Antiadipogenic Effects of Mixtures of Cornus officinalis and Ribes fasciculatum Extracts on 3T3-L1 Preadipocytes and High-Fat Diet-Induced Mice
Abstract
:1. Introduction
2. Results
2.1. Effects of CO and RF on Adipogenesis in Vitro
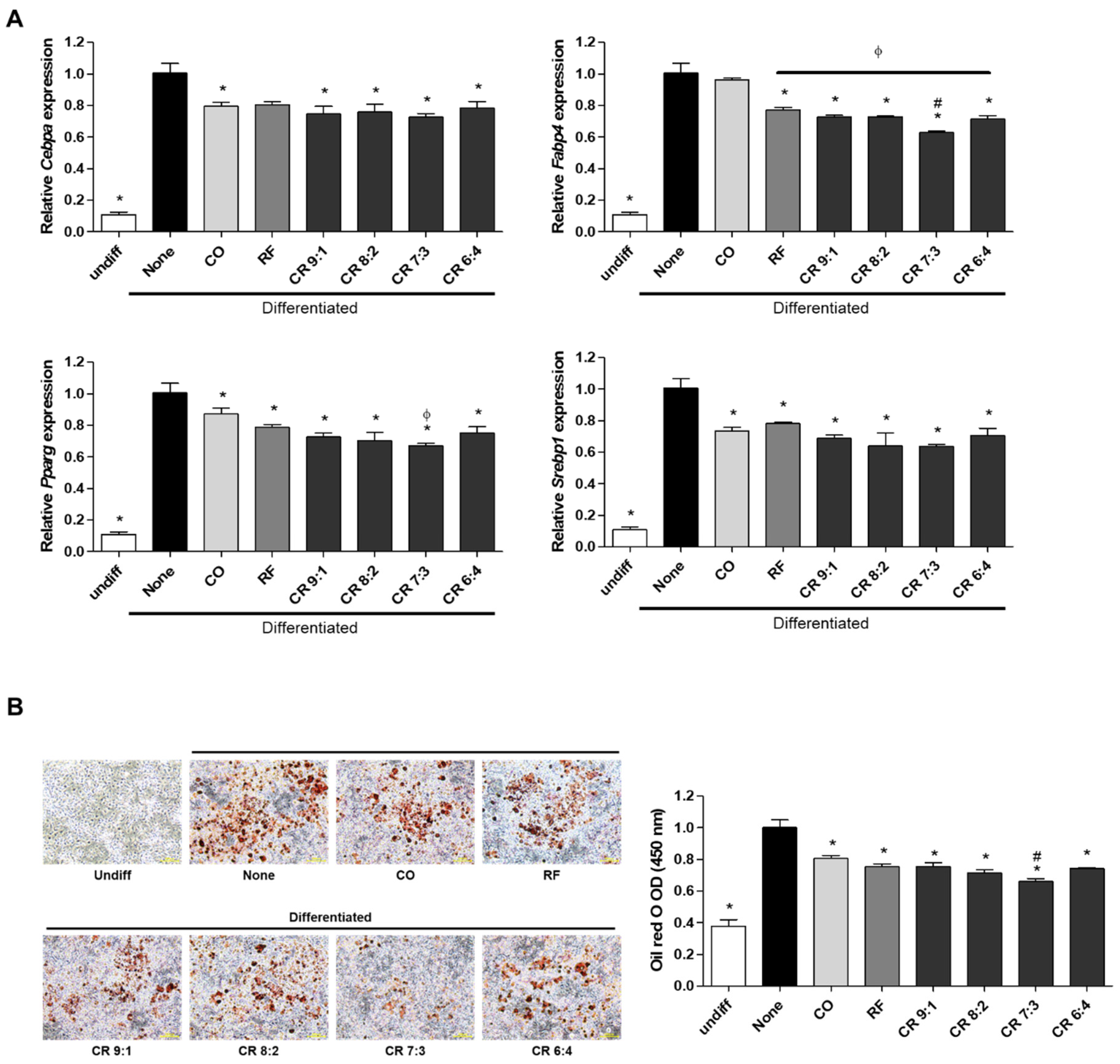
2.2. Effects of CO+RF Extract Mixture on Adipogenesis in 3T3-L1 Cells
2.3. Anti-Obesity Effects of CO and RF in HFD-Induced Murine Obesity Model
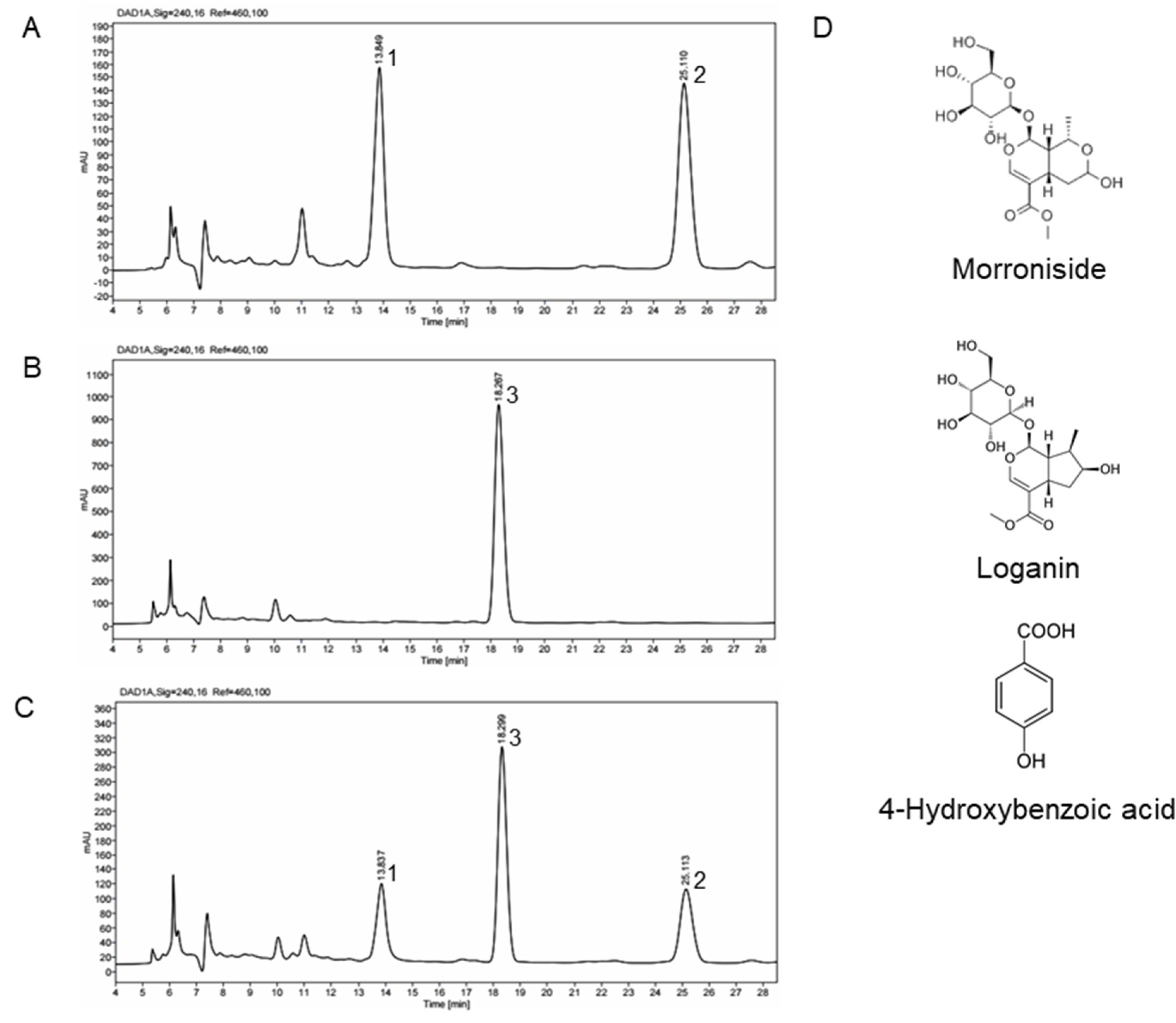
2.4. HPLC Profile of CO and RF Extracts
3. Discussion
4. Materials and Methods
4.1. Preparation of CO and RF Extracts
4.2. HPLC-DAD Analysis of CO and RF Extracts
4.3. Cell Culture and Adipocyte Differentiation
4.4. Cell Viability
4.5. Quantitative Reverse-Transcription Polymerase Chain Reaction
4.6. Oil Red O Staining of Adipocytes
4.7. In Vivo Experiments in an Obesity Murine Model
4.8. Preparation of Tissue Samples and Histology
4.9. Statistical Analysis
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Thaker, V.V. Genetic and epigenetic causes of obesity. Adolesc. Med. State. Art. Rev. 2017, 28, 379–405. [Google Scholar] [PubMed]
- Aleksandar, K. Social causes of obesity. Med. Pregl. 1986, 39, 591–592. [Google Scholar] [PubMed]
- Kotsis, V.; Stabouli, S.; Papakatsika, S.; Rizos, Z.; Parati, G. Mechanisms of obesity-induced hypertension. Hypertens. Res. 2010, 33, 386–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alegria Ezquerra, E.; Castellano Vazquez, J.M.; Alegria Barrero, A. Obesity, metabolic syndrome and diabetes: Cardiovascular implications and therapy. Rev. Esp. Cardiol. 2008, 61, 752–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attie, A.D.; Scherer, P.E. Adipocyte metabolism and obesity. J. Lipid Res. 2009, 50, S395–S399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsushita, K. Mesenchymal stem cells and metabolic syndrome: Current understanding and potential clinical implications. Stem Cells Int. 2016, 2016, 2892840. [Google Scholar] [CrossRef] [Green Version]
- Ryden, M.; Andersson, D.P.; Bernard, S.; Spalding, K.; Arner, P. Adipocyte triglyceride turnover and lipolysis in lean and overweight subjects. J. Lipid Res. 2013, 54, 2909–2913. [Google Scholar] [CrossRef] [Green Version]
