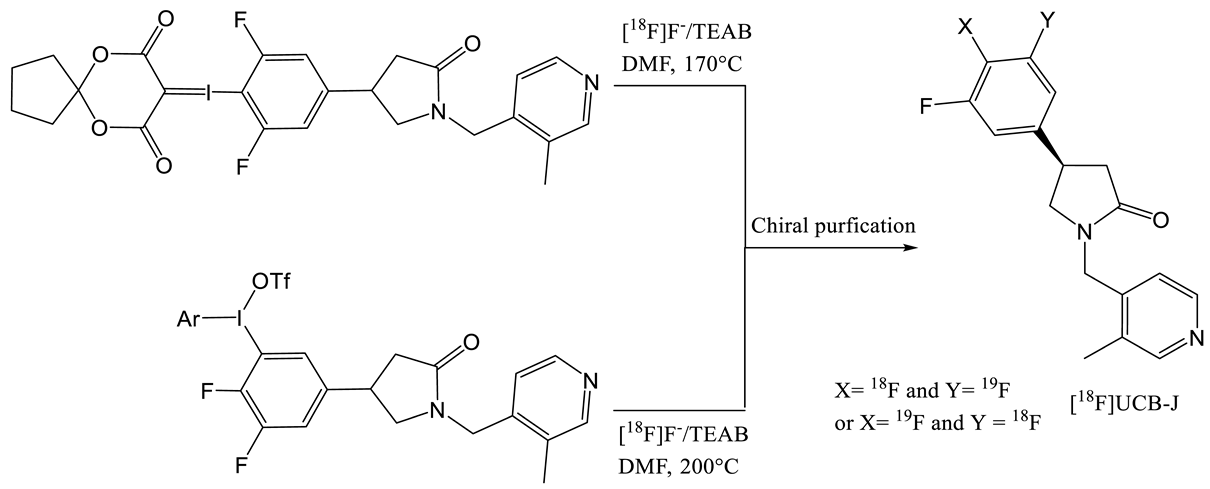 | Similar to [11C]UCB-J | Similar to [11C]UCB-J | 59 ± 36 | 1–2 (ndc) |
| 7 | [18F]SynVesT-1 | [28,29] | 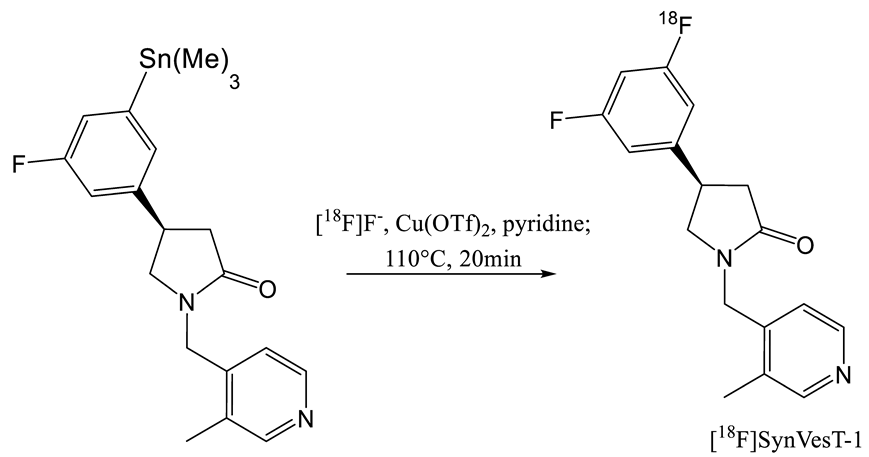 | 8.4 | 2.2–4.7 b | 242 | 19 (ndc) |
| 8 | [18F]SynVesT-2 | [31] | 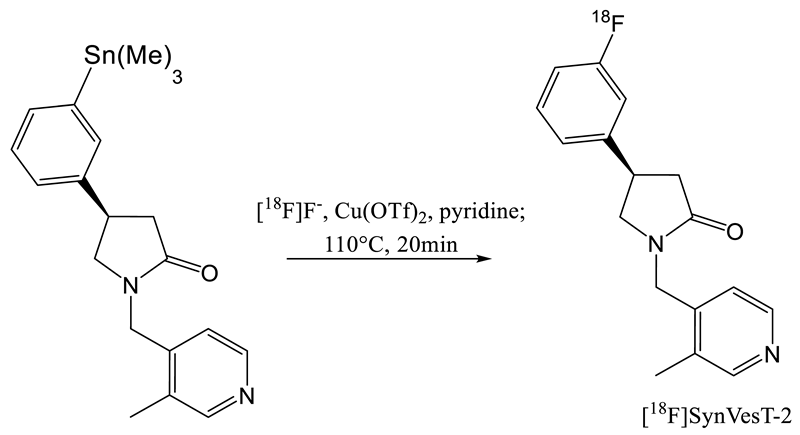 | ND a | 12 | 141 | 7(dc) |
| 9 | [18F]1 | [33] | 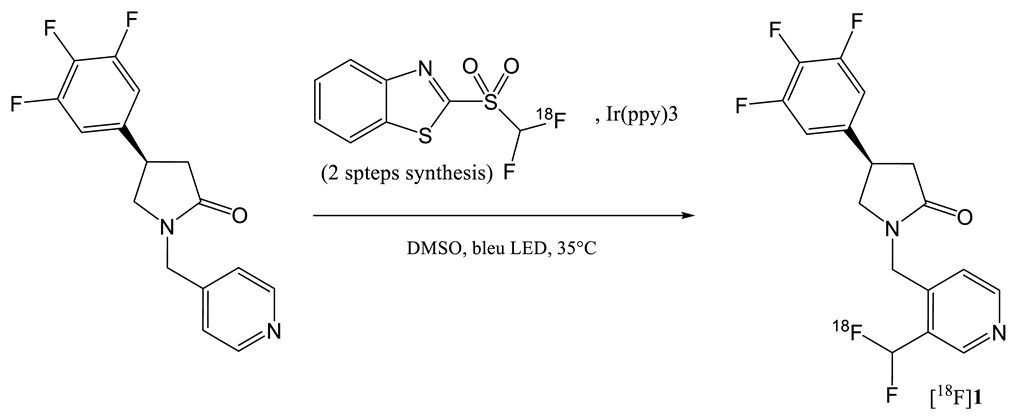 | 8.3 | ND a | 40–80 | 1.5 (dc) |
| Tracers | LogD | ER | Clint µL·min−1·mg·Protein−1 | Fu% Brain | Free B/P Ratio |
|---|---|---|---|---|---|
| [11C]UCB-A | 1.4 | 1.2 | 20 | 12 | 0.6 |
| [18F]UCB-H | 2.3 | 0.7 | 12 | 8 | 1.6 |
| [11C]UCB-J | 2.5 | 0.8 | 16 | 4.5 | 1.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becker, G.; Dammicco, S.; Bahri, M.A.; Salmon, E. The Rise of Synaptic Density PET Imaging. Molecules 2020, 25, 2303. https://doi.org/10.3390/molecules25102303
Becker G, Dammicco S, Bahri MA, Salmon E. The Rise of Synaptic Density PET Imaging. Molecules. 2020; 25(10):2303. https://doi.org/10.3390/molecules25102303
Chicago/Turabian StyleBecker, Guillaume, Sylvestre Dammicco, Mohamed Ali Bahri, and Eric Salmon. 2020. "The Rise of Synaptic Density PET Imaging" Molecules 25, no. 10: 2303. https://doi.org/10.3390/molecules25102303
APA StyleBecker, G., Dammicco, S., Bahri, M. A., & Salmon, E. (2020). The Rise of Synaptic Density PET Imaging. Molecules, 25(10), 2303. https://doi.org/10.3390/molecules25102303






