Porous Activated Carbon from Lignocellulosic Agricultural Waste for the Removal of Acetampirid Pesticide from Aqueous Solutions
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of TPAC
2.1.1. FTIR Analysis
2.1.2. Specific Surface Area and Particle Size
2.1.3. XRD Analysis
2.2. Effect of Different Factors on the Adsorption Process
2.3. Adsorption Kinetic Modeling
2.4. Adsorption Isotherms Modeling
2.5. Thermodynamics
2.6. Comparison of Acetamiprid Adsorption Capacity with Other Adsorbents
2.7. Reusability of the Activated Carbon
3. Materials and Methods
3.1. Apparatus
3.2. Chemicals
3.3. Preparation of Activated Carbon
3.4. Acetamiprid Uptake Study
3.5. Batch Adsorption Experiments
3.6. Adsorption Isotherms and Kinetics
3.7. Adsorption Thermodynamics
3.8. Reusability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- EPA. U.S. Office of Pesticide Programs, Environmental Fate and Efect Division. Revised EFED Risk Assessment of Carbaryl in Support of the Registration Eligibility Decision; EPA: Washington, DC, USA, 2003.
- Seccia, S.; Fidente, P.; Barbini, D.A.; Morrica, P. Multiresidue determination of nicotinoid insecticide residues in drinking water by liquid chromatography with electrospray ionization mass spectrometry. Anal. Chim. Acta 2005, 553, 21–26. [Google Scholar] [CrossRef]
- Morrissey, C.A.; Mineau, P.; Devries, J.H.; Sanchez-Bayo, F.; Liess, M.; Cavallaro, M.C.; Liber, K. Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: A review. Environ. Int. 2015, 74, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Pesticide Fact Sheet: Acetamiprid, EPA (United States Environmental Protection Agency) 2002. Available online: https://www3.epa.gov/pesticides/%20chem_search/reg_actions/%20registration/%20fs_PC-099050_15-Mar-02.pdf (accessed on 25 April 2020).
- Hoyle, S.C.A. Neonicotinoids in California’s Surface Waters: A Preliminary Review of Potential Risk to Aquatic Invertebrates; The Xerces Society for Invertebrate Conservation: Portland, OR, USA, 2016; pp. 1–17. [Google Scholar]
- Barbosa, M.O.; Moreira, N.F.F.; Ribeiro, A.R.; Pereira, M.F.R.; Silva, A.M.T. Occurrence and removal of organicmicropollutants: an overview of the watch list of EU Decision 2015/495. Water Res. 2016, 94, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, L.; Huang, X.; Zheng, S.; Xu, X.; Liu, Z.; Zhang, Y.; Wang, J.; Lin, H.; Xua, D. Adsorption and removal of organophosphorus pesticides from environmental water and soil samples by using magnetic multi-walled carbon nanotubes@organic framework ZIF-8. J. Mater. Sci. 2018, 53, 10772–10783. [Google Scholar] [CrossRef]
- Saeed, M.; Nadeem, R.; Yousaf, M. Removal of industrial pollutant (Reactive Orange 122 dye) using environment-friendly sorbent Trapa bispinosa’s peel and fruit. Int. J. Environ. Sci. Technol. 2014, 12, 1223–1234. [Google Scholar] [CrossRef]
- Carra, I.; Sánchez Pérez, J.A.; Malato, S.; Autin, O.; Jefferson, B.; Jarvis, P. Application of high intensity UVC-LED for the removal of acetamipridwith the photo-Fenton process. Chem. Eng. J. 2015, 264, 690–696. [Google Scholar] [CrossRef]