- Ndumele, C.E.; Nasir, K.; Conceicao, R.D.; Carvalho, J.A.; Blumenthal, R.S.; Santos, R.D. Hepatic steatosis, obesity, and the metabolic syndrome are independently and additively associated with increased systemic inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1927–1932. [Google Scholar] [CrossRef] [Green Version]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef]
- Hensrud, D.D. Pharmacotherapy for obesity. Med. Clin. North Am. 2000, 84, 463–476. [Google Scholar] [CrossRef]
- Kim, M.K.; Lee, W.Y.; Kang, J.H.; Kang, J.H.; Kim, B.T.; Kim, S.M.; Kim, E.M.; Suh, S.H.; Shin, H.J.; Lee, K.R.; et al. 2014 clinical practice guidelines for overweight and obesity in Korea. Endocrinol. Metab. (Seoul) 2014, 29, 405–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancini, M.C.; Halpern, A. Pharmacological treatment of obesity. Arq. Bras. Endocrinol. Metabol. 2006, 50, 377–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.F.; Cheung, B.M. Rise and fall of anti-obesity drugs. World J. Diabetes 2011, 2, 19–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Ishaq, A.; Alshawsh, M.A.; Chik, Z.B. Evaluating the oestrogenic activities of aqueous root extract of Asparagus africanus Lam in female Sprague-Dawley rats and its phytochemical screening using Gas Chromatography-Mass Spectrometry (GC/MS). PeerJ. 2019, 7, e7254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teiten, M.H.; Gaascht, F.; Dicato, M.; Diederich, M. Anticancer bioactivity of compounds from medicinal plants used in European medieval traditions. Biochem. Pharmacol. 2013, 86, 1239–1247. [Google Scholar] [CrossRef]
- Che, C.T.; Zhang, H. Plant natural products for human health. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.A.A.; Akhter, N.; Auckloo, B.N.; Khan, I.; Lu, Y.; Wang, K.; Wu, B.; Guo, Y.W. Structural diversity, biological properties and applications of natural products from cyanobacteria. A review. Mar. Drugs 2017, 15. [Google Scholar] [CrossRef] [Green Version]
- Mathur, S.; Hoskins, C. Drug development: Lessons from nature. Biomed. Rep. 2017, 6, 612–614. [Google Scholar] [CrossRef] [Green Version]
- Ulrich-Merzenich, G.; Panek, D.; Zeitler, H.; Wagner, H.; Vetter, H. New perspectives for synergy research with the "omic"-technologies. Phytomedicine 2009, 16, 495–508. [Google Scholar] [CrossRef]
- Rejhova, A.; Opattova, A.; Cumova, A.; Sliva, D.; Vodicka, P. Natural compounds and combination therapy in colorectal cancer treatment. Eur. J. Med. Chem. 2018, 144, 582–594. [Google Scholar] [CrossRef]
- Lv, X.; Dai, G.; Lv, G.; Chen, Y.; Wu, Y.; Shen, H.; Xu, H. Synergistic interaction of effective parts in Rehmanniae radix and Cornus officinalis ameliorates renal injury in C57BL/KsJ-db/db diabetic mice: Involvement of suppression of AGEs/RAGE/SphK1 signaling pathway. J. Ethnopharmacol. 2016, 185, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Mechesso, A.F.; Lee, S.J.; Park, N.H.; Kim, J.Y.; Im, Z.E.; Suh, J.W.; Park, S.C. Preventive effects of a novel herbal mixture on atopic dermatitis-like skin lesions in BALB/C mice. BMC Complement. Altern. Med. 2019, 19, 25. [Google Scholar] [CrossRef] [PubMed]
- Telang, N.T.; Nair, H.B.; Wong, G.Y.C. Growth inhibitory efficacy of Cornus officinalis in a cell culture model for triple-negative breast cancer. Oncol. Lett. 2019, 17, 5261–5266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, N.H.; Seo, C.S.; Lee, H.Y.; Jung, D.Y.; Lee, J.K.; Lee, J.A.; Song, K.Y.; Shin, H.K.; Lee, M.Y.; Seo, Y.B.; et al. Hepatoprotective and antioxidative activities of Cornus officinalis against acetaminophen-induced hepatotoxicity in mice. Evid. Based Complement. Alternat. Med. 2012, 2012, 804924. [Google Scholar] [PubMed] [Green Version]
- Kim, J.Y.; Kim, Y.K.; Choi, M.K.; Oh, J.; Kwak, H.B.; Kim, J.J. Effect of Cornus officinalis on receptor activator of nuclear factor-kappaB ligand (RANKL)-induced osteoclast differentiation. J. Bone Metab. 2012, 19, 121–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, W.; Zhao, J.; Lee, J.H.; Akanda, M.R.; Cho, J.H.; Kim, S.K.; Choi, Y.J.; Park, B.Y. Neuroprotective effects of Cornus officinalis on stress-induced hippocampal deficits in rats and H2O2-induced neurotoxicity in SH-SY5Y neuroblastoma cells. Antioxidants (Basel) 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.W.; Kim, S.J.; Ahn, E.M.; Oh, S.R.; Lee, H.J.; Jeong, J.A.; Lee, J.Y. Ribes fasciculatum var. chinense attenuated allergic inflammation in vivo and in vitro. Biomol. Ther. (Seoul) 2014, 22, 547–552. [Google Scholar] [CrossRef] [Green Version]