- Mitsika, E.E.; Christophoridis, C.E.; Fytianos, K. Fenton and Fenton-like oxidation of pesticide acetamiprid in water samples: Kinetic study of the degradation and optimization using response surface methodology. Chemosphere 2013, 93, 1818–1825. [Google Scholar] [CrossRef]
- Fenoll, J.; Garrido, I.; Hellín, P.; Flores, P.; Navarro, S. Photodegradation of neonicotinoid insecticides in water by semiconductor oxides. Environ. Sci. Pollut. Res. 2015, 22, 15055–15066. [Google Scholar] [CrossRef]
- Guzsvány, V.; Rajic, L.; Jović, B.; Orčić, D.; Csanádi, J.; Lazić, S.; Abramović, B.F. Spectroscopic monitoring of photocatalytic degradation of the insecticide acetamiprid and its degradation product 6-chloronicotinic acid on TiO2 catalyst. J. Environ. Sci. Heal. Part. A 2012, 47, 1919–1929. [Google Scholar] [CrossRef]
- Choumane, F.Z.; Benguella, B. Removal of acetamiprid from aqueous solutions with low-cost sorbents. DESALINATION Water Treat. 2014, 1–12. [Google Scholar] [CrossRef]
- Li, S.; Ma, X.; Jiang, Y.; Cao, X. Acetamiprid removal in wastewater by the low-temperature plasma using dielectric barrier discharge. Ecotoxicol. Environ. Saf. 2014, 106, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, N. Investigation of adsorption kinetics and isotherms of imidacloprid as a pollutant from aqueous solution by adsorption onto industrial granular activated carbon. J. Food Agric. Environ. 2007, 5, 425–429. [Google Scholar]
- Zhang, J.; Zhou, A.-N.; Shao, L.; He, P. The use of biochar-amended composting to improve the humification and degradation of sewage sludge. Bioresour. Technol. 2014, 168, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gao, P.; Cui, J.; Zhang, F.; Wang, F.; Cheng, J. Preparation and Cr(VI) removal performance of corncob activated carbon. Environ. Sci. Pollut. Res. 2018, 25, 20743–20755. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S. Egyptian Apricot Stone (Prunus armeniaca) as a Low Cost and Eco-friendly Biosorbent for Oxamyl Removal from Aqueous Solutions. Am. J. Exp. Agric. 2014, 4, 302–321. [Google Scholar] [CrossRef]
- Mohammad, S.; Ahmed, S.M.; Badawi, A.F.M. A comparative adsorption study with different agricultural waste adsorbents for removal of oxamyl pesticide. DESALINATION Water Treat. 2014, 55, 1–12. [Google Scholar] [CrossRef]
- Haq, A.U.; Saeed, M.; Usman, M.; Muneer, M.; Adeel, S.; Abbas, S.; Iqbal, A. Removal of butachlor from aqueous solution using cantaloupe seed shell powder: kinetic, equilibrium and thermodynamic studies. Int. J. Environ. Sci. Technol. 2018, 16, 6029–6042. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Kamalian, S.; Shayeghi, M.; Yousefi, M.; Heidarinejad, Z.; Agarwal, S.; Gupta, V.K. High-performance removal of diazinon pesticide from water using multi-walled carbon nanotubes. Microchem. J. 2019, 145, 486–491. [Google Scholar] [CrossRef]
- Vithanage, M.; Mayakaduwa, S.; Herath, I.; Ok, Y.S.; Mohan, D. Kinetics, thermodynamics and mechanistic studies of carbofuran removal using biochars from tea waste and rice husks. Chemosphere 2016, 150, 781–789. [Google Scholar] [CrossRef]
- Mohammad, S.; Ahmed, S.M. Adsorptive removal of acetamiprid pesticide from aqueous solution using environmentally friendly natural and agricultural wastes. DESALINATION Water Treat. 2019, 145, 280–290. [Google Scholar] [CrossRef]