- Jeon, H.; Cha, D.S. Anti-aging properties of Ribes fasciculatum in Caenorhabditis elegans. Chin. J. Nat. Med. 2016, 14, 335–342. [Google Scholar]
- Dat, N.T.; Cai, X.F.; Shen, Q.; Lee, I.S.; Kim, Y.H. New inhibitor against nuclear factor of activated T cells transcription from Ribes fasciculatum var. chinense. Chem. Pharm. Bull. (Tokyo) 2005, 53, 114–117. [Google Scholar] [CrossRef] [Green Version]
- Park, E.; Kim, J.; Yeo, S.; Kim, G.; Ko, E.H.; Lee, S.W.; Li, W.Y.; Choi, C.W.; Jeong, S.Y. Antiadipogenic effects of loganic acid in 3T3-L1 preadipocytes and ovariectomized mice. Molecules 2018, 23. [Google Scholar] [CrossRef] [Green Version]
- Peltz, G.; Aguirre, M.T.; Sanderson, M.; Fadden, M.K. The role of fat mass index in determining obesity. Am. J. Hum. Biol. 2010, 22, 639–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, A.; Misra, A. Hepatic steatosis, insulin resistance, and adipose tissue disorders. J. Clin. Endocrinol. Metab. 2002, 87, 3019–3022. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism 2019, 92, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sun, A.; Wu, S.; Liu, R. Preparative purification of morroniside and loganin from Fructus corni by combination of macroporous absorption resin and HSCCC. J. Chromatogr. Sci. 2009, 47, 333–336. [Google Scholar]
- Li, M.; Wang, W.; Wang, P.; Yang, K.; Sun, H.; Wang, X. The pharmacological effects of morroniside and loganin isolated from Liuweidihuang Wan, on MC3T3-E1 cells. Molecules 2010, 15, 7403–7414. [Google Scholar] [CrossRef]
- Park, E.; Ryu, M.J.; Kim, N.K.; Bae, M.H.; Seo, Y.; Kim, J.; Yeo, S.; Kanwal, M.; Choi, C.W.; Heo, J.Y.; et al. Synergistic neuroprotective effect of schisandra chinensis and Ribes fasciculatum on neuronal cell death and scopolamine-induced cognitive impairment in rats. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [Green Version]
- Winter, A.N.; Brenner, M.C.; Punessen, N.; Snodgrass, M.; Byars, C.; Arora, Y.; Linseman, D.A. Comparison of the Neuroprotective and Anti-Inflammatory Effects of the Anthocyanin Metabolites, Protocatechuic Acid and 4-Hydroxybenzoic Acid. Oxid. Med. Cell. Longev. 2017, 2017, 6297080. [Google Scholar] [CrossRef] [Green Version]
- Knight, J.A. Diseases and disorders associated with excess body weight. Ann. Clin. Lab. Sci. 2011, 41, 107–121. [Google Scholar]
- Sharma, A.M.; Staels, B. Review: Peroxisome proliferator-activated receptor gamma and adipose tissue--understanding obesity-related changes in regulation of lipid and glucose metabolism. J. Clin. Endocrinol. Metab. 2007, 92, 386–395. [Google Scholar] [CrossRef]
- He, W.; Barak, Y.; Hevener, A.; Olson, P.; Liao, D.; Le, J.; Nelson, M.; Ong, E.; Olefsky, J.M.; Evans, R.M. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc. Natl. Acad. Sci. U S A 2003, 100, 15712–15717. [Google Scholar] [CrossRef] [Green Version]
- Garin-Shkolnik, T.; Rudich, A.; Hotamisligil, G.S.; Rubinstein, M. FABP4 attenuates PPARgamma and adipogenesis and is inversely correlated with PPARgamma in adipose tissues. Diabetes 2014, 63, 900–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darlington, G.J.; Ross, S.E.; MacDougald, O.A. The role of C/EBP genes in adipocyte differentiation. J. Biol. Chem. 1998, 273, 30057–30060. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.D.; Finegold, M.J.; Bradley, A.; Ou, C.N.; Abdelsayed, S.V.; Wilde, M.D.; Taylor, L.R.; Wilson, D.R.; Darlington, G.J. Impaired energy homeostasis in C/EBP alpha knockout mice. Science 1995, 269, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Rosen, E.D.; Brun, R.; Hauser, S.; Adelmant, G.; Troy, A.E.; McKeon, C.; Darlington, G.J.; Spiegelman, B.M. Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol. Cell 1999, 3, 151–158. [Google Scholar] [CrossRef]
- Rosen, E.D.; Hsu, C.H.; Wang, X.; Sakai, S.; Freeman, M.W.; Gonzalez, F.J.; Spiegelman, B.M. C/EBPalpha induces adipogenesis through PPARgamma: A unified pathway. Genes Dev. 2002, 16, 22–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eberle, D.; Hegarty, B.; Bossard, P.; Ferre, P.; Foufelle, F. SREBP transcription factors: Master regulators of lipid homeostasis. Biochimie 2004, 86, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.L.; Jeon, Y.D.; Park, J.; Rim, H.K.; Jeong, M.Y.; Lim, H.; Ko, S.G.; Jang, H.J.; Lee, B.C.; Lee, K.T.; et al. Corni Fructus Containing Formulation Attenuates Weight Gain in Mice with Diet-Induced Obesity and Regulates Adipogenesis through AMPK. Evid. Based Complementary Altern. Med. 2013, 2013, 423741. [Google Scholar] [CrossRef] [Green Version]