- Qiu, K.; Song, X.; Tang, G.; Wu, L.; Min, S. Determination of Fipronil in Acetamiprid Formulation by Attenuated Total Reflectance-Mid-Infrared Spectroscopy Combined with Partial Least Squares Regression. Anal. Lett. 2013, 46, 2388–2399. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Xiuli, H.; Haixia, J.; Yong, Z.; Weifeng, H.; Yangfan, Z.; Ping, G.; Rui, D.; Enhui, L. A high performance nitrogen-doped porous activated carbon for supercapacitor derived from pueraria. J. Alloys Comp. 2018, 744, 544–551. [Google Scholar]
- Kılıç, M.Ö.; Apaydın-Varol, E.; Pütün, A.E. Adsorptive removal of phenol from aqueous solutions on activated carbon prepared from tobacco residues: Equilibrium, kinetics and thermodynamics. J. Hazard. Mater. 2011, 189, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafez, A.A.; Li, J. Removal of Pb(II) from aqueous solution by using biochars derived from sugar cane bagasse and orange peel. J. Taiwan Inst. Chem. Eng. 2016, 61, 367–375. [Google Scholar] [CrossRef]
- Sahithya, K.; Das, D.; Das, N. Efective removal of dichlorvos from aqueous solution using biopolymer modifed MMT–CuO composites: equilibrium, kinetic and thermodynamic studies. J. Mol. Liq. 2015, 211, 821–830. [Google Scholar] [CrossRef]
- Njoku, V.; Foo, K.Y.; Hameed, B. Microwave-assisted preparation of pumpkin seed hull activated carbon and its application for the adsorptive removal of 2,4-dichlorophenoxyacetic acid. Chem. Eng. J. 2013, 215, 383–388. [Google Scholar] [CrossRef]
- Doczekalska, B.; Kuśmierek, K.; Swiatkowski, A.; Bartkowiak, M. Adsorption of 2,4-dichlorophenoxyacetic acid and 4-chloro-2-metylphenoxyacetic acid onto activated carbons derived from various lignocellulosic materials. J. Environ. Sci. Heal. Part. B 2018, 53, 290–297. [Google Scholar] [CrossRef]
- Długosz, O.; Banach, M. Kinetic, isotherm and thermodynamic investigations of the adsorption of Ag+ and Cu2+ on vermiculite. J. Mol. Liq. 2018, 258, 295–309. [Google Scholar] [CrossRef]
- Simonin, J.-P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef]
- Kumar, M.; Tamilarasan, R. Kinetics, equilibrium data and modeling studies for the sorption of chromium by Prosopis juliflora bark carbon. Arab. J. Chem. 2017, 10, S1567–S1577. [Google Scholar] [CrossRef]
- Omri, A.; Wali, A.; Benzina, M. Adsorption of bentazon on activated carbon prepared from Lawsonia inermis wood: Equilibrium, kinetic and thermodynamic studies. Arab. J. Chem. 2016, 9, S1729–S1739. [Google Scholar] [CrossRef]
- Ghaedi, M.; Hajati, S.; Karimi, F.; Barazesh, B.; Ghezelbash, G. Equilibrium, kinetic and isotherm of some metal ion biosorption. J. Ind. Eng. Chem. 2013, 19, 987–992. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef]
- Li, F.; Shen, K.; Long, X.; Wen, J.; Xie, X.; Zeng, X.; Liang, Y.; Wei, Y.; Lin, Z.; Huang, W.; et al. Preparation and Characterization of Biochars from Eichornia crassipes for Cadmium Removal in Aqueous Solutions. PLoS ONE 2016, 11, e0148132. [Google Scholar] [CrossRef]
- Fan, S.; Wang, Y.; Wang, Z.; Tang, J.; Tang, J.; Li, X. Removal of methylene blue from aqueous solution by sewage sludge-derived biochar: Adsorption kinetics, equilibrium, thermodynamics and mechanism. J. Environ. Chem. Eng. 2017, 5, 601–611. [Google Scholar] [CrossRef]