- Park, E.; Lim, E.; Yeo, S.; Yong, Y.; Yang, J.; Jeong, S.Y. Anti-Menopausal Effects of Cornus officinalis and Ribes fasciculatum Extract In Vitro and In Vivo. Nutrients 2020, 12. [Google Scholar] [CrossRef] [Green Version]
- Inoue, M.; Ohtake, T.; Motomura, W.; Takahashi, N.; Hosoki, Y.; Miyoshi, S.; Suzuki, Y.; Saito, H.; Kohgo, Y.; Okumura, T. Increased expression of PPARgamma in high fat diet-induced liver steatosis in mice. Biochem. Biophys. Res. Commun. 2005, 336, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Walker, A.K.; Atkin, J.D. Stress signaling from the endoplasmic reticulum: A central player in the pathogenesis of amyotrophic lateral sclerosis. IUBMB Life 2011, 63, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Kubota, N.; Terauchi, Y.; Miki, H.; Tamemoto, H.; Yamauchi, T.; Komeda, K.; Satoh, S.; Nakano, R.; Ishii, C.; Sugiyama, T.; et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol. Cell 1999, 4, 597–609. [Google Scholar] [CrossRef]
- Millward, C.A.; Heaney, J.D.; Sinasac, D.S.; Chu, E.C.; Bederman, I.R.; Gilge, D.A.; Previs, S.F.; Croniger, C.M. Mice with a deletion in the gene for CCAAT/enhancer-binding protein beta are protected against diet-induced obesity. Diabetes 2007, 56, 161–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, E.; Lee, C.G.; Jeong, H.; Yeo, S.; Kim, J.A.; Jeong, S.-Y. Antiadipogenic Effects of Mixtures of Cornus officinalis and Ribes fasciculatum Extracts on 3T3-L1 Preadipocytes and High-Fat Diet-Induced Mice. Molecules 2020, 25, 2350. https://doi.org/10.3390/molecules25102350
Park E, Lee CG, Jeong H, Yeo S, Kim JA, Jeong S-Y. Antiadipogenic Effects of Mixtures of Cornus officinalis and Ribes fasciculatum Extracts on 3T3-L1 Preadipocytes and High-Fat Diet-Induced Mice. Molecules. 2020; 25(10):2350. https://doi.org/10.3390/molecules25102350
Chicago/Turabian StylePark, Eunkuk, Chang Gun Lee, Hyesoo Jeong, Subin Yeo, Ji Ae Kim, and Seon-Yong Jeong. 2020. "Antiadipogenic Effects of Mixtures of Cornus officinalis and Ribes fasciculatum Extracts on 3T3-L1 Preadipocytes and High-Fat Diet-Induced Mice" Molecules 25, no. 10: 2350. https://doi.org/10.3390/molecules25102350