- Lima, E.C.A.M.; Machado, F.M. Chapter 3: Kinetic and equilibrium models of adsorption. In Carbon Nanomaterials as Adsorbents for Environmental and Biological Applications; Bergmann, C.P., Machado, F.M., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 33–69. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |



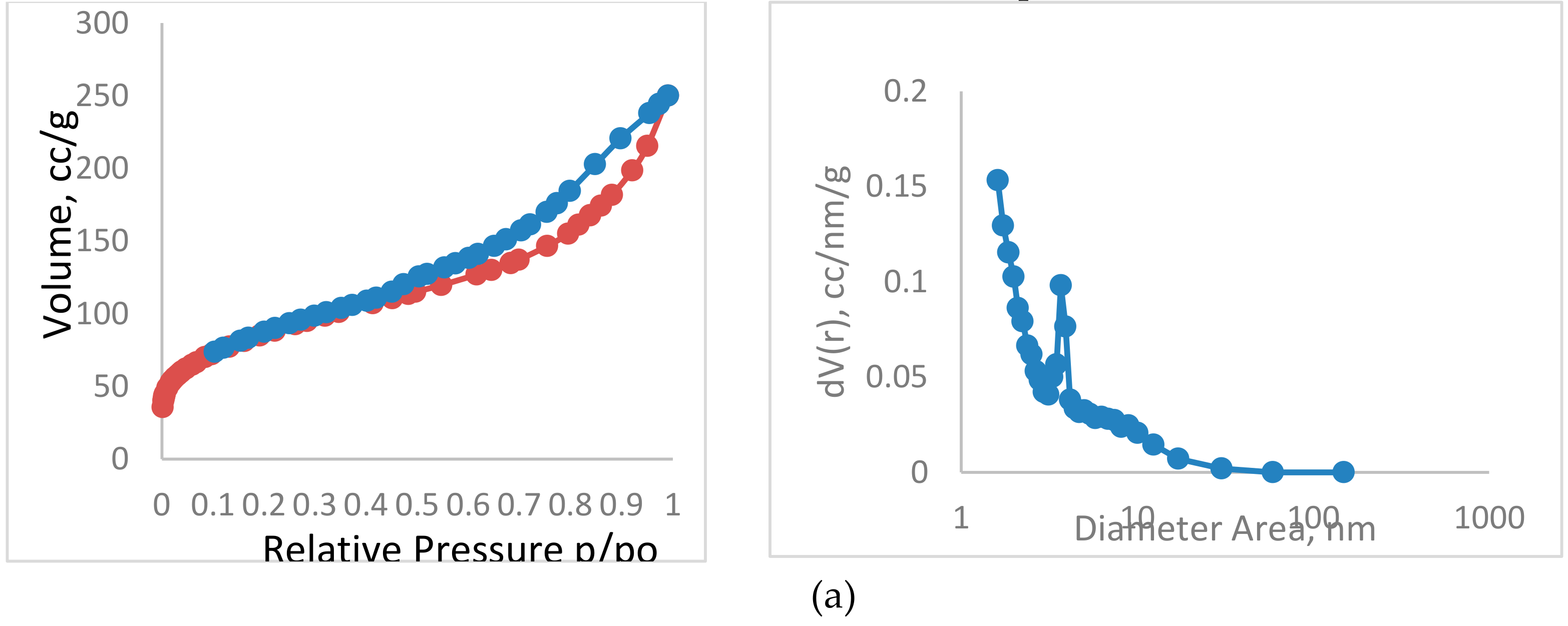








| Samples | Mesopore Size, nm | Micropore Size, nm | Mesopore Volume, cc/g | Micropore Volume, cc/g | Total Pore Volume, cc/g | Mesopore Area, m2/g | Micropore Area, m2/g | Total Surface Area, m2/g |
|---|---|---|---|---|---|---|---|---|
| TPAC (A) | 3.6 | 1.61 | 0.47 | 0.17 | 0.64 | 385.9 | 301.9 | 687.8 |
| TPAC (B) | 3.7 | 1.6 | 0.35 | 0.03 | 0.38 | 196.4 | 99.9 | 296.4 |
| Kinetic Model | Parameter | Values |
|---|---|---|
| Pseudo-first-order | k1(1/min) | 0.01 ± 0.02 |
| qe (mg/g) | 7.3 ± 0.01 | |
| R2 | 0.884 | |
| Pseudo-second-order | k2 (g/mg.min) | 0.005 ± 0.001 |
| qe (mg/g) | 23.3 ± 0.717 | |
| R2 | 0.998 |
| Isotherm Model | Parameter | Values |
|---|---|---|
| Freundlich | Kf | 4.8 ± 0.03 |
| R2 | 0.919 | |
| 1/n | 0.02 ± 0.001 | |
| Langmuir | B (L/mg) | 1.01 ± 0.02 |
| qm (mg/g) | 35.7 ± 0.05 | |
| R2 | 0.997 |
| Temperature (K) | ΔG° (KJ/mol) | ΔH° (KJ/mol) | ΔS° (KJmol−1 K−1) |
|---|---|---|---|
| 298 | −71.7 | −37.8 | −113.5 |
| 308 | −72.8 | ||
| 323 | −74.5 |
| Adsorbent Type | Maximum Adsorption Capacity (mg/g) | Initial Concentration (mg/L) | Contact Time (min) | Optimum pH | Isotherm Fitted | Adsorbent Dose (g) | % Removal | Ref. |
|---|---|---|---|---|---|---|---|---|
| Bentonite | 9.1 | 100 | 30 | 7.0 | Langmuir | 1 | – | [13] |
| Bentonite and clay | 7.8 | 100 | 30 | 7.0 | Langmuir | 1 | – | [13] |
| Kaolin | 7.7 | 100 | 30 | 7.0 | Langmuir | 1 | – | [13] |
| Orange peels activated carbon | 151.5 | 300 | 120 | 5.6 | Freundlich | 1 | 99.4 | [23] |
| Almond shells activated carbon | 370.3 | 300 | 120 | 5.6 | Freundlich | 0.5 | 99.4 | [23] |
| Tangerine peels activated carbon | 35.7 | 25 | 240 | 5.6 | Langmuir | 0.1 | 92.0 | This Study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammad, S.G.; Ahmed, S.M.; Amr, A.E.-G.E.; Kamel, A.H. Porous Activated Carbon from Lignocellulosic Agricultural Waste for the Removal of Acetampirid Pesticide from Aqueous Solutions. Molecules 2020, 25, 2339. https://doi.org/10.3390/molecules25102339
Mohammad SG, Ahmed SM, Amr AE-GE, Kamel AH. Porous Activated Carbon from Lignocellulosic Agricultural Waste for the Removal of Acetampirid Pesticide from Aqueous Solutions. Molecules. 2020; 25(10):2339. https://doi.org/10.3390/molecules25102339
Chicago/Turabian StyleMohammad, Somaia G., Sahar M. Ahmed, Abd El-Galil E. Amr, and Ayman H. Kamel. 2020. "Porous Activated Carbon from Lignocellulosic Agricultural Waste for the Removal of Acetampirid Pesticide from Aqueous Solutions" Molecules 25, no. 10: 2339. https://doi.org/10.3390/molecules25102339
APA StyleMohammad, S. G., Ahmed, S. M., Amr, A. E.-G. E., & Kamel, A. H. (2020). Porous Activated Carbon from Lignocellulosic Agricultural Waste for the Removal of Acetampirid Pesticide from Aqueous Solutions. Molecules, 25(10), 2339. https://doi.org/10.3390/molecules25102339








